Abstract
Introduction and hypothesis
eHealth interventions represent a promising novel strategy in pelvic floor management for women. Nevertheless, the effectiveness of eHealth interventions among women with or at risk of pelvic floor dysfunction (PFD) has not been adequately discussed to date. This study aimed to determine the effectiveness of eHealth interventions in preventing and treating PFD among women.
Methods
Eleven electronic databases were searched for randomized controlled trials (RCTs) from inception until August 28, 2021.
Results
Twenty-four RCTs were included in this meta-analysis that included 3691 women. The meta-analysis showed that eHealth interventions were not only vital for preventing PFD (pregnant women: pooled OR = 0.25, 95% CI: 0.14 to 0.45, p < 0.001; postnatal women: pooled OR = 0.19, 95% CI: 0.06 to 0.60, p = 0.005), but also for reducing the severity of PFD (pooled SMD = -0.63, 95% CI: -1.20 to -0.06, p = 0.031). In addition, compared with traditional care, eHealth interventions showed significant positive effects on several outcome indicators, including quality of life (pooled SMD = 0.49, 95% CI: 0.19 to 0.80, p = 0.002), pelvic floor type I muscle strength (pooled OR = 1.92, 95% CI: 1.30 to 2.82, p = 0.001), pelvic floor type II muscle strength (pooled OR = 2.04, 95% CI: 1.38 to 3.01, p < 0.001), sexual function (pooled SMD = 0.51, 95% CI: 0.29 to 0.73, p < 0.001), satisfaction (pooled OR = 3.93, 95% CI: 2.73 to 5.66, p < 0.001), and self-efficacy (pooled SMD = 2.62, 95% CI: 2.12 to 3.13, p < 0.001).
Conclusions
eHealth interventions are an effective emerging treatment and preventive modality for female PFD. Higher quality, larger scale, and strictly designed RCTs are warranted to evaluate the effectiveness of eHealth interventions on female pelvic floor management.
Similar content being viewed by others
Introduction
Pelvic floor dysfunction (PFD) is one of the most common gynecological diseases among women worldwide, caused by the weakening of pelvic floor supporting tissue, and consists of a group of degenerative conditions such as urinary incontinence, pelvic organ prolapse, fecal incontinence, sexual dysfunction, and other urogenital symptoms. Disorders of the pelvic floor are known to affect millions of women worldwide. The general population proportion of women with one or more pelvic floor disorders has been reported at 25%, and this markedly increases with age [1]. It has been estimated that the total number of individuals suffering from PFD in developed and developing countries will increase to approximately 43.8 million by 2050 [2]. Many risk factors are associated with PFD progression, including pregnancy, vaginal delivery, age, menopause, chronic cough, obesity, etc. [3]. Among them, it is generally thought that pregnancy and delivery-related pelvic floor trauma are essential risk factors for PFD [4, 5]. PFD can negatively affect the social and physical functions of women, restrict women in their daily activities, impair sexual function, and ultimately reduce their overall quality of life while putting a considerable economic burden on healthcare resources [6, 7].
Effective prevention and treatment are both efficient strategies that are critical to PFD management. PFD is usually treated with conservative methods such as pelvic floor muscle training, bladder retraining, electrostimulation, and lifestyle interventions [8, 9]. Among them, pelvic floor muscle training is not only an effective method to prevent PFD, but also internationally recommended as the first-line treatment for urinary incontinence and pelvic organ prolapse [10, 11]. Despite the existence of evidence-based management approaches to the prevention and treatment of PFD [12, 13], findings from existing literature sources show that the best prevention and management practices for PFD in women have not been routinely enforced in most healthcare settings [14]. Most women experiencing PFD symptoms try to take control of the condition without seeking medical care [15]. According to studies, < 30% of women seek the help of healthcare professionals [16, 17]. Previous researchers have revealed that several reasons for women not seeking professional treatment include stigma and embarrassment, the lack of knowledge about PFD, the high cost of treatment, excessive wait times, limited access to health care services, and concerns about perceived consequences, which hinder the implementation of pelvic floor rehabilitation and are detrimental to individual health and quality of life [18, 19]. As a result, developing innovative strategies or modalities of pelvic floor management for women with or at risk of PFD is critical.
With the rapid advancement of information technology, eHealth has recently attracted considerable attention and is currently being promoted as a way for individuals and healthcare providers to improve health care [20]. It represents a promising method that can reduce barriers for women who do not seek medical care and potentially improve pelvic floor self-management ability and pelvic floor muscle training compliance for women with or at risk of PFD. eHealth, defined as “health services and information delivered or enhanced through the internet and related technologies” [21], includes, for example, teleconsultation, remote monitoring, virtual reality, internet-based interventions, mobile phone apps, videoconferencing, etc. [22, 23]. Through the internet and other electronic-related technologies, eHealth interventions are not limited by the lack of time and space when providing knowledge of disease management and can help women obtain relevant information about pelvic floor management quickly and easily, which is different from the traditional face-to-face intervention. Moreover, due to its anonymity, flexibility, and accessibility, eHealth interventions can also reduce women’s sense of shame and embarrassment associated with seeking professional help, reduce the cost and time, and increase their access to healthcare services [24,25,26].
Some studies have provided evidence that eHealth interventions exert a beneficial influence on women’s pelvic floor symptom management [27, 28], while others could not find any significant improvement [29]. More recently, a systematic review evaluated the efficiency of eHealth interventions in the rehabilitation of female PFD [30]. Nevertheless, there were several important limitations to this systematic review. To begin with, the review included only four related references for analysis. Second, no quantitative summary assessment was performed. Third, the review did not provide evidence to support the effectiveness of eHealth interventions for PFD prevention.
Up to now, a comprehensive review on the effectiveness of eHealth interventions among women with or at risk of pelvic floor disorders has been lacking. Thus, given the limited scope of previous studies, the aim of this meta-analysis was to determine the effectiveness of eHealth interventions in preventing and treating PFD among women compared with traditional care.
Methods
The PRISMA guidelines were followed for this meta-analysis [31], and a protocol was registered on the PROSPERO database (CRD42021287322).
Data sources and searches
We systemically performed a systematic search in 11 electronic databases: PubMed, Web of Science, CINAHL, Embase, PsycINFO, The Cochrane Library databases, Scopus, CNKI, WanFang, VIP databases, and CBM from inception until August 28, 2021. The Medical Subject Headings (MeSH) and keywords were used as follows: “telemedicine,” “telehealth,” “e-health,” “mobile health,” “teleconsultation,” “telecommunications,” “multimedia,” “mobile application,” “smartphone,” “urinary incontinence,” “pelvic organ prolapse,” “uterine prolapse,” “rectocele,” “cystocele,” “fecal incontinence,” “pelvic floor,” “pelvic floor disorders,” and “randomized controlled trial.” The detailed retrieval strategies are available in the Appendix 1. To identify additional records, we also manually searched for potentially eligible publications. In the literature screening process, the results of the searches from different electronic databases were imported into EndNote Version X9, where duplicate studies were deleted. After excluding duplicates, two reviewers independently screened the retrieved studies according to the inclusion and exclusion criteria.
Inclusion and exclusion criteria
We included all studies evaluating the effects of eHealth interventions on women who have been diagnosed with pelvic floor disorders or are at risk of PFD for either prevention or treatment of the disease. Studies were considered for inclusion based on the PICOS framework if the following criteria were met: (1) participants: participants were women who were diagnosed with pelvic floor disorders or at risk of PFD (such as postnatal women or pregnant women); (2) intervention: in the intervention group, participants received any form of eHealth intervention (e.g., distance counseling, mobile applications, videoconferencing, text messaging) to help women treat or prevent PFD through self-management; (3) comparison: traditional care or waiting list control were provided to participants in the control group; (4) outcome: one or more of the following interesting outcomes have been reported (e.g., in prevention studies, the incidence of PFD was assessed as an outcome measure; in treatment studies, the severity of pelvic floor symptoms and the patient’s global impression of improvement were included as outcome indicators; other outcome measures were as follows: quality of life, self-efficacy, satisfaction with the intervention, sexual function, and the rate of qualification for pelvic floor muscle strength) (5) study design: the study was a randomized controlled trial.
The exclusion criteria for studies were as follows: (1) cohort studies, case-control studies, qualitative studies, reviews, conference abstracts, study protocols, or ongoing studies; (2) publications in languages other than English and Chinese; (3) incomplete data; (4) follow-up studies if studies from the same population were published; (5) the outcomes of interest were not reported.
Data extraction
Data from the included studies was extracted and summarized independently by two of the reviewers using a standardized data extraction form. The extracted study information included the first author, year of publication, country, study design, sample size (eHealth/usual care), purpose of administration, study population, mean age, details of the intervention, control content, outcome measures, and data collection time points. The original authors were contacted to obtain any missing data if possible.
Quality assessment
The methodological quality of all individual studies was appraised for study quality using The Cochrane Risk of Bias Assessment Tool by two independent reviewers, with a third researcher was used where discrepancies persisted. The Cochrane Risk of Bias Assessment Tool was used to rate the overall quality of evidence based on six domains: (1) generation of random sequence; (2) concealment of allocation; (3) blinding of participants and personnel; (4) blinding of outcome assessors; (5) adequately addressed incomplete outcome data; (6) selective outcome reporting; (7) other bias (e.g., baseline comparability, early stopping, and possible bias due to funding). All domains were evaluated using the Cochrane criteria to classify the risk of bias as: (1) low risk of bias; (2) high risk of bias; (3) unclear.
Statistical analysis
All the statistical analyses were performed with the Review Manager Software 5.3 and STATA 15.1. According to whether the outcomes were measured with the same scales or different scales, mean difference (MD) and standardized mean difference (SMD) with the 95% confidence interval (CI) were used to analyze continuous variable data. Odds ratio and 95% confidence interval were used for dichotomous outcomes. Statistical heterogeneity among the studies was assessed using the chi-square test and the I2 statistic; if p < 0.10 and/or I2 > 50%, a random effects model would be used because of substantial heterogeneity; otherwise, a fixed-effects model of analysis would be used if heterogeneity between studies was recognized as being low. Subgroup analyses were conducted to determine the effects of different eHealth modalities.
Results
Study selection
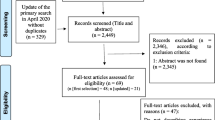
A total of 8592 relevant studies were retrieved from the literature search, of which 2334 were considered duplicate literature. After excluding duplicate articles, 6258 titles and abstracts were screened, and 6126 studies were excluded. Thus, 132 of the full-text articles were selected for further consideration, of which 108 were excluded. Finally, 24 RCTs met the inclusion criteria and were included in this meta-analysis [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. The study flow diagram is displayed in Fig. 1.
Characteristics of the included studies
Selected studies were published between 2013 and 2021, of which 10 studies [32,33,34,35,36, 41, 43, 44, 47, 48] were written in English and the remaining were written in Chinese, originating from 7 countries, including China (15 studies) [37,38,39,40, 42, 45,46,47, 49,50,51,52,53,54,55], Brazil (2 studies) [32, 34], Sweden (2 studies) [33, 44], the US (2 studies) [43, 48], the UK (1 study) [36], Canada (1 study) [35], and The Netherlands (1 study) [41]. The 24 studies included 3691 women who were diagnosed with pelvic floor disorders or at risk of PFD, among whom 1862 were allocated to the eHealth group and 1829 to the control group. Among all the studies, various eHealth technologies were observed, including: mobile phone or tablet applications (7 studies) [32, 33, 36, 38, 40, 41, 45], internet (9 studies) [37, 39, 42, 44, 46, 49,50,51, 55], telemetry device (1 study) [34], video (2 studies) [43, 54], audio (1 study) [47], telephone (2 studies) [52, 53], and mixed technologies, where more than one eHealth modality was reported (2 studies combined telemetry devices with applications) [35, 48]. The characteristics of eligible studies are displayed in Tables 1 and 2.
Assessment of the risk of bias for included studies
The risk of bias graph and the risk of bias summary are shown in Figs. 2 and 3. For random sequence generation, 16 studies were evaluated to be at low risk since the methods of random sequence generation were described in detail [32,33,34,35,36, 38,39,40, 44,45,46,47,48,49,50,51]. One of the 24 studies was assessed as high risk because it was grouped according to the odd and even numbers of health care cards [53]. Five studies reported adequate allocation concealment [32, 33, 35, 41, 47], and 18 studies were judged at unclear risk due to insufficient descriptions [34, 37,38,39,40, 42,43,44,45,46, 48,49,50,51,52,53,54,55]. As for blinding of participants and personnel, most included studies (83.3%) were judged to be at an unclear risk of bias. For the blinding of outcome assessment, seven studies were assessed as low risk [32, 34,35,36, 41, 47, 48]. Three studies were rated as having a high risk of attrition bias as the attrition rates were > 15%, and intention-to-treat analysis was not performed [32, 43, 48]. A study was judged to be at high risk of reporting bias because “coordination of the pelvic floor muscles” was given as an outcome indicator in the clinical trial protocol but was not stated in the study [34]. Seventeen studies had an unclear risk of reporting bias due to a lack of detailed information on study protocols and trial registrations for further assessment [37,38,39,40,41,42,43, 45,46,47, 49,50,51,52,53,54,55]. A high risk of reporting other bias was given to two studies because there were baseline differences between the intervention group and control group [32, 41].
Effects of eHealth interventions on PFD prevention
The incidence of PFD
Five preventative studies involving 846 participants who were at risk of PFD investigated the incidence of PFD, and we noticed that all of the women included in preventative RCTs were postnatal or pregnant [39, 40, 46, 52, 55].
Three studies involving 579 pregnant women reported the efficiency of eHealth interventions on the incidence of PFD [39, 40, 52]. As no heterogeneity was noted (I2 = 0%) (Fig. 4a), a fixed-effects model was chosen. There was a statistically significant effect of eHealth interventions compared with control groups in preventing the occurrence of PFD for pregnant women (pooled OR = 0.25, 95% CI: 0.14 to 0.45, z = 4.65, p < 0.001).
Two studies involving 267 postnatal women reported the incidence of PFD between the eHealth and control groups [46, 55]. A random-effects model was selected for data synthesis because of significant heterogeneity (I2 = 76.3%) across the studies, and a statistically significant difference was found between the two groups (pooled OR = 0.19, 95% CI: 0.06 to 0.60, z = 2.82, p = 0.005) (Fig. 4b).
Effects of eHealth interventions on PFD treatment
The severity of pelvic floor symptoms
Eight studies involving 769 participants examined the efficiency of eHealth interventions on the severity of pelvic floor symptoms [32,33,34, 38, 44, 47, 48, 53]. Owing to the significant heterogeneity (I2 = 92.5%) across the studies, a random-effects model was applied (Fig. 5). The meta-analysis revealed that there was a statistically significant difference in the severity of pelvic floor symptoms between the eHealth and control groups (pooled SMD = -0.63, 95% CI: -1.20 to -0.06, z = 2.15, p = 0.031).
The patient’s global impression of improvement
We included 5 studies involving 627 participants that reported the patient’s global impression of improvement between the eHealth and control groups [33, 34, 41, 44, 48]. The meta-analysis showed that no significant difference was found in the patient’s global impression of improvement between the two groups (pooled OR = 1.90, 95% CI: 0.64 to 5.59, z = 1.16, p = 0.246) (Fig. 6).
Effects of eHealth interventions on other outcome measures
Quality of life
Eight studies involving 1089 participants evaluated quality of life [33, 35, 42,43,44, 49, 50, 53]. Due to the significant heterogeneity (I2 = 81.8%), a random-effects model was applied (Fig. 7), and a statistically significant difference was found between the eHealth and control groups (pooled SMD = 0.49, 95% CI: 0.19 to 0.80, z = 3.14, p = 0.002). Compared with the control group, quality of life scores in the eHealth group were higher.
Self-efficacy
We included 3 studies involving 775 participants that assessed self-efficacy between the eHealth and control groups [38, 47, 54]. A random-effects model was applied because of significant heterogeneity (I2 = 78.3%) across the studies, and a statistically significant difference was found between the two groups (pooled SMD = 2.62, 95% CI: 2.12 to 3.13, z = 10.15, p < 0.001) (Fig. 8).
Satisfaction with the intervention
We included 5 studies involving 1350 participants that assessed satisfaction with the intervention between the eHealth and control groups [37, 40, 44, 45, 54]. As no heterogeneity was noted (I2 = 0%) (Fig. 9), a fixed-effects model was chosen. A statistically significant difference was observed between the two groups (pooled OR = 3.93, 95% CI: 2.73 to 5.66, z = 7.36, p < 0.001), indicating that the eHealth group had better satisfaction with the intervention than the control group.
Sexual function
Three studies involving 318 participants were included to assess sexual function between the eHealth and control groups [36, 47, 51]. As no heterogeneity was noted (I2 = 0%) (Fig. 10), a fixed-effects model was chosen. A statistically significant difference was observed between the two groups (pooled SMD = 0.51, 95% CI: 0.29 to 0.73, z = 4.52, p < 0.001), indicating that the eHealth group had better sexual function than the control group.
The rate of qualification for pelvic floor muscle strength
Type I muscle strength
Three studies involving 480 participants were included to assess the rate of qualification for type I pelvic floor muscle strength between the eHealth and control groups [47, 51, 55]. A fixed-effects model was selected for data synthesis as the heterogeneity between studies was low (I2 = 4%) (Fig. 11). A statistically significant difference was observed between the two groups (pooled OR = 1.92, 95% CI: 1.30 to 2.82, z = 3.30, p = 0.001).
Type II muscle strength
Three studies involving 480 participants were included to assess the rate of qualification for type II pelvic floor muscle strength between the eHealth and control groups [47, 51, 55]. As no heterogeneity was noted (I2 = 0%) (Fig. 12), a fixed-effects model was chosen. The meta-analysis showed that there was a statistically significant difference in the rate of qualification for type II muscle strength between the eHealth and control groups (pooled OR = 2.04, 95% CI: 1.38 to 3.01, z = 3.56, p < 0.001).
Subgroup analyses
Subgroup analyses were conducted based on different eHealth modalities (application, telephone, internet, video, audio, telemetry device, and mixed technologies) (Table 3). In subgroup analyses, the results were consistent with the results of the pooled analysis. The results of subgroup analyses showed that the application-based interventions could reduce the severity of PFD (pooled SMD = -1.02, 95% CI: -1.84 to -0.20, z = 2.43, p = 0.015) and improve satisfaction with the intervention (pooled OR = 5.68, 95% CI: 2.05 to 15.75, z = 3.34, p = 0.001) when compared with the control group. Besides, compared with traditional care, the internet-based interventions revealed significant positive effects on several outcome indicators, including quality of life (pooled SMD = 0.47, 95% CI: 0.16 to 0.78, z = 3.00, p = 0.003), satisfaction (pooled OR = 3.74, 95% CI: 2.01 to 6.95, z = 4.17, p < 0.001), pelvic floor type I muscle strength (pooled OR = 1.71, 95% CI: 1.12 to 2.60, z = 2.51, p = 0.012), and pelvic floor type II muscle strength (pooled OR = 1.89, 95% CI: 1.26 to 2.84, z = 3.06, p = 0.002).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to summarize the effectiveness of eHealth interventions in the prevention and treatment of female PFD, based on English and Chinese publications. In this study, we included and comprehensively analyzed 24 RCTs from 11 electronic databases, most of which (91.7%) were published within the past 5 years, indicating that the number of women choosing to use mobile-technology health services for pelvic floor intervention has increased recently. The meta-analysis showed that eHealth interventions were vital not only for preventing PFD but also for reducing the severity of PFD. In addition, compared with traditional care, eHealth interventions showed significant positive effects on several outcome indicators, including quality of life, pelvic floor muscle strength, sexual function, satisfaction with the intervention, and self-efficacy.
Our findings showed that, compared with the control group, the intervention group had a lower incidence of pelvic floor dysfunction-related diseases, indicating that eHealth interventions can play a role in preventing PFD in postnatal and pregnant women.
Pregnancy and delivery are independent risk factors for pelvic floor dysfunction. Peripheral nerves, muscles, and connective tissue will be compressed, stretched, or torn during pregnancy and delivery, significantly increasing the risk of pelvic floor injury [56]. Previous studies pointed out that pelvic floor muscle training during the prenatal and postpartum periods is beneficial for the prevention of PFD [57, 58]. As per the information available from UpToDate, women who have no contraindications should perform daily pelvic floor muscle training during pregnancy, and pelvic floor muscle training should be carried out at the appropriate time during postpartum according to the mode of delivery and personal tolerance [59].
Currently, women have several problems with regard to adhering to pelvic floor muscle training, such as forgetting to perform the exercises and a lack of knowledge or skills to perform pelvic floor muscle training [60, 61]. With the emergence of electronic technology, eHealth interventions are beginning to represent a promising novel approach for addressing these issues. Through the internet, apps, and other eHealth-related technologies, women can get reminders and teach themselves about diseases, which improves the accessibility of pelvic floor muscle training [62, 63]. Because pregnancy and childbirth are known as the key periods of pelvic floor muscle recovery, effective eHealth interventions should be provided to postnatal and pregnant women to promote their pelvic floor rehabilitation.
Although the summary results of the randomized control trials revealed that eHealth interventions had no significant effect on the patient’s global impression of improvement, the meta-analysis showed that eHealth interventions improved the severity of pelvic floor disorder symptoms. The discrepancy between these two outcomes can be explained as follows. The patient's global impression of improvement (PGI-I) was based on a single question in which the respondent was asked to recall their pre-treatment symptom condition and compare it with their current status, with responses ranging from "much better" to "very much worse." A previous study has shown that the strength of the association between the patient's global assessments and symptom measures for urinary patients is potentially affected by the recall period [64]. Our findings showed that the intervention duration in the relevant studies that used the patient's global impression of improvement as the outcome measure ranged from 8 weeks to 4 months. It is possible that the data could be affected by recall bias resulting from the long intervention period, which may be the cause of the discrepancy between the patient's global impression of improvement and the severity of symptoms. We found that patients reporting the severity of pelvic floor symptoms included in the study had urinary incontinence, most of whom suffered from stress urinary incontinence. Our results confirmed the effectiveness of eHealth interventions in reducing the severity of urinary incontinence symptoms, which is consistent with earlier studies [65, 66]. The importance of eHealth interventions has been demonstrated [63], and patients can improve their cognition of the disease in real time by using apps and other similar eHealth technologies [67]. Furthermore, the high-efficiency interactive feedback and reminder function not only provides a convenient platform for doctor-patient communication but also improves the compliance of patients with pelvic floor muscle exercise regimes [68]. It should be noted that a prior study has shown that the severity of patients’ urinary incontinence symptoms has an impact on the effectiveness of eHealth interventions in treating urinary incontinence [69]. Individuals with severe urine incontinence show less improvement while using eHealth interventions than patients with minor symptoms. As a result, patients suffering from severe urine incontinence should seek professional medical advice and assistance for appropriate treatment.
Compared with traditional care, eHealth interventions showed significant positive effects on several outcome indicators, including quality of life, pelvic floor muscle strength, sexual function, satisfaction, and self-efficacy of women with or at risk of pelvic floor disorders. Women with or at risk of pelvic floor disease may worry about a series of adverse consequences linked to PFD, including a restricted ability to perform activities, skin rashes, and pruritus in the genital area [66]. A previous survey showed that most individuals would like to gain more knowledge about their pelvic floor status by using the internet, social networking sites, and other online resources [70]. By providing information on lifestyle choices and effective pelvic floor muscle exercises, eHealth interventions enable women to have better capacity and mental reserves to cope with difficulties, thereby promoting women’s self-management of their pelvic floor health in the short and long term. Moreover, healthcare professionals can provide personalized guidance to patients through the platform, which may improve the effectiveness of patients’ rehabilitation training, enhance their pelvic floor muscle strength, and improve their satisfaction with the intervention.
We found that application-based and internet-based interventions were the two main eHealth interventions used, and the results of the subgroup analysis supported the beneficial effects of both intervention types on pelvic floor rehabilitation. To date, there is no consensus on the relative superiority of application-based or internet-based interventions as eHealth modalities [71, 72]. Research indicates that internet-based interventions are considered compatible, and application-based interventions are considered flexible and personalized [71]. Further comparisons of the differences across the various eHealth modalities were difficult because of the limited number of studies included in each group of the subgroup analyses, and the effectiveness of other types of eHealth interventions needs to be further confirmed by more studies.
There were several advantages to this study. First, this review offers a literary resource on the impact of eHealth interventions on female pelvic floor health. As far as we know, only one review has focused on this issue before, and it was limited to qualitative analysis of the results [30]. One article quantitatively evaluated the impact of telemedicine on urinary incontinence, but the number of articles included was insufficient [65]. We expanded the scope of the systematic evaluation, included more studies, and evaluated multiple outcome indicators related to the prevention and treatment of PFD, including the incidence of PFD, pelvic floor muscle strength, satisfaction, etc. Second, we reviewed 11 databases and used a comprehensive search strategy to systematically retrieve the articles. Third, this meta-analysis only included studies that were designed as randomized controlled trials as this is a strict method used to determine causality and ensure scientific effectiveness, and therefore the conclusion is more reliable.
However, this meta-analysis also had some limitations. First, the literature search identified articles published in English and Chinese, potentially resulting in publication bias. Moreover, there was significant heterogeneity among the multiple outcome indicators in this review. This may be related to differences in the different eHealth interventions, including different intervention durations, intensities, frequencies, intervention contents, target populations, and intervention media, which may affect the accuracy of the evidence obtained from the summary analysis of the articles. Furthermore, most studies lacked sufficient details to assess the possible bias in reporting and detection, and the methodological quality of the included studies was limited and had potential bias, which may affect the quality of evidence obtained in this review.
In the future, large-scale and well-designed RCTs are warranted to evaluate the effects of eHealth interventions among women with or at risk of pelvic floor disorders. Researchers should consider the potential impact of behavior change technologies (such as self-monitoring and feedback) and the theoretical framework of the individual application of eHealth resources. To clarify the impact of different intervention components of eHealth on pelvic floor rehabilitation and to facilitate a more comprehensive systematic review in the future, researchers are encouraged to provide the full details of all intervention components and specifically evaluate the different intensities, frequencies, durations, and types of eHealth services. In addition, the influence of eHealth interventions on other pelvic floor disorders, including fecal incontinence and pelvic organ prolapse, should be investigated. Furthermore, evidence-based pelvic floor muscle training parameters should be embedded in apps when developing and designing relevant pelvic floor electronic health applications to perform standardized pelvic floor rehabilitation exercises [73]. During the COVID-19 pandemic, as patients were not permitted to receive face-to-face treatments, eHealth became an important method for pelvic floor management [74]. Since the success of pelvic floor self-management largely depends on individual adherence [63], some principles can be used in eHealth design, such as interestingness and information sharing, which can increase user participation [71]. According to a study, the willingness to use eHealth was linked to computer literacy [75]. Being unfamiliar with electronic medical applications may also affect the enthusiasm of participants. Women, particularly elderly women, should be educated on how to better use eHealth modalities to increase their information literacy.
Conclusions
This meta-analysis demonstrated that eHealth interventions are an effective emerging treatment and preventive modality for female PFD. Higher quality, larger scale, and strictly designed RCTs are required to further evaluate the efficacy of eHealth interventions on pelvic floor management.
Abbreviations
- PFD:
-
pelvic floor dysfunction
- RCT:
-
randomized controlled trial
References
Dieter AA, Wilkins MF, Wu JM. Epidemiological trends and future care needs for pelvic floor disorders. Curr Opin Obstet Gynecol. 2015;27(5):380–4. https://doi.org/10.1097/GCO.0000000000000200.
Zuchelo LTS, Bezerra IMP, Da Silva ATM, Gomes JM, Soares JM, Baracat EC, et al. Questionnaires to evaluate pelvic floor dysfunction in the postpartum period: a systematic review. Int J Women's Health. 2018;10:409–24. https://doi.org/10.2147/IJWH.S164266.
Vergeldt TFM, Weemhoff M, IntHout J, Kluivers KB. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J. 2015;26(11):1559–73. https://doi.org/10.1007/s00192-015-2695-8.
Hage-Fransen MAH, Wiezer M, Otto A, Wieffer-Platvoet MS, Slotman MH, Nijhuis-van der Sanden MWG, et al. Pregnancy- and obstetric-related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2021;100(3):373–82. https://doi.org/10.1111/aogs.14027.
Zhong F, Miao W, Yu Z, Hong L, Deng N. Clinical effect of electrical stimulation biofeedback therapy combined with pelvic floor functional exercise on postpartum pelvic organ prolapse. Am J Transl Res. 2021;13(6):6629–37.
Mendes A, Hoga L, Goncalves B, Silva P, Pereira P. Adult women's experiences of urinary incontinence: a systematic review of qualitative evidence. JBI Database System Rev Implement Rep. 2017;15(5):1350–408. https://doi.org/10.11124/JBISRIR-2017-003389.
Verbeek M, Hayward L. Pelvic floor dysfunction and its effect on quality of sexual life. Sex Med Rev. 2019;7(4):559–64. https://doi.org/10.1016/j.sxmr.2019.05.007.
Konstantinos H, Eleni K, Dimitrios H. Dilemmas in the management of female stress incontinence: the role of pelvic floor muscle training. Int Urol Nephrol. 2006;38(3-4):513–25. https://doi.org/10.1007/s11255-006-0085-3.
Bo K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol. 2012;30(4):437–43. https://doi.org/10.1007/s00345-011-0779-8.
Dumoulin C, Cacciari LP, Mercier J. Keeping the pelvic floor healthy. Climacteric. 2019;22(3):257–62. https://doi.org/10.1080/13697137.2018.1552934.
Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10(10):CD005654. https://doi.org/10.1002/14651858.CD005654.pub4.
Abrams P, Andersson KE, Apostolidis A, Birder L, Bliss D, Brubaker L, et al. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol Urodyn. 2018;37(7):2271–2. https://doi.org/10.1002/nau.23551.
Woodley SJ, Boyle R, Cody JD, Morkved S, Hay-Smith EJC. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2017;12(12):CD007471. https://doi.org/10.1002/14651858.CD007471.pub3.
Wagg A, Duckett J, McClurg D, Harari D, Lowe D. To what extent are national guidelines for the management of urinary incontinence in women adhered? Data from a national audit. BJOG. 2011;118(13):1592–600. https://doi.org/10.1111/j.1471-0528.2011.03100.x.
Wojtowicz U, Plaszewska-Zywko L, Stangel-Wojcikiewicz K, Basta A. Barriers in entering treatment among women with urinary incontinence. Ginekol Pol. 2014;85(5):342–7. https://doi.org/10.17772/gp/1734.
Yu HJ, Wong WY, Chen J, Chie WC. Quality of life impact and treatment seeking of Chinese women with urinary incontinence. Qual Life Res. 2003;12(3):327–33. https://doi.org/10.1023/a:1023250632395.
Hagglund D, Walker-Engstrom ML, Larsson G, Leppert J. Quality of life and seeking help in women with urinary incontinence - a population-based study. Acta Obstet Gynecol Scand. 2001;80(11):1051–5. https://doi.org/10.1034/j.1600-0412.2001.801117.x.
Fakari FR, Hajian S, Darvish S, Majd HA. Explaining factors affecting help-seeking behaviors in women with urinary incontinence: a qualitative study. BMC Health Serv Res. 2021;21(1):60. https://doi.org/10.1186/s12913-020-06047-y.
Buurman MBR, Lagro-Janssen ALM. Women's perception of postpartum pelvic floor dysfunction and their help-seeking behaviour: a qualitative interview study. Scand J Caring Sci. 2013;27(2):406–13. https://doi.org/10.1111/j.1471-6712.2012.01044.x.
Neijenhuijs KI, van der Hout A, Veldhuijzen E, Scholten-Peeters GGM, van Uden-Kraan CF, Cuijpers P, et al. Translation of the eHealth impact questionnaire for a population of dutch electronic health users: validation study. J Med Internet Res. 2019;21(8):e13408. https://doi.org/10.2196/13408.
Eysenbach G. What is e-health? J Med Internet Res. 2001;3(2):E20. https://doi.org/10.2196/jmir.3.2.e20.
van den Heuvel JFM, Groenhof TK, Veerbeek JHW, van Solinge WW, Lely AT, Franx A, et al. eHealth as the next-generation perinatal care: an overview of the literature. J Med Internet Res. 2018;20(6):e202. https://doi.org/10.2196/jmir.9262.
Slattery BW, Haugh S, O'Connor L, Francis K, Dwyer CP, O'Higgins S, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. J Med Internet Res. 2019;21(7):e11086. https://doi.org/10.2196/11086.
Elbert NJ, van Os-Medendorp H, van Renselaar W, Ekeland AG, Hakkaart-van Roijen L, Raat H, et al. Effectiveness and cost-effectiveness of eHealth interventions in somatic diseases: a systematic review of systematic reviews and meta-analyses. J Med Internet Res. 2014;16(4):182–204. https://doi.org/10.2196/jmir.2790.
Wessels NJ, Hulshof L, Loohuis AMM, van Gemert-Pijnen L, Jellema P, van der Worp H, et al. User experiences and preferences regarding an app for the treatment of urinary incontinence in adult women: qualitative study. JMIR Mhealth Uhealth. 2020;8(6):e17114. https://doi.org/10.2196/17114.
Asklund I, Samuelsson E, Hamberg K, Umefjord G, Sjostrom M. User experience of an app-based treatment for stress urinary incontinence: qualitative interview study. J Med Internet Res. 2019;21(3):e11296. https://doi.org/10.2196/11296.
Nagib ABL, Riccetto C, Martinho NM, Pennisi PRC, Blumenberg C, Paranhos LR, et al. Use of mobile apps for controlling of the urinary incontinence: a systematic review. Neurourol Urodyn. 2020;39(4):1036–48. https://doi.org/10.1002/nau.24335.
Hoffman V, Soderstrom L, Samuelsson E. Self-management of stress urinary incontinence via a mobile app: two-year follow-up of a randomized controlled trial. Acta Obstet Gynecol Scand. 2017;96(10):1180–7. https://doi.org/10.1111/aogs.13192.
Hui E, Lee PS, Woo J. Management of urinary incontinence in older women using videoconferencing versus conventional management: a randomized controlled trial. J Telemed Telecare. 2006;12(7):343–7. https://doi.org/10.1258/135763306778682413.
da Mata KRU, Costa RCM, Carbone EDM, Gimenez MM, Bortolini MAT, Castro RA, et al. Telehealth in the rehabilitation of female pelvic floor dysfunction: a systematic literature review. Int Urogynecol J. 2021;32(2):249–59. https://doi.org/10.1007/s00192-020-04588-8.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. https://doi.org/10.1136/bmj.b2700.
Araujo CC, Marques ADA, Juliato CRT. The adherence of home pelvic floor muscles training using a mobile device application for women with urinary incontinence: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2020;26(11):697–703. https://doi.org/10.1097/SPV.0000000000000670.
Asklund I, Nystrom E, Sjostrom M, Umefjord G, Stenlund H, Samuelsson E. Mobile app for treatment of stress urinary incontinence: a randomized controlled trial. Neurourol Urodyn. 2017;36(5):1369–76. https://doi.org/10.1002/nau.23116.
Bezerra LO, de Oliveira MCE, da Silva Filho EM, Vicente da Silva HK, Menezes de Oliveira GF, da Silveira Goncalves AK, et al. Impact of pelvic floor muscles training isolated and associated with game therapy on mixed urinary incontinence: a randomized controlled trial. Games Health J. 2021;10(1):43–9. https://doi.org/10.1089/g4h.2019.0207.
Dufour S, Fedorkow D, Kun J, Deng SX, Fang Q. Exploring the impact of a mobile health solution for postpartum pelvic floor muscle training: pilot randomized controlled feasibility study. JMIR Mhealth Uhealth. 2019;7(7):e12587. https://doi.org/10.2196/12587.
Forbes G, Newton S, Cantalapiedra Calvete C, Birch J, Dodds J, Steed L, et al. MEMPHIS: a smartphone app using psychological approaches for women with chronic pelvic pain presenting to gynaecology clinics: a randomised feasibility trial. BMJ Open. 2020;10(3):e030164. https://doi.org/10.1136/bmjopen-2019-030164.
Geng F, Xu Q. Effect of wechat interactive intervention model on postpartum pelvic floor rehabilitation management and sexual life quality of women after vaginal delivery. Int J Nurs. 2020;39(9):1704–7.
Jia J, Xu J, Qiu X. Application of hospital-community-family home care mobiIe App in patients with stress urinary incontinence. Chin J Nurs. 2018;53(5):533–6.
Jin M. Clinical research of WeChat public platform in the prevention and control of stress urinary incontinence during pregnancy and postpartum. Master Thesis, Southern Medical University, China, 2019.
Li Y, Zhu Z, Huang L. Application of information-based management platform in continuing care services of pelvic floor rehabilitation for pregnant women. Chin Gen Pract Nursing. 2020;18(34):4842–4.
Loohuis AMM, Wessels NJ, Dekker JH, van Merode NAM, Slieker-Ten Hove MCP, Kollen BJ, et al. App-based treatment in primary care for urinary incontinence: a pragmatic, randomized controlled trial. Ann Fam Med. 2021;19(2):102–9. https://doi.org/10.1370/afm.2585.
Mu X, Ding Y, Liu S, Chen H, Xia C. Influence of extended nursing service based on micro channel platform on quality of life of female patients with urinary incontinence. Chin Nurs Res. 2016;30(15):1878–9.
Schroeder M, Plotner EA, Sharma S, Hunter K, Spector S, Lipetskaia L. A randomized controlled trial of a multimedia patient education tool for stress versus urgency urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27(7):403–8. https://doi.org/10.1097/SPV.0000000000000946.
Sjostrom M, Umefjord G, Stenlund H, Carlbring P, Andersson G, Samuelsson E. Internet-based treatment of stress urinary incontinence: a randomised controlled study with focus on pelvic floor muscle training. BJU Int. 2013;112(3):362–72. https://doi.org/10.1111/j.1464-410X.2012.11713.x.
Sun J. Application of hospital-community-family incontinence care platform in the management of patients with stress urinary incontinence. Media Commun. 2018;17:63.
Wang A, Lin C, Xu D. Evaluation of the effect of group follow-up intervention of "Dingding" in postpartum urinary incontinence. Prev Med. 2019;31(9):956–8.
Wang X, Xu X, Luo J, Chen Z, Feng S. Effect of app-based audio guidance pelvic floor muscle training on treatment of stress urinary incontinence in primiparas: a randomized controlled trial. Int J Nurs Stud. 2020;104:103527. https://doi.org/10.1016/j.ijnurstu.2020.103527.
Weinstein MM, Collins S, Quiroz L, Anger JT, Paraiso MFR, DeLong J, et al. Multicenter randomized controlled trial of pelvic floor muscle training with a motion-based digital therapeutic device versus pelvic floor muscle training alone for treatment of stress-predominant urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;28(1):1–6. https://doi.org/10.1097/SPV.0000000000001052.
Wu Z, Ye M, Xu M, Huai L, Wang L. Effect of WeChat-based group guidance on quality of life and emotions of postpartum women with stress urinary incontinence. Hospital Management Forum. 2019; 36 (8): 75-77+69.
Xu L, Zhang X. The effect of web-based interactive educational management on the compliance behavior of patients with postpartum pelvic floor rehabilitation. Chin Rural Health Service Administration. 2018;38(8):1039–41.
Ye Q, Ni J, Zhang Q. Effect of WeChat interactive intervention on postpartum pelvic floor rehabilitation for second child parturients in vaginal delivery. Chin J Pract Nurs. 2017;33(18):1401–3.
Zhang W. Effect of persistent guidance with telephone follow-up after discharge on pelvic floor muscle exercise for pregnant women. Modern Med J. 2014;42(7):818–20.
Zhang X, Linli Z, Chen S. Effect of telephone follow-up on the treatment of elderly female patients with urinary incontinence. Xinjiang Med J. 2017;47(4):444–7.
Zheng X, Wang F, Gao M, Wang F. The effect of WeChat video on promoting the recovery of postpartum pelvic floor muscles. Electronic Journal Of Practical Clinical. Nurs Sci. 2019;4(42):184–90.
Zhong Y, Tu X. Application of mobile information technology health education model in postpartum pelvic floor rehabilitation management. Modern Diagnosis and Treatment. 2019;30(10):1743–5.
Mørkved S, Bø K. Effect of pelvic floor muscle training during pregnancy and after childbirth on prevention and treatment of urinary incontinence: a systematic review. Br J Sports Med. 2014;48(4):299–310. https://doi.org/10.1136/bjsports-2012-091758.
Romeikiene KE, Bartkeviciene D. Pelvic-floor dysfunction prevention in prepartum and postpartum periods. Medicina-Lithuania. 2021;57(4):387. https://doi.org/10.3390/medicina57040387.
Bozkurt M, Yumru AE, Şahin L. Pelvic floor dysfunction, and effects of pregnancy and mode of delivery on pelvic floor. Taiwan J Obstet Gynecol. 2014;53(4):452–8. https://doi.org/10.1016/j.tjog.2014.08.001.
Raul A. Exercise during pregnancy and the postpartum period, https://www.uptodate.cn/contents/exercise-during-pregnancy-and-the-postpartum-period (2021, Accessed 02 November 2021).
Temtanakitpaisan T, Bunyavejchevin S, Buppasiri P, Chongsomchai C. Knowledge, attitude, and practices (KAP) survey towards pelvic floor muscle training (PFMT) among pregnant women. Int J Women's Health. 2020;12:295–9. https://doi.org/10.2147/IJWH.S242432.
Sidik SM, Jaffar A, Foo CN, Muhammad NA, Abdul Manaf R, Ismail SIF, et al. KEPT-app trial: a pragmatic, single-blind, parallel, cluster-randomised effectiveness study of pelvic floor muscle training among incontinent pregnant women: study protocol. BMJ Open. 2021;11(1):e039076. https://doi.org/10.1136/bmjopen-2020-039076.
Wallwiener S, Müller M, Doster A, Laserer W, Reck C, Pauluschke-Fröhlich J, et al. Pregnancy eHealth and mHealth: user proportions and characteristics of pregnant women using web-based information sources-a cross-sectional study. Arch Gynecol Obstet. 2016;294(5):937–44. https://doi.org/10.1007/s00404-016-4093-y.
Bernard S, Boucher S, McLean L, Moffet H. Mobile technologies for the conservative self-management of urinary incontinence: a systematic scoping review. Int Urogynecol J. 2020;31(6):1163–74. https://doi.org/10.1007/s00192-019-04012-w.
Viktrup L, Hayes RP, Wang P, Shen W. Construct validation of patient global impression of severity (PGI-S) and improvement (PGI-I) questionnaires in the treatment of men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. BMC Urol. 2012;12:30. https://doi.org/10.1186/1471-2490-12-30.
Huang Z, Wu S, Yu T, Hu A. Efficacy of telemedicine for urinary incontinence in women: a systematic review and meta-analysis of randomized controlled trials. Int Urogynecol J. 2020;31(8):1507–13. https://doi.org/10.1007/s00192-020-04340-2.
Seshan V, Muliira JK, Krishnamurthy R, Sivaram V. Using a video assisted teaching program to reduce the severity of urinary incontinence symptoms in women. Int J Urol Nurs. 2013;7(1):33–42. https://doi.org/10.1111/j.1749-771X.2012.01166.x.
Conde TP, Barker MA. Effect of electronic video education on patient's self-assessed knowledge about obesity and pelvic floor disorders: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2019;25(3):257–61. https://doi.org/10.1097/SPV.0000000000000537.
Goode PS, Markland AD, Echt KV, Slay L, Barnacastle S, Hale G, et al. A mobile telehealth program for behavioral treatment of urinary incontinence in women veterans: development and pilot evaluation of MyHealtheBladder. Neurourol Urodyn. 2020;39(1):432–9. https://doi.org/10.1002/nau.24226.
Pepper J, Zhang A, Li R, Wang XH. Usage results of a mobile app for managing urinary incontinence. J Urol. 2015;193(4):1292–7. https://doi.org/10.1016/j.juro.2014.10.009.
Mazloomdoost D, Kanter G, Chan RC, Deveaneau N, Wyman AM, Von Bargen EC, et al. Social networking and Internet use among pelvic floor patients: a multicenter survey. Am J Obstet Gynecol. 2016;215(5):654.e1–654.e10. https://doi.org/10.1016/j.ajog.2016.06.011.
Du S, Liu W, Cai S, Hu Y, Dong J. The efficacy of e-health in the self-management of chronic low back pain: a meta analysis. Int J Nurs Stud. 2020;106:103507. https://doi.org/10.1016/j.ijnurstu.2019.103507.
Gliddon E, Barnes SJ, Murray G, Michalak EE. Online and mobile technologies for self-management in bipolar disorder: a systematic review. Psychiatr Rehabil J. 2017;40(3):309–19. https://doi.org/10.1037/prj0000270.
Latorre GFS, de Fraga R, Seleme MR, Mueller CV, Berghmans B. An ideal e-health system for pelvic floor muscle training adherence: systematic review. Neurourol Urodyn. 2019;38(1):63–80. https://doi.org/10.1002/nau.23835.
Novara G, Checcucci E, Crestani A, Abrate A, Esperto F, Pavan N, et al. Telehealth in urology: a systematic review of the literature. How much can telemedicine be useful during and after the COVID-19 pandemic? Eur Urol. 2020;78(6):786–811. https://doi.org/10.1016/j.eururo.2020.06.025.
Firet L, Teunissen D, Verhoeks C, Lagro-Janssen A. Expectations regarding eHealth among women with stress urinary incontinence. Int Urogynecol J. 2019;30(11):1955–63. https://doi.org/10.1007/s00192-018-3849-2.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was funded by the Projects of the Zhejiang Provincial Science and Technology Program in Medicine and Health (grant no. WKJ-ZJ-2211, 2022KY188).
Author information
Authors and Affiliations
Contributions
Study design: Ping Xu and Suwen Feng. Literature searches, data extraction and quality assessment: Xiaojuan Wang and Pingping Guo. Analysis and interpretation of data: Ping Xu and Wei Zhang. Drafting of the manuscript: Ping Xu and Minna Mao. Critical revision of the manuscript: Suwen Feng.
Corresponding author
Ethics declarations
Conflicts of interests
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Search strategies
Search strategies
-
1.
Database: PubMed
Results: 633
Search period: 1950 to August 28, 2021
Number | Search terms | Results |
#1 | "telemedicine"[MeSH Terms] OR tele-medicine[Title/Abstract] OR telehealth[Title/Abstract] OR tele-health[Title/Abstract] OR ehealth[Title/Abstract] OR e-health[Title/Abstract] OR "electronic health"[Title/Abstract] OR "mobile health"[Title/Abstract] OR mhealth[Title/Abstract] OR m-health[Title/Abstract] OR "remote consultation"[MeSH Terms] OR "distance counseling"[MeSH Terms] OR "wearable electronic devices"[MeSH Terms] OR teleconsultation[Title/Abstract] OR tele-consultation[Title/Abstract] OR "telecommunications"[MeSH Terms] OR "social media"[MeSH Terms] OR "social medium"[Title/Abstract] OR multi-media[Title/Abstract] OR "multimedia"[MeSH Terms] OR "mobile applications"[MeSH Terms] OR "mobile app*"[Title/Abstract] OR "mobile application"[Title/Abstract] OR "mobile device"[Title/Abstract] OR phone[Title/Abstract] OR "mobile phone"[Title/Abstract] OR "smartphone"[MeSH Terms] OR "smart phone"[Title/Abstract] OR "cellular phone"[Title/Abstract] OR cellphone[Title/Abstract] OR "cell phone"[MeSH Terms] OR "telephone"[MeSH Terms] OR "text messaging"[MeSH Terms] OR "text- messag*"[Title/Abstract] OR textmessag*[Title/Abstract] OR "text messag*"[Title/Abstract] OR SMS[Title/Abstract] OR texting*[Title/Abstract] OR "short message service*"[Title/Abstract] OR "electronic mail"[MeSH Terms] OR e-mail*[Title/Abstract] OR email*[Title/Abstract] OR game*[Title/Abstract] OR gaming[Title/Abstract] OR gamification[Title/Abstract] OR "video games"[MeSH Terms] OR videogame*[Title/Abstract] OR "computer game*"[Title/Abstract] OR "internet based intervention"[MeSH Terms] OR internet-based[Title/Abstract] OR web-based[Title/Abstract] OR web based[Title/Abstract] OR computer based[Title/Abstract] OR computer-based[Title/Abstract] OR internet-guided[Title/Abstract] OR internet guided[Title/Abstract] OR "videoconferencing"[MeSH Terms] OR video[Title/Abstract] OR website[Title/Abstract] OR web[Title/Abstract] OR online*[Title/Abstract] OR on-line[Title/Abstract] OR internet*[Title/Abstract] OR app[Title/Abstract] OR application*[Title/Abstract] OR computer*[Title/Abstract] OR electronic*[Title/Abstract] OR digital*[Title/Abstract] | 2,554,839 |
#2 | incontinence[Title/Abstract] OR "urinary incontinence"[MeSH Terms] OR "urinary incontinence, stress"[MeSH Terms] OR "urinary incontinence, urge"[MeSH Terms] OR "mixed urinary incontinence"[Title/Abstract] OR "pelvic organ prolapse"[MeSH Terms] OR "genital prolapse"[Title/Abstract] OR "uterine prolapse"[MeSH Terms] OR "cervical prolapse"[Title/Abstract] OR "urogenital prolapse"[Title/Abstract] OR "vaginal prolapse"[Title/Abstract] OR "vaginal apex prolapse"[Title/Abstract] OR "vaginal vault prolapse"[Title/Abstract] OR "utero vaginal prolapse"[Title/Abstract] OR "anterior vaginal wall prolapse"[Title/Abstract] OR "posterior wall prolapse"[Title/Abstract] OR "posterior vaginal wall prolapse"[Title/Abstract] OR "rectocele"[MeSH Terms] OR proctocele*[Title/Abstract] OR "cystocele"[MeSH Terms] OR "urinary bladder prolapse"[Title/Abstract] OR enterocele[Title/Abstract] OR "fecal incontinence"[MeSH Terms] OR "anal incontinence"[Title/Abstract] OR "bowel incontinence"[Title/Abstract] OR "sexual dysfunction, physiological"[MeSH Terms] OR "physiological sexual dysfunction*"[Title/Abstract] OR "sexual dysfunction*"[Title/Abstract] OR "sexual disorder*"[Title/Abstract] OR "dyspareunia"[MeSH Terms] OR "pelvic pain"[MeSH Terms] OR "urinary retention"[MeSH Terms] OR "urinary bladder diseases"[MeSH Terms] OR "urinary bladder, neurogenic"[MeSH Terms] OR "urinary bladder"[MeSH Terms] OR "urinary bladder, overactive"[MeSH Terms] OR "overactive bladder"[Title/Abstract] OR "overactive detrusor"[Title/Abstract] OR "overactive bladder syndrome"[Title/Abstract] OR "urinary bladder, neurogenic"[MeSH Terms] OR "neurogenic bladder"[Title/Abstract] OR "bladder dysfunction"[Title/Abstract] OR "diurnal enuresis"[MeSH Terms] OR "nocturnal enuresis"[MeSH Terms] OR "lower urinary tract symptoms"[MeSH Terms] OR "lower urinary tract dysfunction"[Title/Abstract] OR "lower urinary tract abnormalities"[Title/Abstract] OR "pelvic floor"[MeSH Terms] OR "pelvic floor disorders"[MeSH Terms] OR "pelvic floor disorder*"[Title/Abstract] OR "pelvic floor disease*"[Title/Abstract] OR "pelvic floor dysfunction*"[Title/Abstract] | 256,085 |
#3 | #1 AND #2 | 14,754 |
#4 | Filters: randomized controlled trial | 633 |
-
2.
Database: Web of Science (ALL Database)
Results: 1462
Search period: 1864 to August 28, 2021
Number | Search terms | Results |
#1 | TS = (telemedicine OR tele-medicine OR telehealth OR tele-health OR ehealth OR e-health OR "electronic health" OR "mobile health" OR mhealth OR m-health OR "remote consultation" OR "distance counseling" OR "wearable electronic devices" OR teleconsultation OR tele-consultation OR telecommunications OR "social media" OR "social medium" OR multi-media OR multimedia OR "mobile applications" OR "mobile app*" OR "mobile application" OR "mobile device" OR phone OR "mobile phone" OR smartphone OR "smart phone" OR "cellular phone" OR cellphone OR "cell phone" OR telephone OR "text messaging" OR text-messag* OR textmessag* OR "text messag*" OR sms OR texting* OR "short message service*" OR "electronic mail" OR e-mail* OR email* OR game* OR gaming OR gamification OR "video games" OR videogame* OR "computer game*" OR "internet based intervention" OR internet-based OR web-based OR "web based" OR "computer based" OR computer-based OR internet-guided OR "internet guided" OR videoconferencing OR video OR website OR web OR online* OR on-line OR internet* OR app OR application* OR computer* OR electronic* OR digital*) | 23,995,037 |
#2 | TS = (incontinence OR "urinary incontinence" OR "urinary incontinence, stress" OR "urinary incontinence, urge" OR "mixed urinary incontinence" OR "pelvic organ prolapse" OR "genital prolapse" OR "uterine prolapse" OR "cervical prolapse" OR "urogenital prolapse" OR "vaginal prolapse" OR "vaginal apex prolapse" OR "vaginal vault prolapse" OR "utero-vaginal prolapse" OR "anterior vaginal wall prolapse" OR "posterior wall prolapse" OR "posterior vaginal wall prolapse" OR rectocele OR proctocele* OR cystocele OR "urinary bladder prolapse" OR enterocele OR "fecal incontinence" OR "anal incontinence" OR "bowel incontinence" OR "sexual dysfunction, physiological" OR "physiological sexual dysfunction*" OR "sexual dysfunction*" OR "sexual disorder*" OR dyspareunia OR "pelvic pain" OR "urinary retention" OR "urinary bladder diseases" OR "urinary bladder, neurogenic" OR "urinary bladder" OR "urinary bladder, overactive" OR "overactive bladder" OR "overactive detrusor" OR "overactive bladder syndrome" OR "urinary bladder, neurogenic" OR "neurogenic bladder" OR "bladder dysfunction" OR "diurnal enuresis" OR "nocturnal enuresis" OR "lower urinary tract symptoms" OR "lower urinary tract dysfunction" OR "lower urinary tract abnormalities" OR "pelvic floor" OR "pelvic floor disorders" OR "pelvic floor disorder*" OR "pelvic floor disease*" OR "pelvic floor dysfunction*") | 369,300 |
#3 | TS =("randomized controlled trial" OR "random allocation" OR random∗) | 1,654,611 |
#4 | #1 AND #2 AND #3 | 1462 |
TS = Topic.
-
3.
Database: CINAHL (EBSCO CINAHL Plus with Full Text)
Results: 35
Search period: 1937 to August 28, 2021
Number | Search terms | Results |
S1 | MH “telemedicine” OR SU tele-medicine OR SU telehealth OR SU tele-health OR SU ehealth OR SU e-health OR SU “electronic health” OR SU “mobile health” OR SU mhealth OR SU m-health OR MH “remote consultation” OR MH “distance counseling” OR MH “wearable electronic devices” OR SU teleconsultation OR SU tele-consultation OR MH “telecommunications” OR MH “social media” OR SU “social medium” OR SU multi-media OR MH “multimedia” OR MH “mobile applications” OR SU “mobile app*” OR SU “mobile application” OR SU “mobile device” OR SU phone OR SU “mobile phone” OR MH “smartphone” OR SU “smart phone” OR SU “cellular phone” OR SU cellphone OR MH “cell phone” OR MH “telephone” OR MH “text messaging” OR SU text-messag* OR SU textmessag* OR SU “text messag*” OR SU SMS OR SU texting* OR SU “short message service*” OR MH “electronic mail” OR SU e-mail* OR SU email* OR SU game* OR SU gaming OR SU gamification OR MH “video games” OR SU videogame* OR SU “computer game*” OR MH “internet based intervention” OR SU internet-based OR SU web-based OR SU “web based” OR SU “computer based” OR SU computer-based OR SU internet-guided OR SU “internet guided” OR MH “videoconferencing” OR SU video OR SU website OR SU web OR SU online* OR SU on-line OR SU internet* OR SU app OR SU application* OR SU computer* OR SU electronic* OR SU digital* | 412,747 |
S2 | SU incontinence OR MH “urinary incontinence” OR MH “urinary incontinence, stress” OR MH “urinary incontinence, urge” OR SU “mixed urinary incontinence” OR MH “pelvic organ prolapse” OR SU “genital prolapse” OR MH “uterine prolapse” OR SU “cervical prolapse” OR SU “urogenital prolapse” OR SU “vaginal prolapse” OR SU “vaginal apex prolapse” OR SU “vaginal vault prolapse” OR SU “utero-vaginal prolapse” OR SU “anterior vaginal wall prolapse” OR SU “posterior wall prolapse” OR SU “posterior vaginal wall prolapse” OR MH “rectocele” OR SU proctocele* OR MH “cystocele” OR SU “urinary bladder prolapse” OR SU enterocele OR MH “fecal incontinence” OR SU “anal incontinence” OR SU “bowel incontinence” OR MH “sexual dysfunction, physiological” OR SU “physiological sexual dysfunction*” OR SU “sexual dysfunction*” OR SU “sexual disorder*” OR MH “dyspareunia” OR MH “pelvic pain” OR MH “urinary retention” OR MH “urinary bladder diseases” OR MH “urinary bladder, neurogenic” OR MH “urinary bladder” OR MH “urinary bladder, overactive” OR SU “overactive bladder” OR SU “overactive detrusor” OR SU “overactive bladder syndrome” OR MH “urinary bladder, neurogenic” OR SU “neurogenic bladder” OR SU “bladder dysfunction” OR MH “diurnal enuresis” OR MH “nocturnal enuresis” OR MH “lower urinary tract symptoms” OR SU “lower urinary tract dysfunction” OR SU “lower urinary tract abnormalities” OR MH “pelvic floor” OR MH “pelvic floor disorders” OR SU “pelvic floor disorder*” OR SU “pelvic floor disease*” OR SU “pelvic floor dysfunction*” | 29,119 |
S3 | PT “randomized controlled trial” OR SU “random allocation” OR SU random∗ | 214,169 |
S4 | S1 AND S2 AND S3 | 35 |
MH = exact subject heading; SU = subject; PT = publication type
-
4.
Database: Embase
Results: 521
Search period: 1946 to August 28, 2021
Number | Search terms | Results |
#1 | 'telemedicine'/exp OR 'tele-medicine':ab,ti OR telehealth:ab,ti OR 'tele-health':ab,ti OR ehealth:ab,ti OR 'e-health':ab,ti OR 'electronic health':ab,ti OR 'mobile health':ab,ti OR mhealth:ab,ti OR 'm-health':ab,ti OR 'remote consultation'/exp OR 'distance counseling'/exp OR 'wearable electronic devices'/exp OR teleconsultation:ab,ti OR 'tele-consultation':ab,ti OR 'telecommunications'/exp OR 'social media'/exp OR 'social medium':ab,ti OR 'multi-media':ab,ti OR 'multimedia'/exp OR 'mobile applications'/exp OR 'mobile app*':ab,ti OR 'mobile application':ab,ti OR 'mobile device':ab,ti OR phone:ab,ti OR 'mobile phone':ab,ti OR 'smartphone'/exp OR 'smart phone':ab,ti OR 'cellular phone':ab,ti OR cellphone:ab,ti OR 'cell phone'/exp OR 'telephone'/exp OR 'text messaging'/exp OR 'text-messag*' OR textmessag*:ab,ti OR 'text messag*':ab,ti OR sms:ab,ti OR texting*:ab,ti OR 'short message service*':ab,ti OR 'electronic mail'/exp OR 'e-mail*':ab,ti OR email*:ab,ti OR game*:ab,ti OR gaming:ab,ti OR gamification:ab,ti OR 'video games'/exp OR videogame*:ab,ti OR 'computer game*':ab,ti OR 'internet based intervention'/exp OR 'internet-based':ab,ti OR 'web-based':ab,ti OR 'web based':ab,ti OR 'computer based':ab,ti OR 'computer-based':ab,ti OR 'internet-guided':ab,ti OR 'internet guided':ab,ti OR 'videoconferencing'/exp OR video:ab,ti OR website:ab,ti OR web:ab,ti OR online*:ab,ti OR 'on- line':ab,ti OR internet*:ab,ti OR app:ab,ti OR application*:ab,ti OR computer*:ab,ti OR electronic*:ab,ti OR digital*:ab,ti | 3,210,161 |
#2 | incontinence:ab,ti OR 'urinary incontinence'/exp OR 'urinary incontinence, stress'/exp OR 'urinary incontinence, urge'/exp OR 'mixed urinary incontinence':ab,ti OR 'pelvic organ prolapse'/exp OR 'genital prolapse':ab,ti OR 'uterine prolapse'/exp OR 'cervical prolapse':ab,ti OR 'urogenital prolapse':ab,ti OR 'vaginal prolapse':ab,ti OR 'vaginal apex prolapse':ab,ti OR 'vaginal vault prolapse':ab,ti OR 'utero-vaginal prolapse':ab,ti OR 'anterior vaginal wall prolapse':ab,ti OR 'posterior wall prolapse':ab,ti OR 'posterior vaginal wall prolapse':ab,ti OR 'rectocele'/exp OR proctocele*:ab,ti OR 'cystocele'/exp OR 'urinary bladder prolapse':ab,ti OR enterocele:ab,ti OR 'fecal incontinence'/exp OR 'anal incontinence':ab,ti OR 'bowel incontinence':ab,ti OR 'sexual dysfunction, physiological'/exp OR 'physiological sexual dysfunction*':ab,ti OR 'sexual dysfunction*':ab,ti OR 'sexual disorder*':ab,ti OR 'dyspareunia'/exp OR 'pelvic pain'/exp OR 'urinary retention'/exp OR 'urinary bladder diseases'/exp OR 'urinary bladder'/exp OR 'urinary bladder, overactive'/exp OR 'overactive bladder':ab,ti OR 'overactive detrusor':ab,ti OR 'overactive bladder syndrome':ab,ti OR 'urinary bladder, neurogenic'/exp OR 'neurogenic bladder':ab,ti OR 'bladder dysfunction':ab,ti OR 'diurnal enuresis'/exp OR 'nocturnal enuresis'/exp OR 'lower urinary tract symptoms'/exp OR 'lower urinary tract dysfunction':ab,ti OR 'lower urinary tract abnormalities':ab,ti OR 'pelvic floor'/exp OR 'pelvic floor disorders'/exp OR 'pelvic floor disorder*':ab,ti OR 'pelvic floor disease*':ab,ti OR 'pelvic floor dysfunction*':ab,ti | 489,323 |
#3 | 'randomized controlled trial':ab,ti OR 'random allocation':ab,ti OR random∗:ab,ti | 113,995 |
#4 | #1 AND #2 AND #3 | 521 |
Exp = Emtree term-exploded; ab,ti = title or abstract
-
5.
Database: The Cochrane Library
Results: 1890
Search period: 1946 to August 28, 2021
Number | Search terms | Results |
#1 | MeSH descriptor: [Telemedicine] explode all trees | 2896 |
#2 | MeSH descriptor: [Remote Consultation] explode all trees | 401 |
#3 | MeSH descriptor: [Distance Counseling] explode all trees | 20 |
#4 | MeSH descriptor: [Wearable Electronic Devices] explode all trees | 505 |
#5 | MeSH descriptor: [Telecommunications] explode all trees | 7362 |
#6 | MeSH descriptor: [Social Media] explode all trees | 197 |
#7 | MeSH descriptor: [Multimedia] explode all trees | 239 |
#8 | MeSH descriptor: [Mobile Applications] explode all trees | 864 |
#9 | MeSH descriptor: [Smartphone] explode all trees | 448 |
#10 | MeSH descriptor: [Cell Phone] explode all trees | 1955 |
#11 | MeSH descriptor: [Telephone] explode all trees | 4110 |
#12 | MeSH descriptor: [Text Messaging] explode all trees | 991 |
#13 | MeSH descriptor: [Electronic Mail] explode all trees | 342 |
#14 | MeSH descriptor: [Video Games] explode all trees | 743 |
#15 | MeSH descriptor: [Internet-Based Intervention] explode all trees | 223 |
#16 | MeSH descriptor: [Videoconferencing] explode all trees | 227 |
#17 | (tele-medicine OR telehealth OR tele-health OR ehealth OR e-health OR “electronic health” OR “mobile health” OR mhealth OR m-health OR teleconltation OR tele-conltation OR “social medium” OR multi-media OR “mobile app*” OR “mobile application” OR “mobile device” OR phone OR “mobile phone” OR “smart phone” OR “cellular phone” OR cellphone OR text-messag* OR textmessag* OR “text messag*” OR SMS OR texting* OR “short message service*” OR e-mail* OR email* OR game* OR gaming OR gamification OR videogame* OR “computer game*” OR internet-based OR web-based OR “web based” OR “computer based” OR computer-based OR internet-guided OR “internet guided” OR video OR website OR web OR online* OR on-line OR internet* OR app OR application* OR computer* OR electronic* OR digital*):ti,ab,kw | 192,684 |
#18 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 | 195,535 |
#19 | MeSH descriptor: [Urinary Incontinence] explode all trees | 2363 |
#20 | MeSH descriptor: [Urinary Incontinence, Stress] explode all trees | 1007 |
#21 | MeSH descriptor: [Urinary Incontinence, Urge] explode all trees | 200 |
#22 | MeSH descriptor: [Pelvic Organ Prolapse] explode all trees | 619 |
#23 | MeSH descriptor: [Uterine Prolapse] explode all trees | 215 |
#24 | MeSH descriptor: [Rectocele] explode all trees | 45 |
#25 | MeSH descriptor: [Cystocele] explode all trees | 40 |
#26 | MeSH descriptor: [Fecal Incontinence] explode all trees | 511 |
#27 | MeSH descriptor: [Sexual Dysfunction, Physiological] explode all trees | 2313 |
#28 | MeSH descriptor: [Dyspareunia] explode all trees | 213 |
#29 | MeSH descriptor: [Pelvic Pain] explode all trees | 1236 |
#30 | MeSH descriptor: [Urinary Retention] explode all trees | 423 |
#31 | MeSH descriptor: [Urinary Bladder Diseases] explode all trees | 3506 |
#32 | MeSH descriptor: [Urinary Bladder, Neurogenic] explode all trees | 238 |
#33 | MeSH descriptor: [Urinary Bladder] explode all trees | 829 |
#34 | MeSH descriptor: [Urinary Bladder, Overactive] explode all trees | 787 |
#35 | MeSH descriptor: [Urinary Bladder, Neurogenic] explode all trees | 238 |
#36 | MeSH descriptor: [Diurnal Enuresis] explode all trees | 7 |
#37 | MeSH descriptor: [Nocturnal Enuresis] explode all trees | 104 |
#38 | MeSH descriptor: [Lower Urinary Tract Symptoms] explode all trees | 3430 |
#39 | MeSH descriptor: [Pelvic Floor] explode all trees | 554 |
#40 | MeSH descriptor: [Pelvic Floor Disorders] explode all trees | 88 |
#41 | (incontinence OR “mixed urinary incontinence” OR “genital prolapse” OR “cervical prolapse” OR “urogenital prolapse” OR “vaginal prolapse” OR “vaginal apex prolapse” OR “vaginal vault prolapse” OR “utero-vaginal prolapse” OR “anterior vaginal wall prolapse” OR “posterior wall prolapse” OR “posterior vaginal wall prolapse” OR proctocele* OR “urinary bladder prolapse” OR enterocele OR “anal incontinence” OR “bowel incontinence” OR “physiological sexual dysfunction*” OR “sexual dysfunction*” OR “sexual disorder*” OR “overactive bladder” OR “overactive detrusor” OR “overactive bladder syndrome” OR “neurogenic bladder” OR “bladder dysfunction” OR “lower urinary tract dysfunction” OR “lower urinary tract abnormalities” OR “pelvic floor disorder*” OR “pelvic floor disease*” OR “pelvic floor dysfunction*”):ti,ab,kw | 15,727 |
#42 | #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 | 22,225 |
#43 | (“randomized controlled trial”):pt OR (“random allocation”):ti,ab,kw OR (random∗):ti,ab,kw | 1,154,246 |
#44 | #18 AND #42 AND #43 | 1890 |
ti,ab,kw = title, abstract, keyword; pt = publication type
-
6.
Database: PsycINFO
Results: 4
Search period: until August 28, 2021
Number | Search terms | Results |
S1 | MA “telemedicine” OR SU tele-medicine OR SU telehealth OR SU tele-health OR SU ehealth OR SU e-health OR SU “electronic health” OR SU “mobile health” OR SU mhealth OR SU m-health OR MA “remote consultation” OR MA “distance counseling” OR MA “wearable electronic devices” OR SU teleconsultation OR SU tele-consultation OR MA “telecommunications” OR MA “social media” OR SU “social medium” OR SU multi-media OR MA “multimedia” OR MA “mobile applications” OR SU “mobile app*” OR SU “mobile application” OR SU “mobile device” OR SU phone OR SU “mobile phone” OR MA “smartphone” OR SU “smart phone” OR SU “cellular phone” OR SU cellphone OR MA “cell phone” OR MA “telephone” OR MA “text messaging” OR SU text-messag* OR SU textmessag* OR SU “text messag*” OR SU SMS OR SU texting* OR SU “short message service*” OR MA “electronic mail” OR SU e-mail* OR SU email* OR SU game* OR SU gaming OR SU gamification OR MA “video games” OR SU videogame* OR SU “computer game*” OR MA “internet based intervention” OR SU internet-based OR SU web-based OR SU “web based” OR SU “computer based” OR SU computer-based OR SU internet-guided OR SU “internet guided” OR MA “videoconferencing” OR SU video OR SU website OR SU web OR SU online* OR SU on-line OR SU internet* OR SU app OR SU application* OR SU computer* OR SU electronic* OR SU digital* | 267,044 |
S2 | SU incontinence OR MA “urinary incontinence” OR MA “urinary incontinence, stress” OR MA “urinary incontinence, urge” OR SU “mixed urinary incontinence” OR MA “pelvic organ prolapse” OR SU “genital prolapse” OR MA “uterine prolapse” OR SU “cervical prolapse” OR SU “urogenital prolapse” OR SU “vaginal prolapse” OR SU “vaginal apex prolapse” OR SU “vaginal vault prolapse” OR SU “utero-vaginal prolapse” OR SU “anterior vaginal wall prolapse” OR SU “posterior wall prolapse” OR SU “posterior vaginal wall prolapse” OR MA “rectocele” OR SU proctocele* OR MA “cystocele” OR SU “urinary bladder prolapse” OR SU enterocele OR MA “fecal incontinence” OR SU “anal incontinence” OR SU “bowel incontinence” OR MA “sexual dysfunction, physiological” OR SU “physiological sexual dysfunction*” OR SU “sexual dysfunction*” OR SU “sexual disorder*” OR MA “dyspareunia” OR MA “pelvic pain” OR MA “urinary retention” OR MA “urinary bladder diseases” OR MA “urinary bladder, neurogenic” OR MA “urinary bladder” OR MA “urinary bladder, overactive” OR SU “overactive bladder” OR SU “overactive detrusor” OR SU “overactive bladder syndrome” OR MA “urinary bladder, neurogenic” OR SU “neurogenic bladder” OR SU “bladder dysfunction” OR MA “diurnal enuresis” OR MA “nocturnal enuresis” OR MA “lower urinary tract symptoms” OR SU “lower urinary tract dysfunction” OR SU “lower urinary tract abnormalities” OR MA “pelvic floor” OR MA “pelvic floor disorders” OR SU “pelvic floor disorder*” OR SU “pelvic floor disease*” OR SU “pelvic floor dysfunction*” | 10,990 |
S3 | SU “randomized controlled trial” OR SU “random allocation” OR SU random∗ | 16,540 |
S4 | S1 AND S2 AND S3 | 4 |
MA = MeSH subject heading; SU = subjects
-
7.
Database: Scopus
Results: 1556
Search period: 1970 to August 28, 2021
Number | Search terms | Results |
#1 | TITLE-ABS-KEY(telemedicine OR tele-medicine OR telehealth OR tele-health OR ehealth OR e-health OR "electronic health" OR "mobile health" OR mhealth OR m-health OR "remote consultation" OR "distance counseling" OR "wearable electronic devices" OR teleconsultation OR tele-consultation OR telecommunications OR "social media" OR "social medium" OR multi-media OR multimedia OR "mobile applications" OR "mobile app*" OR "mobile application" OR "mobile device" OR phone OR "mobile phone" OR smartphone OR "smart phone" OR "cellular phone" OR cellphone OR "cell phone" OR telephone OR "text messaging" OR text-messag* OR textmessag* OR "text messag*" OR sms OR texting* OR "short message service*" OR "electronic mail" OR e-mail* OR email* OR game* OR gaming OR gamification OR "video games" OR videogame* OR "computer game*" OR "internet based intervention" OR internet-based OR web-based OR "web based" OR "computer based" OR computer-based OR internet-guided OR "internet guided" OR videoconferencing OR video OR website OR web OR online* OR on-line OR internet* OR app OR application* OR computer* OR electronic* OR digital*) | 15,071,640 |
#2 | TITLE-ABS-KEY (incontinence OR "urinary incontinence" OR "urinary incontinence, stress" OR "urinary incontinence, urge" OR "mixed urinary incontinence" OR "pelvic organ prolapse" OR "genital prolapse" OR "uterine prolapse" OR "cervical prolapse" OR "urogenital prolapse" OR "vaginal prolapse" OR "vaginal apex prolapse" OR "vaginal vault prolapse" OR "utero-vaginal prolapse" OR "anterior vaginal wall prolapse" OR "posterior wall prolapse" OR "posterior vaginal wall prolapse" OR rectocele OR proctocele* OR cystocele OR "urinary bladder prolapse" OR enterocele OR "fecal incontinence" OR "anal incontinence" OR "bowel incontinence" OR "sexual dysfunction, physiological" OR "physiological sexual dysfunction*" OR "sexual dysfunction*" OR "sexual disorder*" OR dyspareunia OR "pelvic pain" OR "urinary retention" OR "urinary bladder diseases" OR "urinary bladder, neurogenic" OR "urinary bladder" OR "urinary bladder, overactive" OR "overactive bladder" OR "overactive detrusor" OR "overactive bladder syndrome" OR "urinary bladder, neurogenic" OR "neurogenic bladder" OR "bladder dysfunction" OR "diurnal enuresis" OR "nocturnal enuresis" OR "lower urinary tract symptoms" OR "lower urinary tract dysfunction" OR "lower urinary tract abnormalities" OR "pelvic floor" OR "pelvic floor disorders" OR "pelvic floor disorder*" OR "pelvic floor disease*" OR "pelvic floor dysfunction*") | 285,139 |
#3 | TITLE-ABS-KEY ("randomized controlled trial" OR "random allocation" OR random∗ ) | 931,842 |
#4 | #1 AND #2 AND #3 (2357) Refine results: Exclude: Document type-Review ( TITLE-ABS-KEY ( telemedicine OR tele-medicine OR telehealth OR tele-health OR ehealth OR e-health OR "electronic health" OR "mobile health" OR mhealth OR m-health OR "remote consultation" OR "distance counseling" OR "wearable electronic devices" OR teleconsultation OR tele-consultation OR telecommunications OR "social media" OR "social medium" OR multi-media OR multimedia OR "mobile applications" OR "mobile app*" OR "mobile application" OR "mobile device" OR phone OR "mobile phone" OR smartphone OR "smart phone" OR "cellular phone" OR cellphone OR "cell phone" OR telephone OR "text messaging" OR text-messag* OR textmessag* OR "text messag*" OR sms OR texting* OR "short message service*" OR "electronic mail" OR e-mail* OR email* OR game* OR gaming OR gamification OR "video games" OR videogame* OR "computer game*" OR "internet based intervention" OR internet-based OR web-based OR "web based" OR "computer based" OR computer-based OR internet-guided OR "internet guided" OR videoconferencing OR video OR website OR web OR online* OR on-line OR internet* OR app OR application* OR computer* OR electronic* OR digital* ) ) AND ( TITLE-ABS-KEY ( incontinence OR "urinary incontinence" OR "urinary incontinence, stress" OR "urinary incontinence, urge" OR "mixed urinary incontinence" OR "pelvic organ prolapse" OR "genital prolapse" OR "uterine prolapse" OR "cervical prolapse" OR "urogenital prolapse" OR "vaginal prolapse" OR "vaginal apex prolapse" OR "vaginal vault prolapse" OR "utero-vaginal prolapse" OR "anterior vaginal wall prolapse" OR "posterior wall prolapse" OR "posterior vaginal wall prolapse" OR rectocele OR proctocele* OR cystocele OR "urinary bladder prolapse" OR enterocele OR "fecal incontinence" OR "anal incontinence" OR "bowel incontinence" OR "sexual dysfunction, physiological" OR "physiological sexual dysfunction*" OR "sexual dysfunction*" OR "sexual disorder*" OR dyspareunia OR "pelvic pain" OR "urinary retention" OR "urinary bladder diseases" OR "urinary bladder, neurogenic" OR "urinary bladder" OR "urinary bladder, overactive" OR "overactive bladder" OR "overactive detrusor" OR "overactive bladder syndrome" OR "urinary bladder, neurogenic" OR "neurogenic bladder" OR "bladder dysfunction" OR "diurnal enuresis" OR "nocturnal enuresis" OR "lower urinary tract symptoms" OR "lower urinary tract dysfunction" OR "lower urinary tract abnormalities" OR "pelvic floor" OR "pelvic floor disorders" OR "pelvic floor disorder*" OR "pelvic floor disease*" OR "pelvic floor dysfunction*" ) ) AND ( TITLE-ABS-KEY ( "randomized controlled trial" OR "random allocation" OR random∗ ) ) AND ( EXCLUDE ( DOCTYPE , "re" ) ) | 1556 |
TITLE-ABS-KEY = article title, abstract, keywords; DOCTYPE = document type; "re" = Review
-
8.
Database: Chinese National Knowledge Infrastructure (CNKI)
Results: 1906
Search period: until August 28, 2021
Number | Search terms | Results |
#1 | SU = (远程医疗 + 远程健康 + 电子健康 + 移动健康 + 移动医疗 + 互联网医疗 + 远程咨询 + 远程医疗咨询 + 可穿戴电子设备 + 社交媒体 + 多媒体 + App + 移动应用 + 移动应用程序 + 移动设备 + 手机 + 智能手机 + 电话 + 移动电话 + 短信 + 电子邮件 + 游戏 + 视频游戏 + 电子游戏 + 计算机游戏 + 基于互联网 + 基于网络 + 基于计算机 + 视频会议 + 网站 + 网络 + 社交网络 + 网络平台 + 移动网络 + 在线 + 线上 + 互联网 + 微信 + 论坛 + 平台) AND SU= (失禁 + 尿失禁 + 漏尿 + 尿失禁, 压力性 + 压力性尿失禁 + 尿失禁, 急迫性 + 急迫性尿失禁 + 混合性尿失禁 + 盆腔器官脱垂 + 盆腔脏器脱垂 + 脱垂 + 膨出 + 生殖器脱垂 + 生殖道脱垂 + 子宫脱垂 + 宫颈脱垂 + 阴道脱垂 + 阴道顶端脱垂 + 阴道穹窿脱垂 + 阴道前壁脱垂 + 阴道后壁脱垂 + 阴道前壁膨出 + 阴道后壁膨出 + 直肠前突 + 膀胱脱垂 + 膀胱膨出 + 肠膨出 + 大便失禁 + 肛门失禁 + 性功能障碍 + 性功能障碍, 生理性 + 性交困难 + 骨盆疼痛 + 尿潴留 + 膀胱疾病 + 神经源性膀胱 + 膀胱过度活跃 + 过度活跃膀胱 + 逼尿肌过度活跃 + 膀胱功能障碍 + 遗尿 + 下尿路症状 + 下尿路功能障碍 + 下尿路异常 + 骨盆底 + 盆底 + 盆底疾病 + 盆底功能障碍 + 盆底功能障碍性疾病) AND AB= (随机对照试验 + 随机分配 + 随机) | 1906 |
SU = subject; AB = abstract
-
9.
Database: Wanfang Database
Results: 88
Search period: until August 28, 2021
Number | Search terms | Results |
#1 | 题名或关键词:(远程医疗 or 远程健康 or 电子健康 or 移动健康 or 移动医疗 or 互联网医疗 or 远程咨询 or 远程医疗咨询 or 可穿戴电子设备 or 社交媒体 or 多媒体 or App or 移动应用 or 移动应用程序 or 移动设备 or 手机 or 智能手机 or 电话 or 移动电话 or 短信 or 电子邮件 or 游戏 or 视频游戏 or 电子游戏 or 计算机游戏 or 基于互联网 or 基于网络 or 基于计算机 or 视频会议 or 网站 or 网络 or 社交网络 or 网络平台 or 移动网络 or 在线 or 线上 or 互联网 or 微信 or 论坛 or 平台) and 题名或关键词:(失禁 or 尿失禁 or 漏尿 or 尿失禁, 压力性 or 压力性尿失禁 or 尿失禁, 急迫性 or 急迫性尿失禁 or 混合性尿失禁 or 盆腔器官脱垂 or 盆腔脏器脱垂 or 脱垂 or 膨出 or 生殖器脱垂 or 生殖道脱垂 or 子宫脱垂 or 宫颈脱垂 or 阴道脱垂 or 阴道顶端脱垂 or 阴道穹窿脱垂 or 阴道前壁脱垂 or 阴道后壁脱垂 or 阴道前壁膨出 or 阴道后壁膨出 or 直肠前突 or 膀胱脱垂 or 膀胱膨出 or 肠膨出 or 大便失禁 or 肛门失禁 or 性功能障碍 or 性功能障碍, 生理性 or 性交困难 or 骨盆疼痛 or 尿潴留 or 膀胱疾病 or 神经源性膀胱 or 膀胱过度活跃 or 过度活跃膀胱 or 逼尿肌过度活跃 or 膀胱功能障碍 or 遗尿 or 下尿路症状 or 下尿路功能障碍 or 下尿路异常 or 骨盆底 or 盆底 or 盆底疾病 or 盆底功能障碍 or 盆底功能障碍性疾病) and 主题:(随机对照试验 or 随机分配 or 随机) | 88 |
-
10.
Database: VIP database
Results: 216
Search period: 1989 to August 28, 2021
Number | Search terms | Results |
#1 | (M= (远程医疗 OR 远程健康 OR 电子健康 OR 移动健康 OR 移动医疗 OR 互联网医疗 OR 远程咨询 OR 远程医疗咨询 OR 可穿戴电子设备 OR 社交媒体 OR 多媒体 OR App OR 移动应用 OR 移动应用程序 OR 移动设备 OR 手机 OR 智能手机 OR 电话 OR 移动电话 OR 短信 OR 电子邮件 OR 游戏 OR 视频游戏 OR 电子游戏 OR 计算机游戏 OR 基于互联网 OR 基于网络 OR 基于计算机 OR 视频会议 OR 网站 OR 网络 OR 社交网络 OR 网络平台 OR 移动网络 OR 在线 OR 线上 OR 互联网 OR 微信 OR 论坛 OR 平台)) AND (M=(失禁 OR 尿失禁 OR 漏尿 OR 尿失禁, 压力性 OR 压力性尿失禁 OR 尿失禁, 急迫性 OR 急迫性尿失禁 OR 混合性尿失禁 OR 盆腔器官脱垂 OR 盆腔脏器脱垂 OR 脱垂 OR 膨出 OR 生殖器脱垂 OR 生殖道脱垂 OR 子宫脱垂 OR 宫颈脱垂 OR 阴道脱垂 OR 阴道顶端脱垂 OR 阴道穹窿脱垂 OR 阴道前壁脱垂 OR 阴道后壁脱垂 OR 阴道前壁膨出 OR 阴道后壁膨出 OR 直肠前突 OR 膀胱脱垂 OR 膀胱膨出 OR 肠膨出 OR 大便失禁 OR 肛门失禁 OR 性功能障碍 OR 性功能障碍, 生理性 OR 性交困难 OR 骨盆疼痛 OR 尿潴留 OR 膀胱疾病 OR 神经源性膀胱 OR 膀胱过度活跃 OR 过度活跃膀胱 OR 逼尿肌过度活跃 OR 膀胱功能障碍 OR 遗尿 OR 下尿路症状 OR 下尿路功能障碍 OR 下尿路异常 OR 骨盆底 OR 盆底 OR 盆底疾病 OR 盆底功能障碍 OR 盆底功能障碍性疾病)) | 216 |
M = title, keyword
11. Database: Chinese Biomedical Literature Database (CBM)
Results: 279
Search period: until August 28, 2021
Number | Search terms | Results |
#1 | ("远程医疗"[核心字段] OR "远程健康"[核心字段] OR "电子健康"[核心字段] OR "移动健康"[核心字段] OR "移动医疗"[核心字段] OR "互联网医疗"[核心字段] OR ("远程咨询"[核心字段] OR "网络咨询"[核心字段] OR "E-治疗"[核心字段] OR "远程咨询"[主题词]) OR "远程医疗咨询"[核心字段] OR "可穿戴电子设备"[核心字段] OR "社交媒体"[核心字段] OR ("多媒体"[核心字段] OR "多媒体"[主题词]) OR "App"[核心字段] OR ("移动应用"[核心字段] OR "移动应用程序"[核心字段] OR "移动应用"[主题词]) OR "移动设备"[核心字段] OR ("手机"[核心字段] OR "便携式电话"[核心字段] OR "汽车无线电话"[核心字段] OR "移动电话"[核心字段] OR "便携式电话"[主题词]) OR ("智能手机"[核心字段] OR "智能手机"[主题词]) OR ("电话"[核心字段] OR "电话总机"[核心字段] OR "电话"[主题词]) OR ("移动电话"[核心字段] OR "便携式电话"[核心字段] OR "汽车无线电话"[核心字段] OR "手机"[核心字段] OR "便携式电话"[主题词]) OR ("短信"[核心字段] OR "短信"[主题词]) OR ("电子邮件"[核心字段] OR "Email"[核心字段] OR "E-Mail"[核心字段] OR "电子邮件"[主题词]) OR ("游戏"[核心字段] OR "游戏和玩具"[核心字段] OR "木偶"[核心字段] OR "玩具"[核心字段] OR "游戏和玩具"[主题词]) OR "视频游戏"[核心字段] OR ("电子游戏"[核心字段] OR "电子游戏"[主题词]) OR "计算机游戏"[核心字段] OR "基于互联网"[核心字段] OR "基于网络"[核心字段] OR "基于计算机"[核心字段] OR ("视频会议"[核心字段] OR "视频会议"[主题词]) OR "网站"[核心字段] OR "网络"[核心字段] OR ("社交网络"[核心字段] OR "社交网络"[主题词]) OR "网络平台"[核心字段] OR "移动网络"[核心字段] OR "在线"[核心字段] OR "线上"[核心字段] OR "互联网"[核心字段] OR "微信"[核心字段] OR "论坛"[核心字段] OR "平台"[核心字段]) | 170,662 |
#2 | ("失禁"[核心字段] OR ("尿失禁"[核心字段] OR "尿失禁"[主题词]) OR "漏尿"[核心字段] OR "尿失禁, 压力性"[核心字段] OR "压力性尿失禁"[核心字段] OR "尿失禁, 急迫性"[核心字段] OR "急迫性尿失禁"[核心字段] OR "混合性尿失禁"[核心字段] OR ("盆腔器官脱垂"[核心字段] OR "盆腔器官脱垂"[主题词]) OR "盆腔脏器脱垂"[核心字段] OR ("脱垂"[核心字段] OR "脱垂"[主题词]) OR "膨出"[核心字段] OR "生殖器脱垂"[核心字段] OR "生殖道脱垂"[核心字段] OR ("子宫脱垂"[核心字段] OR "阴道脱垂"[核心字段] OR "子宫脱垂"[主题词]) OR "宫颈脱垂"[核心字段] OR ("阴道脱垂"[核心字段] OR "子宫脱垂"[核心字段] OR "子宫脱垂"[主题词]) OR "阴道顶端脱垂"[核心字段] OR "阴道穹窿脱垂"[核心字段] OR "阴道前壁脱垂"[核心字段] OR "阴道后壁脱垂"[核心字段] OR "阴道前壁膨出"[核心字段] OR "阴道后壁膨出"[核心字段] OR ("直肠前突"[核心字段] OR "直肠前突"[主题词]) OR ("膀胱脱垂"[核心字段] OR "膀胱膨出"[核心字段] OR "膀胱脱出"[核心字段] OR "膀胱膨出"[主题词]) OR ("膀胱膨出"[核心字段] OR "膀胱脱出"[核心字段] OR "膀胱脱垂"[核心字段] OR "膀胱膨出"[主题词]) OR "肠膨出"[核心字段] OR ("大便失禁"[核心字段] OR "大便失禁"[主题词]) OR "肛门失禁"[核心字段] OR ("性功能障碍"[核心字段] OR "生理性性功能障碍"[核心字段] OR "性功能障碍, 生理性"[主题词]) OR "性功能障碍, 生理性"[核心字段] OR "性交困难"[核心字段] OR "骨盆疼痛"[核心字段] OR ("尿潴留"[核心字段] OR "尿潴留"[主题词]) OR ("膀胱疾病"[核心字段] OR "膀胱疾病"[主题词]) OR "神经源性膀胱"[核心字段] OR "膀胱过度活跃"[核心字段] OR "过度活跃膀胱"[核心字段] OR "逼尿肌过度活跃"[核心字段] OR "膀胱功能障碍"[核心字段] OR ("遗尿"[核心字段] OR "遗尿"[主题词]) OR ("下尿路症状"[核心字段] OR "下尿路症状"[主题词]) OR "下尿路功能障碍"[核心字段] OR "下尿路异常"[核心字段] OR ("骨盆底"[核心字段] OR "骨盆底"[主题词]) OR "盆底"[核心字段] OR ("盆底疾病"[核心字段] OR "盆底疾病"[主题词]) OR "盆底功能障碍"[核心字段] OR "盆底功能障碍性疾病"[核心字段]) | 99,171 |
#3 | (#2) AND (#1) | 279 |
Rights and permissions
About this article
Cite this article
Xu, P., Wang, X., Guo, P. et al. The effectiveness of eHealth interventions on female pelvic floor dysfunction: a systematic review and meta-analysis. Int Urogynecol J 33, 3325–3354 (2022). https://doi.org/10.1007/s00192-022-05222-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05222-5