Abstract
Introduction and hypothesis
We sought to evaluate patient satisfaction with a novel multiplex PCR UTI home collection kit for symptomatic UTI in a urogynecologic population. We secondarily sought to characterize reported uropathogens and resistance profiles of uropathogens in this population. We hypothesized that patients would be satisfied.
Methods
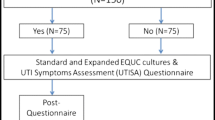
This was a cross-sectional study of women who were surveyed later about their experience undergoing evaluation for a UTI with a home UTI test at a large tertiary care urogynecology practice in 2020. Symptomatic patients were sent a home UTI kit. We assessed patient satisfaction at a later time with a 5-point Likert scale and collected baseline information. The primary outcome was patient satisfaction with this experience. Secondary outcomes included type and number of uropathogens on testing.
Results
A total of 30 patients [73% white race, mean age 71.9 (SD 12.0) years] were surveyed. Patients responded with a mean score of 4.7/5 to all satisfaction questions. Overall, 86% (26/30) of patients would choose this test again. Of those asked if they would choose this test again outside of the COVID-19 pandemic, 86% responded affirmatively. The most common symptoms reported included dysuria (53%), urgency (37%) and frequency (30%). The most common pathogens identified included Escherichia coli (70%), Enterococcus faecalis (60%) and Aerococcus urinae (43%).
Conclusions
Patients were satisfied with home UTI PCR testing and the majority would choose this option again. Home UTI PCR testing revealed common uropathogens for a population with a high proportion of recurrent UTI, but additional research comparing home versus in-office urine PCR testing is necessary.
Similar content being viewed by others
Introduction
Urinary tract infections (UTIs) are very common in women. Approximately 60% of female patients experience at least one UTI in their lifetime, and 20–40% of those have one or more recurrent episodes of UTIs [1]. The most common pathogens causing UTIs include Escherichia coli, Proteus mirabilis, Klebsiella pneumonia and Enterobacter species [2]. For those suffering with recurrent UTIs (rUTI), current guidelines implore clinicians to obtain a urine culture (UCx) and sensitivity with each symptomatic UTI prior to initiating treatment. The gold standard for diagnosis is a Ucx with speciation and sensitivities to ensure that antibiotic treatment offers appropriate coverage. Ucxs take around 48 h for results, are designed to detect a small set of uropathogens, (i.e., Escherichia coli) and require in-person submission of urine specimen either in an office setting or at an outpatient laboratory [2, 3].
Newer technology has allowed molecular diagnostic techniques to be utilized to diagnose common infections. Currently, polymerase chain reaction (PCR) testing is used for identification of sexually transmitted infections, parasitic infections, COVID-19 and others [4]. Limited studies have investigated PCR to identify uropathogens as compared to conventional Ucx methods [4,5,6]. Several companies have created multiplex PCR assays currently undergoing investigation, though a limitation has been inability to quantify pathogens [2]. The largest study to investigate a novel multiplex PCR-based UTI analysis that identifies and quantifies both pathogens and other bacteria not yet confirmed to be uropathogens as well as sensitivities to various antibiotics was recently demonstrated to be noninferior to traditional Ucx [4].
At the start of the COVID-19 pandemic when social distancing was highly recommended, patients in our practice were offered a multiplex PCR test for home collection when they called in or presented via telemedicine with UTI symptoms. The objective of our study was to assess the patient experience and satisfaction with this multiplex PCR home test given its novelty among our patient population. There are no studies that evaluate patient satisfaction of this type of test in a urogynecologic population. We additionally sought to investigate the types and resistance patterns of isolated uropathogens. We hypothesized that patients would be satisfied with this option for UTI evaluation.
Materials and methods
This was a cross-sectional study of women who were surveyed at a later time about their experience undergoing evaluation for a symptomatic UTI with a home UTI test at a large tertiary care urogynecology practice from April 1 through July 15, 2020. Patients were called with a brief telephone survey after they had completed testing and treatment for their UTI symptoms. Patients met inclusion criteria if they reported UTI symptoms by either telemedicine appointment or phone and chose evaluation with multiplex PCR UTI test without having to physically present to a laboratory. Pathnostics Guidance UTI Test was utilized as a home test and provided to patients at no cost. Importantly, there was no financial gain to our practice or providers for utilization of this test as it was chosen because it was the sole home UTI test available during this time. Testing kits were sent to patients via mail and received 24–48 h later. Patients were instructed by nursing staff in addition to the included testing instructions to collect a mid-stream clean catch urine specimen at home. Samples were picked up the next day and returned to the laboratory via overnight shipping. Patients could anticipate a phone call with results and treatment plan in 3–7 days from the time they called with symptoms.
Once in the laboratory, DNA was extracted from the urine specimen and analyzed with PCR utilizing probes and polymers for 24 separate bacteria and 2 bacterial groups (Table 1) [5]. Probes and primers that identified bacterial species also provided semi-quantified organism counts that were reported as cells/ml and correlated to colony-forming units. Bacterial antibiotic resistance genes were also detected with PCR [7]. The multiplex PCR UTI test reports a pooled sensitivity of 19 antibiotics that considers the possible polymicrobial nature of infections. Pooled antibiotic susceptibility testing or P-AST is performed by placing supernatant from centrifuged urine onto growth media, and when growth reached a certain threshold the samples were plated on preloaded antibiotic plates in a similar fashion to standard cultures [7]. While awaiting results women were managed per clinical care at the discretion of their provider, and they were advised to hydrate and/or use over-the-counter therapies for symptom relief (i.e., phenazopyridine).
The primary outcome of this study was patient satisfaction with home testing with follow-up phone calls after completion of the UTI test. Patients were asked to assess patient satisfaction using a 5-point Likert scale. Additional questions in the telephone survey reviewed future willingness to utilize a home multiplex PCR UTI kit, both in and beyond the setting of a pandemic, travel time to laboratory, resolution of UTI symptoms and comments on overall satisfaction. We secondarily sought to characterize the types of reported uropathogens and resistance profiles of uropathogens in this population of patients.
After the telephone survey, the medical chart was queried. Data collected included basic demographic information, history of rUTIs, presenting UTI symptoms, the resultant uropathogen and pooled antibiotic sensitivities. We additionally investigated the time to next health care contact after submission of urine testing.
Descriptive statistics were calculated utilizing Microsoft Excel 2010 (Microsoft, Redman, WA). This project was approved by the University of Pittsburgh Quality Improvement #2691 and the University of Pittsburgh Medical Center Institutional Review Board: STUDY 21020215.
Results
A total of 30 patients, mean age 71.9 (SD 12.0) years, with UTI symptoms had a multiplex PCR UTI test completed. Seventy-three percent (n = 22) of the patients were of White race. At the time of presentation, 47% (n = 14) had a known history of rUTI. Patients often reported multiple symptoms with the most common being dysuria (53%), urgency (37%) and frequency (30%) (Table 2). Patients were called and surveyed about their experience with the test on average 94.8 (range 47–132) days since they submitted their multiplex UTI PCR test.
Regarding patient satisfaction, collective responses on the 5-point Likert satisfaction scale had a mean score of 4.7 (Table 3). Patients responded to convenience of the test with a mean 4.9 (SD 0.3). One patient reported that this option was “wonderful,” and another reported she was “ecstatic” with this option. Another patient responded, “I would have rated this as a 6!” Patients responded to the accuracy of the home test as compared to a laboratory urine culture with a mean 4.5 (SD 0.8). One patient reported that it was a “much slower time frame” because the kit was mailed. Another patient reported that “UPS took one day too long;” however, she was “happy I didn’t have to leave the house.” Patients typically traveled on average 23.3 min to drive to a laboratory to submit a specimen (SD 18.0 min). Overall, 86% reported they would choose this test again. Of the 21 patients asked if they would choose this test again even outside of a pandemic, 86% responded affirmatively.
The PCR identified at least one bacteria with colony counts > 105 in 29/30 (97%) of patients. Conversely, one patient was found to have E. faecalis 50–99,000 colonies and coagulase negative staphylococci 10–49,000 colonies. The three most common pathogens identified included E. coli (70%), E. faecalis (60%) and A. urinae (43%) (Fig. 1). All but three patients had more than one pathogen detected. The average number of pathogens resulting was 5.9 (SD 2.4), and of those 57% noted colony counts > 10 [5]. Known uropathogens E. coli or E. faecalis were identified in 28 (93%) of patients; the remaining 2 patients had tests that resulted in pathogens of whose clinical significance is yet to be determined. On average, uropathogens were resistant to 54% of the 19 tested antibiotics (SD 24). After treatment with antibiotics per the multiplex PCR UTI sensitivities report, 70% of the patients developed recurrence of UTI symptoms with an average time to patients contacting the health care system of 24.5 (SD 28.0) days.
Discussion
In our population of women in an large tertiary care center urogynecology practice undergoing evaluation for symptomatic UTI, patients were overall satisfied with their experience utilizing a home multiplex PCR UTI kit. Additionally, most would elect this option again, especially because of the convenience factor. Considering the high proportion of women with rUTI and the number of evaluations for symptomatic UTI in our practice, our study suggests initial feasibility of a home UTI testing mechanism. Given current guidelines that implore providers to collect pre-treatment UCx for women with rUTI [1], women may be interested in alternative options for specimen collection over traditional urine culture in a laboratory or hospital setting. As 86% of patients would choose this test again, even outside of a pandemic, these data suggest that the multiplex PCR UTI home test, or likely traditional urine culture if available as home testing, is a subjectively acceptable alternative to laboratory urine specimen collection. Patients also expressed satisfaction at the collection process and confidence in the sterility of the process. Again, the majority of patients had experience with providing urine specimens over the past year, and this collection process was not very different. The area with the lowest satisfaction was confidence in the accuracy of results.
While patients overall reported satisfaction, 70% developed recurrence of symptoms on average approximately 1 month after the initial home UTI PCR test. In a population of women with a high proportion of rUTI, this is similar to reported literature with median time between infections ranging from 40–70 days [8]. Regarding results of the multiplex PCR UTI home test, the most common pathogens identified included E. coli, E. faecalis and A. urinae. Escherichia coli as the most prevalent pathogen is consistent with previous findings in the literature [1, 2, 6]. Additionally, the majority of our subjects presented with more discriminatory symptoms (i.e., dysuria), which suggests a higher possibility of the pathogenic nature of the bacteria present on testing [8]. The multiplex PCR UTI kits identified a mean of 5.9 pathogens per patient and identified bacteria in 100% of patients, which is significantly > 60% with positive PCR results in the previous literature [4]. Some of this high number of pathogens per patient may be attributed to vaginal contamination, as a previous study described similar rates of vaginal contamination in patients who performed a mid-stream clean catch sample compared to those without any cleansing, 32% versus 29%, respectively [9]. Regardless, true uropathogens were identified in 93% of patients who presented with UTI symptoms, suggesting a reliable confidence in this test. Additionally, our results surrounding proportion of uropathogens are consistent with the literature, despite a small sample size. Studies from our institution that evaluated urine cultures in symptomatic UTI patients revealed similar uropathogens with high resistance patterns, especially in women with rUTI [10, 11]. Additional research to compare traditional urine cultures with the multiplex PCR home UTI test is necessary along with prospective studies to determine symptomatic response after treatment.
One of the strengths of this study is its novelty. While there has been one study that reviewed patient outcomes and satisfaction with treatment of UTI with antibiotics via telemedicine visit [12], there have not been any other studies published in the literature regarding patient satisfaction with this new technology for the management and evaluation of UTIs. Moreover, the patients in the previously mentioned study were prescribed antibiotics and not required to submit a urine specimen, which would not be helpful for our rUTI patients that, per guidelines, require a pre-treatment urine culture to guide therapy [1, 12]. Additionally, this new technology was implemented during the COVID-19 pandemic in part to promote social distancing and to help patients avoid health care settings. Again, given the recommendation to collect UCx prior to treatment of symptomatic UTI in women with rUTI, this technology allows for access to testing prior to treatment while maintaining a safe environment, though there is room for improvement in efficiency so as not to delay treatment too long [1]. As we continue to live with the pandemic, this test may ultimately be preferable for vulnerable patients suffering from UTIs who are worried about presenting to health care settings for evaluation and management. The overall positive subjective response encourages further research on its usage.
We acknowledge the limitation that our conclusions may include recall bias as subjects were called on average approximately 3 months after the completion of their test. Despite this long interval, patients in and outside of this study cohort continued to express their desire to use the home test kits, especially as the pandemic continued, suggesting their overall satisfaction with the home collection process and results. Prospective studies about the utilization of this test would be helpful to mitigate any bias. There may also be some selection bias as subjects who chose to submit urine culture at a laboratory setting were not included in our study, which could be addressed in the future with a prospective study comparing these two modalities. There was no cost associated with the UTI PCR test as it was provided to our office at the start of the pandemic. While this was not typically discussed with patients, some patients may have inquired and potentially preferentially chosen this option as there was no cost associated with it, introducing some bias. Additionally, we focused on patient satisfaction with this experience, and there are two novelties that may be difficult to distinguish in this experience: submitting a specimen from home and PCR technology, which can be investigated in future research. More research is also needed to further evaluate the sensitivity and specificity of this test compared to standard of care urine cultures. While Wojno et al. found that the multiplex UTI test is noninferior to urine culture for detecting bacteria, prospective data are needed to evaluate patient treatment outcomes after using this test and the subsequent treatment prescribed [4]. In particular, the high number of pathogens identified per patient is somewhat concerning for vaginal contamination, which could be a limitation. However, the identification and subsequent treatment of a well-known uropathogen in almost every patient in a population with a high proportion of rUTI is reassuring for the ability to detect pathogens in symptomatic patients. Finally, the small number of patients in this study limits its generalizability, but this could be addressed in a larger prospective study.
In conclusion, a small cohort of women undergoing evaluation for possible UTI who utilized a multiplex PCR UTI home test were satisfied with the experience. The majority of these patients would use this test again in the evaluation of a UTI, even outside of a pandemic. Given the early positive feedback about this experience, further evaluation regarding patient outcomes with this test compared to traditional UCx is necessary given the novelty of this technology.
Change history
19 August 2022
The name of the second author was incorrectly tagged as S.G. Clark. The correct name is S. Glass Clark.
References
Anger J, Lee U, Ackerman AL, Chou R, Chughtai B, Clemens JQ, Hickling D, Kapoor A, Kenton KS, Kaufman MR, Rondanina MA, Stapleton A, Stothers LCTC. Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J Urol. 2019;202(2):282–9.
Davenport M, Mach KE, Shortliffe LMD, Banaei N, Wang TH, Liao JC. New and developing diagnostic technologies for urinary tract infections. Nat Rev Urol. 2017;14(5):296–310. https://doi.org/10.1038/nrurol.2017.20.
Brubaker LWAJ. The new world of the urinary microbiota in women. Am J Obstet Gynecol. 2015;213(5):644–9.
Wojno KJ, Baunoch D, Luke N, et al. Multiplex based urinary tract infection (UTI) analysis compared to traditional urine culture in identifying significant pathogens in symptomatic patients. Urology. 2020;136:119–26. https://doi.org/10.1016/j.urology.2019.10.018.
Lehmann LE, Hauser S, Malinka T, Klaschik S, Stüber F, Book M. Real-time polymerase chain-reaction detection of pathogens is feasible to supplement the diagnostic sequence for urinary tract infections. BJU Int. 2010;106(1):114–20. https://doi.org/10.1111/j.1464-410X.2009.09017.x.
Brons JK, Vink SN, de Vos MGJ, Reuter S, Dobrindt U, van Elsas JD. Fast identification of Escherichia coli in urinary tract infections using a virulence gene based PCR approach in a novel thermal cycler. J Microbiol Methods. 2020;169:105799. https://doi.org/10.1016/j.mimet.2019.105799.
Daly A, Baunoch D, Rehling K, et al. Utilization of M-PCR and P-AST for diagnosis and management of urinary tract infections in home-based primary care. JOJ Urol Nephrol. 2020;7(2):555707. https://doi.org/10.19080/JOJUN.2020.07.555707.
Dune TJ, Price TK, Hilt EE, et al. Urinary symptoms and their associations with urinary tract infections in urogynecologic patients. Obstet Gynecol. 2017;130(4):718–25. https://doi.org/10.1097/AOG.0000000000002239.
Lifshitz E, Kramer L. Outpatient urine culture: does collection technique matter? Arch Intern Med. 2000;160(16):2537–40. https://doi.org/10.1001/archinte.160.16.2537.
Bradley MS, Cabrera C, Clark SG, Sassani J, Venuti K, Ackenbom MF. Sporadic compared to recurrent urinary tract infections: Considerations for urogynecologic patients. Neurourol Urodyn. 2020;39(8):2186–91. https://doi.org/10.1002/nau.24471.
Venuti K, Cabrera C, Burkett LS, Bradley MS. Impact of menopausal status on uropathogen prevalence and antimicrobial resistance profiles. Female Pelvic Med Reconstr Surg. 2021;27(1):e13–7. https://doi.org/10.1097/SPV.0000000000000778.
Rastogi R, Martinez KA, Gupta N, Rood M, Rothberg MB. Management of urinary tract infections in direct to consumer telemedicine. J Gen Intern Med. 2020;35(3):643–8. https://doi.org/10.1007/s11606-019-05415-7.
Participation
Melnyk: Project development and IRB approval, data collection and analysis, manuscript writing
Toal: Data collection and manuscript writing
Glass Clark: Project development and manuscript writing
Bradley: Project development and manuscript writing
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This project was approved by the University of Pittsburgh Medical Center Institutional Review Board: STUDY 21020215.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Melnyk, A.I., Toal, C., Glass Clark, S. et al. Home urinary tract infection testing: patient experience and satisfaction with polymerase chain reaction kit. Int Urogynecol J 34, 1055–1060 (2023). https://doi.org/10.1007/s00192-022-05309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05309-z