Abstract
Introduction and hypothesis
We aimed to reveal the effectiveness of the combination of behavioral therapy (BT), drug therapy, and pelvic floor muscle training (PFMT) in patients with the diagnosis of overactive bladder (OAB) who did not respond to drug therapy.
Methods
Seventy female patients aged between 18 and 65 years diagnosed with wet-type OAB, who did not respond to drug therapy, were included in our study, which was planned as a prospective randomized controlled trial. The patients were randomly assigned to one of two groups. BT and a combination of anticholinergic + beta3-agonist was implemented in the control group for 12 weeks. BT and PFMT were applied with a combination of anticholinergic + beta3-agonist in the active therapy group for 12 weeks. Post-treatment changes in OAB, ICIQ-SF scores, and frequency and nocturia were compared.
Results
The age and BMI averages of the groups were similar (p>0.01). After the treatment, no significant decrease was observed in OAB scores in the control group (p = 0.06), but a significant decrease was observed in the active therapy group (p<0.01). The mean ICIQ-SF scores and the number of nocturia were found to decrease in both groups after 12 weeks of treatment (p<0.01). There was no significant decrease in frequency in the control group (p = 0.054). It regressed significantly in the active therapy group (p<0.01). After the treatment, 3 of 30 the patients in control group (10%) and 11 of the 32 patients in the active therapy group (34.3%) said that their complaints had regressed and that they were pleased with their current situation. Although after the treatment, 4 patients in the control group were dry (13.3%), 10 patients in the active therapy group were dry (31.25%).
Conclusions
We demonstrated that drug therapy, BT, and PFMT, which are recommended in the first-line treatment of OAB reduce the need for invasive treatments when they are well explained to the patients and combined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is a common condition that significantly affects the quality of life of patients. Prevalence studies conducted reported that OAB with a prevalence between 12% and 17% is observed more common along with aging [1, 2]. According to the definition of the International Continence Society (ICS), it is a condition often accompanied by frequent urination and nocturia, with or without sudden urgency and urinary incontinence [3]. The diagnosis of OAB may be established by excluding pathological conditions with similar symptoms, including urinary infection, neurogenic voiding dysfunction, bladder neck obstruction, and urinary system stone disease [4].
Although many methods are used in the treatment of OAB, fluid intake modification, restriction of caffeine and tea, weight reduction, bladder training, which constitute behavioral therapy, are recommended in the first-line treatment. Furthermore, in addition to behavioral therapy, pelvic floor muscle training (PFMT) and antimuscarinic and/or beta3-agonist therapy may be implemented in the first-line treatment [5, 6]. Patients who were nonrespondent to the first-line treatment may undergo therapeutic methods such as intravesical botulinum toxin administration, posterior tibial nerve stimulation, and sacral neuromodulation [7].
Studies in the literature have shown that behavioral therapy and pelvic floor rehabilitation are beneficial for the treatment of urge incontinence and OAB [8, 9]. Burgio et al. reveal that behavioral therapy is more effective in their study comparing behavioral versus drug therapy [8]. Rizvi et al. also compared bladder training versus PFMT versus PFMT plus biofeedback and revealed that there was similar efficacy in all groups [9]. However, Bo et al. reported that PFMT was effective in most studies, but was not effective enough in some studies. [10]. It was stated that a significant comparison could not be made between studies owing to the heterogeneity between the PFMT protocols applied in the European Association of Urology (EAU) guideline [11]. Also, in the EAU guideline, it was reported that the treatment compliance in the clinical studies was much higher than in the daily practice [11]. We think that this is because the treatments are explained to the patients in more detail in clinical studies and that the patients are better motivated. Furthermore, in cases where behavioral therapy and PFMT are not well explained and applied to patients during daily practice, patients are unnecessarily referred to third-line invasive treatments. Based on this, we aimed to demonstrate the efficacy of lifestyle change and PFMT in combination with antimuscarinic therapy in female patients diagnosed with wet-type OAB referred to as resistant OAB. Thus, we aimed to show that success can be increased when the antimuscarinic + mirabegron combination is applied together with behavioral therapy and PFMT in clinical practice.
Materials and methods
After obtaining a committee of ethics approval for the present study designed as a prospective, randomized controlled study, 70 female patients aged between 18 and 65 years who had been referred to our outpatient clinic and diagnosed with wet-type overactive bladder resistance to drug therapy (persisting complaints against antimuscarinic and/or beta3 agonist treatment for at least 8 weeks). The prior treatments of the patients are shown in Table 1. Patients who had previously received pelvic floor rehabilitation under the guidance of a physiotherapist were not included in the study. Furthermore, patients with neurogenic bladder, bladder outlet obstruction, and urinary system diseases such as stones, tumors, pregnancy, or infection were excluded. Urinary tract infection (UTI) was excluded by performing a urine culture before the treatment. Patients with pelvic organ prolapse or prolapse-incontinence surgery and stress urinary incontinence were not included in the study. Moreover, those using drugs that may affect lower urinary system functions were also excluded.
The patients were divided into two groups through the block randomization method as control group and active therapy group. Figure 1 shows the flow chart prepared according to the Consolidated Standards of Reporting Trials guideline [12]. In the control group, behavioral therapy with a combination of antimuscarinic (tolterodine 4 mg) and beta3 agonist (mirabegron 50 mg) was administered for 12 weeks. In the active therapy group, behavioral therapy and PFMT were administered in a combination of antimuscarinic (tolterodine) and beta3 agonist (mirabegron) for 12 weeks. All patients were informed about the possible side effects of tolterodine and mirabegron before the treatment, and their written informed consent was obtained. The treatment of patients who had previously used antimuscarinics was started at the earliest 15 days after the drug was discontinued. In our study, 3 patients in the active therapy group stated that they left the study because they did not benefit. In the control group, 1 patient left the study owing to COVID-19, whereas 4 patients left the study stating that they did not benefit.
Behavioral therapy
Lifestyle changes were explained in detail to the patients in behavioral therapy applied to both groups. Patients with obesity were advised to lose weight; in all patients it was suggested that they increase their daily physical activities, quit smoking, significantly reduce the consumption of caffeinated beverages and hot and spicy food, and spread their fluid intake throughout the day. The patients were motivated by explaining in detail that if they reduced their fluid consumption, their symptoms could be significantly reduced.
Pelvic floor muscle training
Two sessions of PFMT were applied to the patients in the treatment group for 12 weeks. Patients received 24 sessions in total. The patients could exercise comfortably in this exercise program. Patients with a PFM strength of 3 out of 5 or more were enrolled in the study. The PFM strength by digital palpation was done in the lithotomy position and graded (minimum 0 to maximum 5), as on Oxford scale. A trial session was conducted before the first session to feel the pelvic floor muscles, to get used to the exercise, and to see the contraction. The therapy program duration was 40 min in total. Following the protocol of Ozlu at al. [13], the patients received a total of 80 treatment cycles with 10 s of contraction and 20 s of relaxation in each session. During the session, the contraction and relaxation of the pelvic floor muscles of the patients were displayed on the monitor to receive visual biofeedback. The contraction of the pelvic floor muscles was demonstrated with graphics or figures in front of the computer screen (extracorporeal biofeedback device, Aktive reha systeme®, Frei Medical, Kirchzarten, Germany), and after verbal explanations, correct exercises were taught in the company of a pelvic physiotherapist. All patients in the study were trained by the same physiotherapist.
Measurements
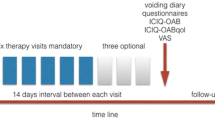
A 3-day voiding diary was implemented for all patients before and after the treatment. Frequency and nocturia counts of the patients were recorded from the voiding diary data. Furthermore, a Turkish-validated OAB query form before and after the treatment and the ICIQ-SF query form questioning the severity of urinary incontinence were filled out [14]. The patients were asked about adverse reactions that developed during the treatment and satisfaction status after the treatment. The patients who wanted to leave the treatment earlier were also asked for the reason for leaving and their answers were noted. The OAB-V8 and ICIQ-SF scores of the groups before and after the treatment, and the change in frequency and nocturia counts were compared.
Statistical analysis
In the G-power analysis performed through the G*Power 3.1 (Heinrich Heine University, Düsseldorf, Germany) program, the significance level was 0.05, the power was 0.95, the effect size was 0.3, the number of measurements was 5, and the required sample size was 30 when the correlation between repeated measurements was accepted as 0.5. Thirty-five subjects were included for each group to take into account the patient possibly leaving. Statistical analysis of the data obtained in the study was performed using the SPSS software version 26 (IBM SPSS, Armonk, NY, USA). The normal distribution state of the variables was investigated through the Kolmogorov–Smirnov/Shapiro–Wilk test. The t test was used to compare continuous variables with normal distribution between groups, whereas Mann–Whitney U test was used for those without normal distribution. Pearson Chi-squared test and Fisher’s exact Chi-squared test were used for variables with normal distribution in categorical variables; however, Wilcoxon signed-rank test was used for those without normal distribution. Repeat measurement ANOVA to evaluate the success of dependent groups before and after treatment. Student t test or Mann–Whitney U test was used to compare pre- and post-treatment conditions between independent groups. Any p value below 0.05 was accepted as significant for statistical significance.
Results
The demographic data of the groups were similar. Also, the consumption of tea, coffee, and hot and spicy food of the patients in both groups was similar (Table 2).
When the pre-treatment OAB-V8 and ICIQ-SF scores of the patients were compared, it was determined that there was no difference between the groups. According to the voiding diary data of the patients taken before treatment, the mean voiding frequency was 12.47±0.73 in the control group and 12.94±0.91 in the active therapy group (p = 0.02). The nocturia count was detected in median (IQR) 2 (1) in both groups (p = 0.767; Table 3). The average number of leaks per day before treatment was 4.43+1.13 in the control group and 5.34+2.28 in the active therapy group (p = 0.054).
Although there was a decrease in the mean OAB-V8 scores in the control group after treatment, no significant difference was detected (p = 0.06). A decrease was observed in OAB-V8 scores in the active therapy group after the treatment (p < 0.01). ICIQ-SF scores were found to be lower in both groups after treatment (p < 0.01). Although the urine frequency obtained from the voiding diary decreased in the control group after treatment, no statistically significant difference was detected (p = 0.054). It was detected that frequency regressed in the active therapy group after the treatment (p < 0.01). The number of nocturia was found to be lower in both groups (p < 0.01). It was also observed that the number of leaks per day decreased significantly in both groups after treatment (p < 0.01).
Investigation of the satisfaction status of the patients after the treatment revealed that 3 of the 30 patients (10%) in the control group and 11 of the 32 patients (34.3%) in the active therapy group had regression of their complaints and were pleased with their current situation. After the treatment, 4 patients in the control group and 10 patients in the active therapy group were dry. Post-treatment satisfaction was significantly higher in the active therapy group than in the control group (p = 0.02; Table 4).
When the treatment success of the groups was compared, it was found that there was a greater decrease in OAB-V8, ICIQ-SF score, and frequency in the active therapy group (p<0.05; Table 5). Also, when the eating and drinking habits were examined after the treatment, it was observed that the consumption of tea and coffee decreased significantly in both groups (p < 0.01). Furthermore, consumption of hot and spicy food significantly decreased in both groups (Table 5).
In the study, no significant adverse reactions were observed that required discontinuation of antimuscarinic drugs during the treatment period. During the treatment, 8 patients complained of dry mouth. These patients were advised to chew gum during the day and their complaints partially decreased. Furthermore, 3 patients had constipation. Constipation was partially resolved with dietary suggestions. Moreover, no adverse effect was observed in patients receiving PFMT.
Discussion
First-line treatment methods of OAB such as PFMT, antimuscarinics, and/or beta3 agonists provide a significant improvement in symptoms in a significant proportion of patients as of today [11]. Burgio et al. compared behavioral therapy supported with biofeedback and drug therapy in OAB and found similar efficacy levels [8]. Burgio et al. showed in another recently published study that the combination of behavioral therapy and drug therapy is more effective than drug therapy alone [15]. However, first-line treatments cannot be used effectively in some cases because patients do not fully understand or apply lifestyle modifications. In our study, when the patients referred owing to resistance to the first-line treatment methods were re-evaluated, we found that the patients did not fully understand or apply lifestyle changes. Afterward, patients were motivated about treatment and behavioral therapy and PFMT were explained in detail. Although there was a decrease in OAB symptoms in patients treated with behavioral therapy and the antimuscarinic + beta3 agonist combination, we could not detect a significant difference. We detected a significant decrease in symptoms in patients who had PFMT. Although 10% of the patients in the behavioral therapy group were satisfied with their current situation and did not want the third-line treatment method, 34% of the patients in the PFMT group were satisfied with their current situation. In other words, we achieved success by combining drug therapy with first-line treatment methods in a significant number of the cases with resistant OAB referred to us for other invasive procedures. We thereby think that we have demonstrated the importance of the effective use of first-line treatment methods.
Obesity, smoking, physical activity level, fluid intake, and consumption of irritant foods such as coffee, tea, and cola may be considered in the etiology of OAB and urge incontinence [8]. In our study, when we explained the dietary restrictions and possible benefits to the patients in detail, we found that their consumption of coffee, tea, and hot and spicy food decreased significantly after the treatment. Another situation we observed in our study was that the consumption of hot and spicy food was quite high. As far as we have examined in the literature, only Alkış et al. reported that consumption of hot and spicy food may increase OAB symptoms [16]. We think that the effects of hot and spicy food consumption on OAB symptoms can be shown in future studies with large numbers of patients. Also, we believe that this is because patients are better motivated when they are informed in detail, their awareness level increases, and they adapt to the restrictions more easily. Many studies have demonstrated that modification of such factors ensures an improvement in symptoms [17,18,19]. In addition to drug therapy, we found a slight decrease in symptoms with lifestyle modification and strict dietary restrictions, in line with the literature. However, we revealed a significant decrease in symptoms when PFMT was added to the treatment. This shows that the treatment success increases significantly when first-line treatments are combined with drug therapy and administered effectively in OAB, and invasive treatments may not be needed.
Although PFMT is mostly recommended in the treatment of SUI in daily practice, it also provides significant benefits in OAB [10]. In SUI, PFMT increases the urethral closure pressure by increasing the structural support of the pelvic floor [20]. However, in OAB, it acts by decreasing the detrusor pressure and increasing the urethral closure pressure [21]. There are studies in the literature demonstrating the efficacy of PFMT in OAB [22, 23]. Furthermore, Kafri et al. found a significant decrease in urinary frequency, UUI episodes and an increase in quality of life through administration of PFMT [23]. Kulaksizoğlu et al. also found a significant decrease in OAB symptoms and an increase in functional bladder capacity with PFMT treatment [24]. However, Bø et al. stated in a systematic review that a meaningful comparison could not be made owing to the heterogeneity in the studies [10]. We revealed in our study that PFMT provides a significant reduction in OAB-V8 and ICIQ-SF scores, frequency, and nocturia when combined with behavioral therapy and drug therapy.
Although anticholinergics have an important place in the treatment of OAB, their efficacy is limited and their side effects are evident. Recent studies showed that, the 12-month adherence rate for oxybutynin was found to be between 68 and 95%, whereas it was 49–84% for fesoterodine [25, 26]. The reasons for discontinuing the treatment were mostly associated with the low level of efficacy (41.3%), side effects (22.4%), and cost (18.7%) [11]. In our study, patients had not benefited from different anticholinergics, mirabegron or a combination of the two. In our study, patients who had previously used different anticholinergics, mirabegron or a combination of both, but did not benefit were included. These patients were informed in detail that behavioral therapy and PFMT, when combined with drug therapy, can be much more beneficial than previous treatments. Although the patients were initially reluctant to re-administer medication, when motivated by the benefits of behavioral therapy and PFMT used effectively, they became willing to participate in the study and use medication. This shows us that OAB treatment options are not very well explained to patients in daily clinical practice. Thus, we think that patients do not persist with the treatment if they have any side effects or if it is ineffective. In clinical studies in the literature, it is reported that treatment adherence is higher than in daily practice [11]. We think that this situation is related to informing the patients in much more detail in the studies and good management of their treatment expectations.
This study has some limitations. One of these is that our therapy outcomes were short term. We think that long-term results in further studies would make a contribution to the literature. Another limitation is the lack of data on the weight changes of patients after behavioral therapy. This could show how effectively the patients followed the diet. Another limitation of our study is the absence of urodynamic studies that reveal objective findings in the diagnosis of OAB. Although this was a prospective, randomized controlled study, the lack of blinding is another limitation.
Conclusion
In our study, we revealed that behavioral therapy in addition to drug therapy is not sufficient in patients with wet-type OAB who did not benefit from previous non-invasive treatments; however, combination with behavioral therapy and PFMT provided a significant reduction in the patients’ symptoms. Consequently, we believe that unnecessary invasive treatments may be prevented in these patients.
References
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–8.
Zumrutbas AE, Bozkurt AI, Tas E, Acar CI, Alkis O, et al. Prevalence of lower urinary tract symptoms, overactive bladder and urinary incontinence in western Turkey: results of a population-based survey. Int J Urol. 2014;21(10):1027–33.
Abrams P, Artibani W, Cardozo L, Dmochowski R, van Kerrebroeck P, et al. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn. 2009;28(4):287. https://doi.org/10.1002/nau.20737.
Wein AJ, Kavoussi LR, Partin AV, Peters CA. Campbell-Walsh urology, 11th edition. Elsevier, Amsterdam; 2016.
Smith A, Bevan D, Douglas HR, James D. Management of urinary incontinence in women: summary of updated NICE guidance. BMJ. 2013;347:f5170.
Baser A, Eliaçık S, Baykam MM, Tan FU. Clinical manifestations of overactive bladder with migraine as a comorbidity: a prospective cross-sectional study. Int Neurourol J. 2020;24(4):375–81. https://doi.org/10.5213/inj.2040186.093.
Alkis O, Sevim M, Güven Kartal İ, Baser A, İbrahim İvelik H, Aras B. Comparison of transcutaneous tibial nerve stimulation (TTNS) protocols for women with refractory overactive bladder (OAB): a prospective randomised trial. Int J Clin Pract. 2021;75(9):e14342. https://doi.org/10.1111/ijcp.14342.
Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Dombrowski M, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA. 1998;280:1995–2000.
Rizvi RM, Chughtai NG, Kapadia N. Effects of bladder training and pelvic floor muscle training in female patients with overactive bladder syndrome: a randomized controlled trial. Urol Int. 2018;100:420–7.
Bo K, Fernandes ACNL, Duarte TB, Brito LGO, Ferreira CHJ. Is pelvic floor muscle training effective for symptoms of overactive bladder in women? A systematic review. Physiotherapy. 2020;106:65–75.
Harding CK, Lapitan MC, Arlandis S, Bø K, Costantini E, Groen J, et al. EAU Guidelines Non-neurogenic Female LUTS. Presented at the EAU Annual Congress Milan 2021. Available at: http://uroweb.org/guidelines/compilations-of-all-guidelines/
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32.
Özlü A, Yıldız N, Öztekin Ö. Comparison of the efficacy of perineal and intravaginal biofeedback assisted pelvic floor muscle exercises in women with urodynamic stress urinary incontinence. Neurourol Urodyn. 2017;36(8):2132–41. https://doi.org/10.1002/nau.23257.
Tarcan T, Mangır N, Ozgur O, Akbal C. OAB-V8 asırı aktif mesane sorgulama formu validasyon çalışması. Uroloji Bulteni. 2012;21:113–6.
Burgio KL, Kraus SR, Johnson TM 2nd, Markland AD, Vaughan CP, Li P, Redden DT, Goode PS. Effectiveness of combined behavioral and drug therapy for overactive bladder symptoms in men: a randomized clinical trial. JAMA Intern Med. 2020;180(3):411–9. https://doi.org/10.1001/jamainternmed.2019.6398.
Alkış O, Başer A, Özlülerden Y, Ölçücü MT, Zümrütbaş AE, Aybek Z. Factors facilitating the emergence of overactive bladder in adults with childhood voiding disorder. New J Urol. 2020;15(3):189–93.
Le Berre M, Presse N, Morin M, et al. What do we really know about the role of caffeine on urinary tract symptoms? A scoping review on caffeine consumption and lower urinary tract symptoms in adults. Neurourol Urodyn. 2020;39(5):1217–33. https://doi.org/10.1002/nau.24344.
Erickson BA, Vaughan-Sarrazin M, Liu X, Breyer BN, Kreder KJ, Cram P. Lower urinary tract symptoms and diet quality: findings from the 2000–2001 National Health and Nutrition Examination Survey. Urology. 2012;79(6):1262–7. https://doi.org/10.1016/j.urology.2012.03.004.
Bradley CS, Erickson BA, Messersmith EE, et al. Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN). Evidence of the impact of diet, fluid intake, caffeine, alcohol and tobacco on lower urinary tract symptoms: a systematic review. J Urol. 2017;198(5):1010–20. https://doi.org/10.1016/j.juro.2017.04.097.
Bø K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work? Int Urogynecol J. 2004;15(2):76–84.
Shafik A, Shafik IA. Overactive bladder inhibition in response to pelvic floor muscle exercises. World J Urol. 2003;20(6):374–7.
Bykoviene L, Kubilius R, Aniuliene R, Bartuseviciene E, Bartusevicius A. Pelvic floor muscle training with or without tibial nerve stimulation and lifestyle changes have comparable effects on the overactive bladder. A randomized clinical trial. Urol J. 2018;15(4):186–92.
Kafri R, Deutscher D, Shames J, Golombp J, Melzer I. Randomized trial of a comparison of rehabilitation or drug therapy for urgency urinary incontinence: 1-year follow-up. Int Urogynecol J. 2013;24(7):1181–9. https://doi.org/10.1007/s00192-012-1992-8.
Kulaksızoğlu H, Akand M, Cakmakci E, Gül M, Seçkin B. Effectiveness of pelvic floor muscle training on symptoms and uroflowmetry parameters in female patients with overactive bladder. Turkish J Med Sci. 2015;45:449–53. https://doi.org/10.3906/sag-1310-95.
Scarpero H, Sand PK, Kelleher CJ, Berriman S, Bavendam T, Carlsson M. Long-term safety, tolerability, and efficacy of fesoterodine treatment in men and women with overactive bladder symptoms. Curr Med Res Opin. 2011;27(5):921–30. https://doi.org/10.1185/03007995.2011.559581.
Sand PK, Heesakkers J, Kraus SR, Carlsson M, Guan Z, Berriman S. Long-term safety, tolerability and efficacy of fesoterodine in subjects with overactive bladder symptoms stratified by age: pooled analysis of two open-label extension studies. Drugs Aging. 2012;29(2):119–31. https://doi.org/10.2165/11597970-000000000-00000.
Author information
Authors and Affiliations
Contributions
O. Alkis: project development, data collection, statistical analysis, manuscript writing; A. Ozlu: project development, manuscript writing, editing; I. Kartal: project development, data collection, editing; M. Sevim: project development, data collection, language editing; A. Baser: project development, data collection, statistical analysis; B. Aras: project development, data collection, editing.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alkis, O., Ozlu, A., Kartal, I.G. et al. How effectively do we apply first-line treatment in overactive bladder?. Int Urogynecol J 33, 2299–2306 (2022). https://doi.org/10.1007/s00192-022-05279-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05279-2