Abstract
Introduction and hypothesis
The objective was to compare the effect of antibiotics versus no antibiotics prophylaxis per-operatively on the frequency of urinary tract infection (UTI) following mid-urethral sling application to treat stress or mixed urinary incontinence.
Methods
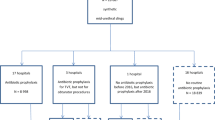
This study was designed as a multicenter prospective randomized trial. Women were included from eight centers in three countries. Women were aged under 60 years and had objectively verified stress urinary incontinence. Women with mixed urinary incontinence were also included. Randomization was held in blocks for operation with either antibiotics or no antibiotics. UTI was defined in accordance with the Centers for Disease Control (CDC) criteria for symptomatic UTI. Women were followed up at 3, 12, and 36 months. This was part of a trial comparing subjective cure rate in relation to application of Ajust® (single-incision mid-urethral slings) versus standard mid-urethral slings.
Results
The main outcome was to evaluate if per-operative antibiotics had any impact on UTI following sling surgery. In total, 305 women were randomized (158 [52%] to antibiotics and 147 [48%] to no antibiotics). Demographic data disclosed no differences between the two groups. The trial did not show any difference between the two groups regarding the frequency of postoperative UTI. Logistic regression analysis disclosed only residual urine volume at 3 months’ follow-up as a significant risk factor for UTIs. Per-operative antibiotics had no influence on the frequency of mesh erosions or any other complication.
Conclusions
Our trial does not suggest any beneficial effect of per-operative antibiotics on the risk of post-operative UTIs.

Similar content being viewed by others
Abbreviations
- MUI:
-
Mixed urinary incontinence
- NFOG:
-
Nordic Federation of Obstetrics and Gynecology
- SIMS:
-
Single-incision mid-urethral slings
- SMUS:
-
Standard mid-urethral slings
- SUI:
-
Stress urinary incontinence
- UTI:
-
Urinary tract infection
References
Suskind AM, Kaufman SR, Dunn RL, Stoffel JT, Clemens JQ, Hollenbeck BK. Population-based trends in ambulatory surgery for urinary incontinence. Int Urogynecol J. 2013;24:207–11.
Anger JT, Litwin MS, Wang Q, Pashos CL, Rodríguez LV. Complications of sling surgery among female Medicare beneficiaries. Obstet Gynecol. 2007;109:707–14.
Paz-Levy D, Weintraub AY, Reuven Y, Yohay Z, Idan I, Elharar D, et al. Prevalence and risk factors for urinary tract infection following stress urinary incontinence surgery with two midurethral sling procedures. Int J Gynaecol Obstet. 2018;143:333–8.
Weintraub AY, Reuven Y, Paz-Levy D, Yohay Z, Idan I, Elharar D, et al. Prevalence and risk factors for urinary tract infection up to one year following midurethral sling incontinence surgery. Eur J Obstet Gynecol Reprod Biol. 2018;222:146–50.
Bermingham SL, Ashe JF. Systematic review of the impact of urinary tract infections on health-related quality of life. BJU Int. 2012;110:E830–6.
Jackson D, Higgins E, Bracken J, Yandell PM, Shull B, Foster RT Sr. Antibiotic prophylaxis for urinary tract infection after midurethral sling: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2013;19:137–41.
Harmanli O, Boyer RL, Metz S, Tunitsky E, Jones KA. Double-blinded randomized trial of preoperative antibiotics in midurethral sling procedures and review of the literature. Int Urogynecol J. 2011;22:1249–53.
Shepherd JP, Jones KA, Harmanli O. Is antibiotic prophylaxis necessary before midurethral sling procedures for female stress incontinence? A decision analysis. Int Urogynecol J. 2014;25:227–33.
El-Hefnawy AS, Nabeeh A, El Sawy E, Wadie BS. Urinary tract infection before and after mid-urethral slings: culture-proven diagnosis and analysis of risk factors. Int Urol Nephrol. 2011;43:345–51.
Rudnicki M, von Bothmer-Ostling K, Holstad A, Magnusson C, Majida M, Merkel C, et al. Adjustable mini-sling compared with conventional mid-urethral slings in women with urinary incontinence. A randomized controlled trial. Acta Obstet Gynecol Scand. 2017;96:1347–56.
National Healthcare Safety Network. Urinary tract infection (catheter-associated urinary tract infection [CAUTI] and noncatheter-associated urinary tract infection [UTI]) and other urinary system infection [USI]) events. National Healthcare Safety Network (NHSN), Device-associated Module; 2015:7–1.
Gehrich AP, Lustik MB, Mehr AA, Patzwald JR. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483–90.
Stec P, Connell R. Sepsis and multiorgan failure following TVT procedure. Ginekol Pol. 2014;85:314–7.
Tate SB, Franco AV, Fynes MM. Tension-free vaginal tape exposure presenting as a recurrent sterile paraurethral abscess. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:420–3.
Lewis FM, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. 2017;124:643–54.
Rogers RG, Kammerer-Doak D, Olsen A, Thompson PK, Walters MD, Lukacz ES, et al. A randomized, double-blind, placebo-controlled comparison of the effect of nitrofurantoin monohydrate macrocrystals on the development of urinary tract infections after surgery for pelvic organ prolapse and/or stress urinary incontinence with suprapubic catheterization. Am J Obstet Gynecol. 2004;191:182–7.
Surgical site infections: prevention and treatment. NICE Guideline, No. 125 NICE Guideline Updates Team (UK); London: National Institute for Health and Care Excellence. 2019, 978–1–4731-3394-5.
Acknowledgements
We thank Katarina von Bothmer Östling from Halmstad Hospital in Sweden, Anja Holstad from Gjøvik Hospital in Norway, Claes Magnusson from Sahlgrenska University Hospital in Sweden, Memona Majida from Ahus University Hospital in Norway, and Constanze Merkel from Regional Hospital of Northern Jutland in Denmark for their vital contributions to the development of this project. We also thank all the women who participated in this study and shared their experiences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There were no conflicts of interest for any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The trial was registered at ClinicalTrials.gov: NCT01754558. URL: clinicaltrials.gov/ct2/show/NCT01754558?term=NCT01754558&rank=1
Rights and permissions
About this article
Cite this article
Rudnicki, M., Jakobsson, U. & Teleman, P. Impact of per-operative antibiotics on the urinary tract infection rate following mid-urethral sling surgery for urinary incontinence: a randomized controlled trial. Int Urogynecol J 31, 1545–1550 (2020). https://doi.org/10.1007/s00192-019-04156-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-019-04156-9