Abstract
Introduction and hypothesis
The impact of body mass index (BMI) on pelvic floor recovery after an obstetric anal sphincter injury (OASI) is unclear. The aim of this study was to evaluate the hypothesis that urinary incontinence (UI) and anal incontinence (AI) are more common in overweight and obese women than in normal-weight women 8 weeks postpartum in women with OASI.
Methods
A population-based cohort study including 6,595 primiparous women, with an OASI, delivered between 2014 and 2019. Exposure and questionnaire data were retrieved from the Swedish Perineal Laceration Registry.
Uni- and multivariate analyses were used to compare normal-weight (BMI ≤24.9, reference), overweight (25.0–29.9), and obese (≥ 30) women with regard to UI and AI at 8 weeks post-partum.
Results
Multivariate analyses showed an increased risk for urinary incontinence (OR 1.54, 95% CI 1.27–1.87) among overweight women as well as among obese women (OR 1.72, 95% CI 1.32–2.24). In contrast to our hypothesis, both overweight women (OR 0.68, 95% CI 0.56–0.83) and obese women (OR 0.65, 95% CI 0.49–0.87) were at a decreased risk for any gas and/or faecal incontinence after adjustment to possible confounding factors.
The absolute rate of AI was 40.1% among normal-weight women, 34.2% among overweight women, and 29.1% in the obese group.
Conclusions
Urinary incontinence is more common, whereas AI is less common among overweight and obese women than in primiparous women with a BMI <24.9, 8 weeks after an OASI. The new finding, that overweight women report less AI than normal-weight women, merits further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstetrical anal sphincter injury (OASI) is a major risk factor for anal incontinence (AI), defined as involuntary loss of feces or gas, fecal incontinence (FI), defined as involuntary loss of feces [1], urinary incontinence (UI), sexual dysfunction, and affected quality of life [2, 3]. The prevalence of OASI among primiparous women varies between 1.4% and 16%, with a large variation between different countries around the world [4]. OASI includes both third- and fourth-degree perineal lacerations, involving the anal sphincter muscle complex and/or the anorectal epithelium [5].
It is well known that maternal overweight and obesity are risk factors for several adverse obstetric outcomes and postpartum complications [6, 7]. However, two large cohort studies have shown that obese women have a decreased risk for OASI compared with normal-weight women. It is not known whether this is a true decreased risk or if it is due to insufficient diagnostics in this group [8, 9]. The prevalence of obesity and overweight is increasing among women of childbearing age globally. In Sweden in 2019, 27.2% of the pregnant women were overweight (body mass index [BMI] 25–29.9) and a further 15.7% were obese (BMI ≥30) [10].
The prevalence of AI and UI after an OASI varies in the literature, as do the definitions of AI and UI used in studies. Risk factors described for developing FI and UI after an OASI are age, race, antenatal UI, and BMI [10]. Another study indicates that maternal obesity is a risk factor for anal incontinence after childbirth regardless of whether an OASI had been diagnosed or not [11]. For women at risk of developing AI and UI after an OASI, it is of great importance to target prevention, treatment and follow-up related to these risks [12]. To the best of our knowledge, no previous population-based studies have examined the association between maternal BMI and AI, UI, and quality of life after a first-time vaginal delivery complicated by an OASI.
Thus, the primary aim of this study was to examine the impact of maternal BMI on pelvic floor dysfunction at 8 weeks after a first-time vaginal delivery complicated by an OASI.
The hypothesis was that AI and UI were more common in obese and overweight women at 8 weeks postpartum than in normal-weight women.
Materials and methods
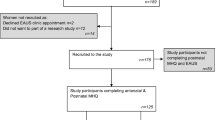
This is a nationwide population-based cohort study based on data from the Swedish Perineal Laceration Registry (PLR) between January 2014 and April 2019 including 6,595 women who had a first-time vaginal birth with an OASI.
The PLR is a section of the Swedish National Quality Register of Gynecological Surgery [13]. The register has previously been described in detail [14, 15]. Briefly, the PLR is a growing nationwide register that started in 2014, and as of 2021 has encompassed all delivery units in Sweden. The Swedish delivery units have joined the register successively since the PLR started.
The PLR provides survey data of patient-reported outcome and experience, as well as data extracted from women’s medical records. Patient-reported data are collected by postal or electronic questionnaires at three points after delivery: first, before discharge from hospital after childbirth concerning pre-pregnancy information, second, at 8 weeks, and finally at 1 year postpartum, concerning pelvic floor function and complications. For the purposes of the present study, the pre-pregnancy and 8-week questionnaires were analysed (Appendices 1 and 2). The questionnaires include questions about several aspects of pelvic floor dysfunction. In the target group interviews preceding the introduction of the PLR, questionnaire brevity was considered very important. Because of that, and to attain rates of completed questionnaires that are as high as possible, only a few selected questions about pelvic floor function were chosen. The questionnaires have been face-, context-, consistency- and content-validated. Those women who reported involuntary leakage of gas or feces were also presented with further questions that comprise the Wexner score for anal incontinence [3]. The Wexner scores of the BMI groups were compared, with a cut-off level ≥2 to define a degree of anal incontinence that affects quality of life after an OASI [3] .
Extracted maternal and obstetrical characteristics were age, BMI, prevalence of diabetes mellitus types I and II (DM), prevalence of inflammatory bowel disease (IBD, Crohn’s disease or ulcerative colitis), prevalence of urinary and anal incontinence, fetal presentation at birth, fetal birth weight, duration of second stage of labour, episiotomy at delivery and mode of vaginal delivery. Data related to the degree and repair of the perineal laceration was anaesthesia during repair and prophylactic antibiotics during repair, suture techniques, suture materials used for the external, internal sphincter and the perineum.
The study population was divided into five BMI groups: underweight (>18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obesity (30–34.9 kg/m2) and morbid obesity (≥35 kg/m2) for analysis concerning maternal and obstetrical characteristics, as well as repair of the perineal laceration.
Outcomes of the present study, in relation to maternal BMI (three groups BMI ≤ 24.9, overweight [25.0–29.9 kg/m2], and obesity [≥30]) were: prevalence of urinary incontinence, defined as urinary incontinence once a week or more often; anal incontinence defined as any anal incontinence; urinary retention yes/no; any infections treated with antibiotics; wound-complications yes/no; re-operation yes/no; unplanned visits at an out-patient unit; and other complications as reported 8 weeks postpartum. The used definition of anal incontinence is reported involuntary loss of feces or flatus [1]. The Wexner score was calculated based on the supplementary questions only in women who answered “yes” to the question about incontinence of gas or feces at 8 weeks postpartum. The Wexner score contains questions about incontinence of gas, liquid stool, solid stool, use of pads, and change of lifestyle, and how often these parameters are present (never/rarely/1–3 times monthly/1–3 times weekly/daily).
For the purposes of dichotomization, women who reported symptoms once a week or more often were categorized into one group. Women who answered no to the question about any gas and/or fecal incontinence, and women with symptoms less frequent than once a week were categorised into a comparative group.
The total Wexner score was also dichotomised into the groups “Wexner score ≥2” and “Wexner score <2” (see Table 3).
Ethical approval
The Regional Ethical Review Board in Linköping approved the study on 20 April 2016; Dnr 2016/144-31.
Statistics
Data were analysed using SPSS version 27 (IBM, Armonk, NY, USA). The descriptive analyses of the categorical background data were presented as total number of patients (N), and percentage of each variable in each BMI group. Continuous data were presented as mean and one standard deviation (SD) or median and interquartile range (IQR), if not normally distributed. Pearson’s Chi-squared test was used to compare categorical variables. Analysis of variance was used to compare mean value of age.
p Values < 0.05 were considered significant. Variables with few available cases were not statistically analysed. Binary logistic regression analyses were used for comparison among the three BMI groups, normal weight (BMI ≤24.9), overweight (25.0–29.9) and obesity (≥30) with BMI ≤24.9 as the reference group. Risk estimates were presented as crude odds ratios (ORs) with 95% confidence intervals (CIs).
Multivariate analysis was used for comparison among the BMI groups. Potential confounders were selected by univariate analysis. Variables with a significant relation to the outcome in the primary multivariate analysis were included in the final adjusted odds ratio (OR) calculation. Risk estimates are presented as adjusted OR with 95% CI.
Non-parametric tests were used to analyse the duration of the second stage of labour, and days to resumption of normal ADL, in the numerical background data. Pearson’s Chi-squared test was used to analyse categorical parameters.
Results
The study population consisted of 6,595 women with a first-time vaginal birth and who had a third- or fourth-degree perineal laceration registered in the Swedish PLR during the study period.
Background data are shown in Table 1. In the study population 3.1% of the women were underweight, 60.4% were normal weight, 25.2% were overweight and 11.3% were obese (8.1% obesity class I and 3.2% total in obesity classes II and III). There was no difference in BMI between responders and non-responders.
The rate of episiotomy was lower with higher BMI (p= 0.046). There was a significant difference in the proportion of episiotomies between the underweight group and the morbidly obese group (p< 0.050). Also, the duration of the second stage of labour decreased with higher BMI (p<0.005). A statistically significant difference was seen in the fetal birth weight between the BMI groups (p<0.050). There were no other differences regarding obstetrical characteristics between the BMI groups. The ratio between the perineal lacerations (third or fourth degree) was similar within the respective BMI group.
Data related to the repair of the perineal laceration are shown in Table 2. The use of prophylactic antibiotics during repair, suture techniques and suture materials did not differ substantially among the BMI groups.
The outcomes of the 8-week follow-up questionnaires are presented in Table 3. Out of the study population, 75% (4,978/6,595) of the women with available data on BMI provided answers to the 8 week follow up questionnaires. There was no difference in BMI between responders and non-responders.
Urinary incontinence was significantly more common among overweight and obese women than in normal-weight women (BMI ≤24.9). The absolute rate of urinary incontinence was 15.6% (N=462) among normal-weight women, 20.9% (N=246) among overweight women and 21.4% (N=111) in the obese group.
Overweight women with an OASI were at a 43% significantly increased risk for urinary incontinence (OR 1.43, 95% CI 1.20–1.69) compared with normal-weight women, the corresponding OR for obese women was 47% (OR 1.47, 95% CI 1.17–1.86). When adjusted for age and pre-pregnancy urinary incontinence, the risk for urinary incontinence remained significant, with an increased risk for urinary incontinence among both overweight women (OR 1.54, 95% CI 1.27–1.87) and obese women (OR 1.72, 95% CI 1.32–2.24; Table 4).
The risk for any anal incontinence was lower among overweight and obese women than in women with BMI ≤24.9. The absolute rate of reported incontinence for gas or feces was 40.1% (N=1,258) among normal weight women, 34.2% (N=431) among overweight women and 29.1% (N=159) in the obese group. Women who were overweight had a 15% decreased risk for incontinence of gas or feces (OR 0.85, 95% CI 0.75–0.97), whereas obese women had a 27% decreased risk, compared with normal-weight women (OR 0.73, 95% CI 0.60–0.88).
Adjustments were made for pre-pregnancy gas and/or fecal incontinence, fetal birth weight, degree of perineal laceration (3rd or 4th degree) and use of prophylactic antibiotics during repair. After adjustments, overweight women were at a decreased risk for any gas and/or fecal incontinence (OR 0.68, 95% CI 0.56–0.83) and obese women were at a decreased risk (OR 0.65, 95% CI 0.49–0.87).
Among the women reporting any incontinence for gas or feces, the type or extent of incontinence according to the Wexner score was not influenced by BMI. A cut-off level at a Wexner score of ≥2 was used to estimate the effect on quality of life in women with AI postpartum [3]. There was no difference in the reported quality of life between the BMI groups.
Overweight women were at a 20% decreased risk of wound complications compared with normal-weight women (OR 0.80, 95% CI 0.67–0.96). After adjustments were made for age, mode of delivery and episiotomy, the decreased risk for patient-reported wound complications remained significant (OR 0.80, 95% CI 0.67–0.95).
The rates of reported infections treated with antibiotics and reported re-operations did not differ over the BMI strata. Median number of days reported to resumption of normal ADL was 10 days among normal-weight women and overweight women, whereas obese women had a median time of return to resumption of normal ADL of 11 days.
The women’s report at 8 weeks regarding any complications was classified by the physician who had repaired the perineal laceration as no, mild or severe complications. The rate of complications was somewhat lower among overweight and obese women than among normal-weight women, but the association was not statistically significant.
Discussion
This study showed that women who were obese or overweight were at a lower risk for developing AI at 8 weeks postpartum, compared with normal-weight women, after a first-time vaginal delivery complicated by an OASI. On the contrary, overweight and obese women were at a higher risk for developing UI at 8 weeks postpartum after a first-time vaginal delivery complicated by an OASI, compared with normal weight women. The result that overweight and obese women are at a lower risk of developing AI 8 weeks after a first-time vaginal delivery complicated by an OASI is a new finding. UI is well known to correlate with higher BMI in older age groups, even after deliveries without OASI [16]. That UI correlates with BMI as early as 8 weeks after OASI has not been shown before.
The results in the literature vary concerning the prevalence and risk factors of AI and UI after an OASI among primiparous women [17,18,19]. Different follow-up questionnaires, follow-up periods and definitions of the incontinence can account for these differences [1, 4]. A few previous studies have reported BMI as a risk factor of developing AI and UI after OASI. Contrary to our study results, a Danish study by Gommesen et al. showed that the risk for developing AI after a first-time delivery with an OASI increased with higher BMI. In their prospective cohort study including 200 primiparous women with an OASI, they found 1 year postpartum that the risk for AI increased by 8% per 1-unit increase in BMI [20]. Further, Burgio et al. investigated risk factors for fecal and urinary incontinence in primiparous women after an OASI [10]. Their study population was interviewed directly postpartum, at 6 weeks after delivery and after 6 months concerning UI and FI. Their study population consisted of 335 primiparous women with an OASI who completed interviews at 6 months postpartum. They used similar questions, regarding pre-delivery symptoms and postpartum symptoms of UI and AI, to those used in the present study. They found that FI was associated with higher pre-delivery BMI as well as race, antenatal UI and older maternal age at delivery. Their findings differ from our study results according to the risk for AI after a first-time vaginal delivery complicated by an OASI. Regarding the risk for UI after an OASI, the results concur. AI is less common than UI in general. Our considerably larger study population with 4,978 women included may explain that our results differ from those of other studies, namely a decreased risk for AI with a higher BMI.
Possibly, what seems to protect overweight and obese women from having an OASI in the first place [8, 9] could also protect them from AI in the aftermath. Women with overweight and obesity have a shorter second stage of labour than normal-weight women [21]. This could have a protective effect on levator muscle injury and the pudendal nerve. Also, overweight and obese nulliparous women have a higher anovaginal distance (AVD) in the active phase of labour. A higher AVD may play a protective role in the anovaginal complex [22], as well as for the levator ani muscle insertions and the pudendal nerve. These possible explanations merit further studies.
The higher risk for UI after a first-time vaginal delivery complicated by an OASI among overweight and obese women is less surprising as obese women have a higher intra-abdominal pressure [23]. Also, overweight and obesity increase the load on the pelvic floor [24]. This could contribute to the higher rates of UI in women who are overweight or obese than in normal-weight women after an OASI.
Wound infections in the postpartum period after having an OASI can cause wound dehiscence [25]. A prospective Danish cohort study including 200 primiparous women with an OASI, found that obese women were at a higher risk for wound infections and wound dehiscence than normal-weight women [26]. We did not find any increased risk for wound infection or wound complications in the higher BMI groups compared with normal-weight women. On the contrary, the overweight group were at a decreased risk for wound complications compared with normal-weight women. This could also contribute to a lower risk for developing AI in this BMI group. The obese group were at a 17% decreased risk for wound complications compared with normal-weight women.
At the 8-week follow-up questionnaire 458 women out of a total of 1,373 women reported changes in life-style due to fecal incontinence in the Wexner questionnaire. There was no difference among the BMI groups regarding the effect of AI on quality of life. Later on in life, both AI and UI have been shown to have a negative effect on the quality of life, regardless of BMI [27].
In summary, the present study highlights pelvic floor complications after OASI in overweight and obese women. These results lead to further questions regarding whether targeted interventions could improve pelvic floor function in this group of women.
A study by Mathé et al suggests that early pelvic floor muscle training (PFMT), starting within 1 month postpartum, after having an OASI, could reduce symptoms of AI and may prevent the medium-term consequences of AI [28].
The efficacy of PFMT postpartum in women who are obese or overweight compared with normal-weight women after OASI is not well described. A Cochrane review from 2020 implies that focus on the impact of BMI on PFMT in women with AI and UI postpartum should be further investigated [12].
A strength of the present study is that the PLR is a population-based register that contains a large cohort available for evaluation. The large number of primiparous women with OASI registered in the PLR makes it possible to analyse subgroups within the population.
Another advantage of this study is that the population in the PLR is gathered from several centres and the findings could be generalisable to settings with similar demographic profiles and delivery practices.
Limitations of register studies are generally incomplete coverage and quality issues in recorded data, such as missing values. The PLR was launched nationally in 2014 and participation has been voluntary for delivery wards. National coverage has increased yearly and as of 2021, all delivery wards have been using the PLR.
The criteria for maternal and obstetrical characteristics may vary to across the country, given the large study population and the high number of health care units involved. However, there is no reason to believe that these variations are impacted by maternal BMI.
Conclusions
This large population-based study showed that women with higher BMI have more UI but less AI 8 weeks after a first-time vaginal birth complicated by an OASI, than women with normal BMI. These are new findings concerning PFD in the immediate postpartum period. The reason for the discrepancy between UI and AI, regarding possible preventive factors associated with a higher BMI, merits further study.
References
Sultan AH, Monga A, Lee J, Emmanuel A, Norton C, Santoro G, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female anorectal dysfunction. Neurourol Urodyn. 2017;36(1):10–34. https://doi.org/10.1002/nau.23055.
Marsh F, Lynne R, Christine L, Alison W. Obstetric anal sphincter injury in the UK and its effect on bowel, bladder and sexual function. Eur J Obstet Gynecol Reprod Biol. 2011;154(2):223–7. https://doi.org/10.1016/j.ejogrb.2010.09.006.
Jangö H, Langhoff-Roos J, Rosthøj S, Sakse A. Wexner score and quality of life in women with obstetric anal sphincter injury. Int Urogynecol J. 2020;31(6):1115–21. https://doi.org/10.1007/s00192-019-04134-1.
Thubert T, Cardaillac C, Fritel X, Winer N, Dochez V. Definition, epidemiology and risk factors of obstetric anal sphincter injuries: CNGOF Perineal Prevention and Protection in Obstetrics Guidelines. Gynecol Obstet Fertil Senol. 2018;46(12):913–21. https://doi.org/10.1016/j.gofs.2018.10.028.
Practice Bulletin No. 165. Prevention and management of obstetric lacerations at vaginal delivery. Obstet Gynecol. 2016;128(1):e1–e15. https://doi.org/10.1097/aog.0000000000001523.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–24. https://doi.org/10.1097/01.Aog.0000107291.46159.00.
Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118(3):561–8. https://doi.org/10.1097/AOG.0b013e31822a6c59.
Blomberg M. Maternal body mass index and risk of obstetric anal sphincter injury. Biomed Res Int. 2014;2014:395803. https://doi.org/10.1155/2014/395803.
Lindholm ES, Altman D. Risk of obstetric anal sphincter lacerations among obese women. BJOG. 2013;120(9):1110–5. https://doi.org/10.1111/1471-0528.12228.
Burgio KL, Borello-France D, Richter HE, Fitzgerald MP, Whitehead W, Handa VL, et al. Risk factors for fecal and urinary incontinence after childbirth: the childbirth and pelvic symptoms study. Am J Gastroenterol. 2007;102(9):1998–2004. https://doi.org/10.1111/j.1572-0241.2007.01364.x.
Cattani L, Neefs L, Verbakel JY, Bosteels J, Deprest J. Obstetric risk factors for anorectal dysfunction after delivery: a systematic review and meta-analysis. Int Urogynecol J. 2021;32(9):2325–36. https://doi.org/10.1007/s00192-021-04723-z.
Woodley SJ, Lawrenson P, Boyle R, Cody JD, Mørkved S, Kernohan A, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;5(5):CD007471. https://doi.org/10.1002/14651858.CD007471.pub4.
Swedish National Quality Register of Gynecological Surgery. 2018. https://www.gynop.se/home/ Accessed 4 July 2022.
Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period; prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. Eur J Obstet Gynecol Reprod Biol. 2020;245:1–6. https://doi.org/10.1016/j.ejogrb.2019.11.030.
Lindqvist M, Persson M, Nilsson M, Uustal E, Lindberg I. 'A worse nightmare than expected'–a Swedish qualitative study of women's experiences two months after obstetric anal sphincter muscle injury. Midwifery. 2018;61:22–8. https://doi.org/10.1016/j.midw.2018.02.015.
Dolan LM, Hilton P. Obstetric risk factors and pelvic floor dysfunction 20 years after first delivery. Int Urogynecol J. 2010;21(5):535–44. https://doi.org/10.1007/s00192-009-1074-8.
Svare JA, Hansen BB, Lose G. Prevalence of anal incontinence during pregnancy and 1 year after delivery in a cohort of primiparous women and a control group of nulliparous women. Acta Obstet Gynecol Scand. 2016;95(8):920–5. https://doi.org/10.1111/aogs.12896.
De Leeuw JW, Vierhout ME, Struijk PC, Hop WC, Wallenburg HC. Anal sphincter damage after vaginal delivery: functional outcome and risk factors for fecal incontinence. Acta Obstet Gynecol Scand. 2001;80(9):830–4.
Laine K, Skjeldestad FE, Sanda B, Horne H, Spydslaug A, Staff AC. Prevalence and risk factors for anal incontinence after obstetric anal sphincter rupture. Acta Obstet Gynecol Scand. 2011;90(4):319–24. https://doi.org/10.1111/j.1600-0412.2010.01057.x.
Gommesen D, Nohr EA, Qvist N, Rasch V. Obstetric perineal ruptures-risk of anal incontinence among primiparous women 12 months postpartum: a prospective cohort study. Am J Obstet Gynecol. 2020;222(2):165.e1–11. https://doi.org/10.1016/j.ajog.2019.08.026.
Carlhäll S, Källén K, Blomberg M. Maternal body mass index and duration of labor. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):49–53. https://doi.org/10.1016/j.ejogrb.2013.08.021.
Hjertberg L, Uustal E, Pihl S, Blomberg M. Maternal body mass index and anovaginal distance in active phase of term labor. Biomed Res Int. 2018;2018:1532949. https://doi.org/10.1155/2018/1532949.
Doumouchtsis SK, Loganathan J, Pergialiotis V. The role of obesity on urinary incontinence and anal incontinence in women: a review. BJOG. 2021;129(1):162–70. https://doi.org/10.1111/1471-0528.16848.
Pomian A, Lisik W, Kosieradzki M, Barcz E. Obesity and pelvic floor disorders: a review of the literature. Med Sci Monit. 2016;22:1880–6. https://doi.org/10.12659/msm.896331.
Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, Kenton K, Gossett DR. Wound complications after obstetric anal sphincter injuries. Obstet Gynecol. 2015;125(5):1088–93. https://doi.org/10.1097/aog.0000000000000833.
Gommesen D, Nohr EA, Drue HC, Qvist N, Rasch V. Obstetric perineal tears: risk factors, wound infection and dehiscence: a prospective cohort study. Arch Gynecol Obstet. 2019;300(1):67–77. https://doi.org/10.1007/s00404-019-05165-1.
Drusany Starič K, Norčič G. Obstetric risk factors for early-onset anal incontinence. Int J Colorectal Dis. 2019;34(1):177–80. https://doi.org/10.1007/s00384-018-3119-2.
Mathé M, Valancogne G, Atallah A, Sciard C, Doret M, Gaucherand P, et al. Early pelvic floor muscle training after obstetrical anal sphincter injuries for the reduction of anal incontinence. Eur J Obstet Gynecol Reprod Biol. 2016;199:201–6. https://doi.org/10.1016/j.ejogrb.2016.01.025.
Acknowledgement
The authors would like to thank Lars Valter, Forum Östergötland, Linköping University, Linköping, Sweden, for assistance with statistics.
Funding
Open access funding provided by Linköping University. Financial support was received from the County Council of Östergötland and Linköping University, Sweden (ALF grants, Region Östergötland)
Author information
Authors and Affiliations
Contributions
L. Hjertberg: protocol and project development, data collection, data analyses and manuscript writing; M. Blomberg: protocol and project development, analysis and interpretation of data and manuscript writing; S. Pihl: protocol and project development and revision of the manuscript; E. Uustal: protocol and project development, analysis and interpretation of data and manuscript writing. All authors gave final approval and agreed to be accountable for all aspects of the work, ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Appendix 1
Pre-pregnancy questionnaire. https://www.gynop.se/wp-content/uploads/2019/01/Health-prior-to-pregnancy-.pdf. Accessed May 10 2022 (PDF 201 kb)
Appendix 2
Eight-week questionnaire. https://www.gynop.se/wp-content/uploads/2019/01/Health-prior-to-pregnancy-.pdf. Accessed 10 May 2022 (PDF 125 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hjertberg, L., Pihl, S., Blomberg, M. et al. Body mass index and complications after obstetric anal sphincter injury, 8 weeks postpartum. Int Urogynecol J 33, 3465–3472 (2022). https://doi.org/10.1007/s00192-022-05328-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05328-w