Abstract
Introduction and hypothesis
Hysterectomy is sometimes considered the cause of lower urinary tract symptoms (LUTS). We hypothesized that hysterectomy for abnormal uterine bleeding and/or symptoms of fibroids is more likely to cause LUTS than a hysteroscopic procedure for the same indications.
Methods
Two groups of women were compared: one group comprised 3,618 women who had had a hysterectomy due to abnormal uterine bleeding or symptoms of fibroids and the other group comprised 238 women who had had hysteroscopic treatment for the same indications. The main outcome measures were occurrence of LUTS before and 1 year after the surgical intervention. The frequencies of LUTS before and after surgery were compared between the groups. Binary logistic regression was used to model the odds of having postoperative urinary leakage and urgency while controlling for uterine size, surgical procedure and preoperative LUTS.
Results
There were no statistically significant differences between women after hysterectomy and after hysteroscopy in the frequencies of LUTS before or after surgery, when uterine size was comparable. However, there was a difference in the rates of de novo urinary incontinence between women with hysterectomy and women with hysteroscopy (7.6%, 95% CI 6.3–9.0, and 3.2%, 95% CI 1.6–6.5, respectively). Of the women with a large uterus, 58.6% (95% CI 51.5–65.5) reported relief of urinary incontinence and 85.5% (95% CI 82.3–88.4) reported relief of urinary urgency postoperatively.
Conclusions
Our results suggest that it is important to individualize preoperative information in women prior to hysterectomy since the outcome concerning LUTS depends on preoperative symptoms and uterine size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hysterectomy is one of the most common major gynaecological procedures. In some countries there is a trend towards a decrease in hysterectomy rates [1–3], but it is still a common procedure. Hysterectomy is an effective way to treat several benign disorders [4] and is often necessary for the treatment of malignant gynaecological conditions. Studies have shown high levels of satisfaction postoperatively [5] and it is considered to be a relatively safe procedure with major complications in approximately 4% of patients [6] and mortality rates in the range 0.2–1 in 1,000 hysterectomies [7, 8]. Lower urinary tract symptoms (LUTS), e.g. urinary leakage, urgency and nocturia, are a common problem in women. The prevalence of LUTS is reported to range from 25% to 45% [3, 9] and negatively affects quality of life [10]. In Sweden, LUTS has been reported to be responsible for approximately 2% of the total health-care costs [11].
There has been and still is a lively discussion amongst gynaecologists about whether hysterectomy enhances the risk of developing LUTS [12–17]. Surgical trauma and/or altered anatomy are considered to be the causes [18]. Treatments other than hysterectomy are becoming more common for some benign gynaecological conditions such as abnormal uterine bleeding or fibroids, and have shown good efficacy [19, 20]. Therefore, it has become even more important to assess the results of hysterectomy and its potential side effects.
Cross-sectional and retrospective studies have tended to show an increased risk of incontinence after hysterectomy [13, 14]. In contrast, prospective studies in which preoperative data are available tend to show the opposite: that hysterectomy does not increase the risk of urinary incontinence [15, 17]. Cross-sectional and retrospective studies usually lack information as to whether the patients had urinary incontinence prior to hysterectomy or rely on patient recall for the information [13, 14, 18]. Prospective studies are more likely to include information regarding preoperative incontinence [15–17].
In this study we evaluated the outcomes regarding urinary incontinence and urgency after a hysterectomy with a focus on both de novo symptoms and problems that existed preoperatively. The study population comprised women with abnormal uterine bleeding and/or symptoms of fibroids who had either a hysterectomy or a hysteroscopic procedure with endometrial resection or ablation or removal of submucosal fibroids. Those who underwent a hysteroscopic procedure served as the control group, since the same conditions are treated without removal of the uterus, and potential damage to the surrounding tissues and alteration of the anatomy in the pelvis are avoided. The choice between procedures often depends on the gynaecologist’s preference rather than the patient’s characteristics, except in relation to certain parameters such as uterine size and location of fibroids.
Materials and methods
This study used data collected through the Swedish National Quality Register for Gynaecological Surgery (GynOp register) that was established in 1997. From the beginning preoperative, perioperative and postoperative information in women undergoing endometrial ablation or hysterectomy has been gathered. Gradually the GynOp register has expanded and today encompasses all major surgical fields in gynaecology. The GynOp register covers approximately 70% of the Swedish female population. Information about the register is available from the GynOp website (http://www.gynop.org/english/english.htm). Data are collected prospectively and consist of both patient questionnaires and forms completed by the gynaecologist. Data concerning general health history as well as current symptoms are collected by questionnaires before and after surgery. Patients’ willingness to participate has been evaluated and the results show that the questionnaires are well accepted and the response rate is over 90% [21]. The gynaecological surgeon records data on clinical status, surgical procedure and postoperative care.
In this study the patients’ answers to questions regarding LUTS were combined with data on the procedure they had undergone. Data used in the present study were collected from the start in 1997 to 2006 when there was a major revision of the GynOp register. During this time period basic questions regarding LUTS included “Do you have either/both of the following bothers from the lower abdomen?”: urinary incontinence/urinary leakage (yes or no), or urinary urgency (yes or no). The questions were subject to face and content validation. The questions were not designed to measure LUTS objectively, only to determine whether respondents were bothered by incontinence or urgency. During the study period the procedure was changed from collecting long-term outcome of the surgical procedure from the patients at 6 months and 2 years postoperatively to one questionnaire at 1 year postoperatively.
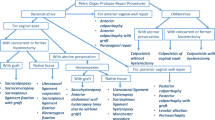
The process of exclusion and inclusion of women who underwent a hysterectomy or hysteroscopy between 1997 and 2006 is shown as a flow chart in Fig. 1. The data were collected in 2009 and included data from the original questionnaires only.
Inclusion criteria were hysterectomy or hysteroscopy due to abnormal uterine bleeding and/or symptoms of uterine fibroids and age 40–52 years and uterine size recorded by the surgeon. In the GynOp register during the study period, abnormal uterine bleeding or fibroids treated with hysteroscopy were seldom found in women older than 52 years. Polyps or postmenopausal bleeding were then a more common reason for hysteroscopy. Also, a hysterectomy before 40 years of age was seldom found in the register for the chosen indications. The surgical indications for hysterectomy in patients older than 52 years included mainly malignant (or suspected malignant) conditions and prolapse. We also wanted to avoid too wide a time span, since age itself is considered to be associated with LUTS.
Exclusion criteria were any previous or concomitant prolapse or incontinence surgery to minimize the impact of a poorly functioning pelvic floor, hysterectomy due to malignant (or suspected malignant) conditions, concomitant adnexal surgery to eliminate the risk of iatrogenic menopause, women with an American Society of Anesthesiologists (ASA) physical status classification of III or worse [22], and those who had not fully answered the questions regarding LUTS.
For the statistical analysis the hysterectomy group was divided into two subgroups, one comprising women with a mean uterine size the same as the mean uterine size of the control group (hysteroscopy) and the other comprising women with a larger uterus. There was a substantial difference in the distribution of uterine sizes between the hysterectomy group and the hysteroscopy control group. The heavily skewed uterine size data prevented matching the uterine sizes in the control group to all uterine sizes in the hysterectomy group and made the use of multivariate analysis inappropriate for determining whether uterine size or mode of surgery had any impact on LUTS.
Statistical methods
The chi-squared test was used to test whether the distribution of the answers on each factor were equal in the different diagnostic groups. The t test was used to test whether the mean age, mean body mass index (BMI), and mean number of births were equal in the different diagnostic groups. A significance level of 0.05 was used for all tests. All p values presented are uncorrected. To adjust for between-group comparisons, the Holm-Bonferroni method was used. If the test was significant after correction, then this is noted in the tables. Binary logistic regression was used to model the odds of having postoperative urinary leakage and urgency while controlling for uterine size, surgical procedure and preoperative LUTS. Receiver operating characteristic curves were used to estimate a threshold for uterine size. SPSS version 22 (IBM Corp., Armonk, NY) and R 3.2.2 (R Core Team, Vienna, Austria) were used for the analyses.
Results
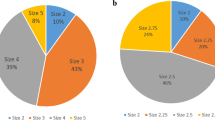
After the inclusion and exclusion process the final cohort comprised 3,850 women (Fig. 1). The majority of women (80%) answered their questionnaires 1–2 years after surgery: 786 (20%) at 6 months, 1,642 (41.8%) at 1 year , and 1,510 (38.2%%)at 2 years. Of the 3,856 women. 3,618 had had a hysterectomy and 238 a hysteroscopic procedure. As described above, the hysterectomy group was divided into two subgroups so a valid comparison with the hysteroscopy group was possible. The cut-off value was set according to the mean uterine size. In the GynOp register, uterine size is estimated in a gynaecological examination prior to the surgical intervention by palpation of the uterus, and is reported in terms of gestational weeks (GW) as small, normal, or 6, 8, 10, 12 or 14 GW, etc. A normal-sized uterus is considered a uterus at 4 GW. Thus the women were classified into three groups: group 1 comprised those with hysterectomy and ≤8 GW, group 2 those with hysterectomy and >8 GW, and group 3 all those with hysteroscopy. The mean uterine sizes of groups 1, 2 and 3 were 5.3 GW (95% CI 5.2–5.4 GW), 13.3 GW (95% CI 13.2–13.5 GW) and 5.3 GW (95% CI 5.0–5.6 GW), respectively. This classification is somewhat arbitrary with regard to LUTS symptoms since there is probably no difference clinically between uterine sizes 8 GW and 9 GW.
We also tested classifiers other than the mean uterine size. A receiver operating characteristic curve was created to determine a possible uterine size threshold for predicting LUTS. The best accuracy (area under the curve 0.52) was noted for 9.5 GW, indicating that uterine size is a poor prognostic factor for postoperative LUTS.
The basic preoperative characteristics of the women are shown in Table 1. Uterine size, age, BMI, and parity were similar in group 1 and group 3. Uterine size was significantly larger in group 2 than in groups 1 and 3. Group 2 had had fewer gynaecological surgery procedures than groups 1 and 3. Further analysis revealed that women in group 2 had had fewer tubal ligations and lesser cervical surgery (dysplasia) than women in groups 1 and 3.
The data regarding urinary symptoms are presented in Table 2. There were no differences between groups 1 and 3. Of women with a larger uterus (group 2), 8.0% reported urinary leakage after hysterectomy, and of those with a smaller uterus (group 1), 11.8% reported leakage (p < 0.001). Of women in group 2, 27.9% reported urgency before surgery compared with 17.7% in group 1 and 11.2% in group 3 (p < 0.001 in both cases). One year after surgery this result was reversed.: only 6.7% of women in group 2 reported urgency (a fourfold decrease), a rate lower than in group 1 (11.4%, p < 0.001) and group 3 (11.6% p = 0.004).
In the multiple regression analysis uterine size was not associated with the postoperative occurrence of LUTS in groups 1 and 3 (odds ratio, OR, 0.97, 95% CI 0.89–1.06, p = 0.567, for incontinence; OR 0.97, 95% CI 0.89–1.05, p = 0.485, for urgency; Fig. 2). In group 2 (>8 GW), an increase in uterine size of 2 GW had a slight protective effect against LUTS (OR 0.93, 95% CI 0.88–0.98, p = 0.011, for incontinence; OR 0.94, 95% CI 0.88–0.99, p = 0.03), for urgency).
The results of analysis of outcomes regarding LUTS according to urinary symptoms reported before surgery are shown in Table 3. There was no difference between groups 1 and 3 with regard to the presence of urinary incontinence before surgery. After surgery, the proportion of women in group 1 reporting de novo urinary incontinence was higher than in group 3 (7.6% vs. 3.2%, p = 0.015). This was also found in the multivariate analysis, which showed that hysteroscopy reduced the risk of de novo incontinence (OR 0.41, 95% CI 0.17–0.82, p = 0.023) more than hysterectomy (Fig. 2). Of women with a small uterus (group 1), 7.6% reported de novo urinary incontinence, a significantly higher proportion than among women with a large uterus (group 2, 4.2%, p < 0.001). If incontinence was present before surgery, the risk of incontinence was significantly higher after hysteroscopy than after hysterectomy (OR 7.49, 95% CI 2.17–30.54, p = 0.0026). Moreover, a high proportion of women (58.6%) with a uterus >8 GW reported relief of urinary leakage 1 year after surgery.
Analysis of urgency revealed no difference between groups 1 and 3 in the frequency of de novo urgency 1 year after surgery (Table 4). The risk of still having bothersome urgency after surgery was higher after hysteroscopy than after hysterectomy (OR 2.67, 95% CI 1.04–6.99, p = 0.042). Group 2 had a lower rate of de novo urgency than groups 1 and 3 (p < 0.001 and p = 0.015). Among women with symptoms present before surgery, 28.6% of those in group 1 had a significant decrease in urgency postoperatively (95%CI 23.7–34.1) compared with 48.4% of those in group 3 (95% CI 32.0–65.2, p = 0.038). An even larger proportion of women in group 2 had a decrease in urgency after surgery: 85.5% reported relief of urgency (p < 0.001 compared with both groups 1 and 3).
The results were further evaluated with regard to the mode of hysterectomy in the total cohort and within each group. There were no significant differences in the frequencies of urinary symptoms before and after hysterectomy among those who underwent abdominal, laparoscopic and vaginal hysterectomy (data not shown). In the patients in this study undergoing hysterectomy, by far the greatest proportion underwent abdominal hysterectomy (67.6% of all procedures); vaginal hysterectomy accounted for 25.8% and laparoscopic only 6.6% of procedures. Vaginal hysterectomy was more common in group 1 (42.2%).
Discussion
Main findings
Several epidemiological studies have shown an increased risk of urinary incontinence after hysterectomy [13, 14, 18]. Our results, which were based on prospective data from a large population of patients registered with GynOp, suggest the need for risk stratification of patients depending on whether LUTS is present before surgery. In addition, uterine size was found to affect outcome. Of women with urinary urgency preoperatively and a uterus with a size >8 GW, 85.5% stated at follow-up that the urgency symptoms had disappeared. This is in line with the findings of several prospective studies in women with known preoperative status regarding LUTS. One study showed a decrease in stress incontinence after surgery from 29.5% to 10% over a 2-year follow-up [15]. Another study showed a decrease in stress urinary incontinence from 44% to 31% over a 3-year follow-up [17]. A randomized controlled study comparing outcomes between partial and total hysterectomy showed decreases in self-reported urge incontinence after surgery from 19% to 11% and 16% to 12%, respectively [23]. In our study, only 3.7% of women with a larger uterus reported de novo urgency 1 year after hysterectomy. A significantly lower proportion of these women had de novo urinary incontinence compared with women with a uterine size ≤ 8 GW.
There was a difference in the frequency of LUTS between women with ≤8 GW and those who underwent hysteroscopy (groups with similar uterine sizes). Notably, the proportion of women with de novo urinary incontinence was higher after a hysterectomy, but if LUTS was present before surgery the proportion of women with symptom relief was also higher.
Strengths and weaknesses
The sample size (3,938 women) is an obvious strength of this study. We were also able to select a homogeneous population because of the large number of registered operations in the GynOp register. The homogeneity reduced the impact of confounding factors such as age, parity and BMI. All data concerning urinary incontinence and urgency were prospectively collected and self-reported preoperatively and postoperatively. This is a great advantage compared to cross-sectional studies that suffer from lack of reliable information regarding preoperative data on LUTS [13, 18, 24]. The mode of data collection also minimized the risk of recall bias, which is a problem in retrospective studies. Taking uterine size into account is important since LUTS has been shown to be less likely in women with a small uterus [25]. Comparing different surgical methods for treating the same disorders in women with equal uterine size makes it possible to rule out the underlying condition as a confounder of the results [26]. We were also able to exclude all women who had concomitant or previous surgery for prolapse or incontinence. As a result, we reduced the impact of already known poor pelvic floor function on the results.
Our reliance solely on patient-reported outcomes and no objective measurements of LUTS could be a limitation. However, other studies have shown a good correlation between patients’ subjective assessment of their continence and objective measurements [27]. In the present study, 26.0% of the women stated preoperatively that they had LUTS. This frequency is in line with the findings of several other studies [3, 9], and hence we regard the questionnaires in the GynOp register as useful for following changes in symptoms after surgery. It is reasonable to believe that each woman described her LUTS symptoms in the same way before and after surgery.
Our follow-up time was short compared to those in several cross-sectional studies that have shown a risk of incontinence after hysterectomy. However, in none of these studies were preoperative symptoms of incontinence recorded [13, 18, 24]. In the present study we showed that women with a normal-sized uterus (mean 5.3 GW) might have an increased risk of incontinence, while women with a larger uterus (mean 13.4 GW) greatly benefited from hysterectomy. Not only were they free of bleeding disorders, but urgency and leakage also decreased substantially. A prospective observational study based on patients’ self-reports after hysterectomy has also shown a decrease in stress urinary incontinence at both 1 year and 3 years [17]. Stratification according to size was not performed in that study. It has been argued that there is a time delay between acute trauma to the pelvic floor at childbirth and onset of symptoms such as urinary incontinence [28]. One can speculate that younger women are able to compensate for trauma to the pelvic floor to a greater extent than older women, so that the time to onset of symptoms after acute trauma would be shorter in older women. The possible influence of the time between hysterectomy and the onset of LUTS needs further evaluation.
Another limitation was the simple nature of the LUTS questions used during the period studied. We were not able to learn if the women who had reported both urinary incontinence and urinary urgency had leaks because of stress or urge incontinence because the original GynOp questionnaire was not designed to determine this. In 2006 a validated instrument with extended questions for LUTS quantification was introduced to the GynOp register [29]. We will soon have data to report from this cohort. Another weakness was the discrepancy in sample size between the two groups of women with similar uterine size: those with hysterectomy (group 1, 1,634 women) and those with hysteroscopy (group 3, 238 women). Hysteroscopic surgery is less common than hysterectomy for bleeding disorders [14], and we had to exclude 30% of otherwise eligible patients who had had a hysteroscopic procedure because uterine size data were missing. In spite of this, a significant difference was found between these groups in de novo incontinence after surgery, a finding well in line with results from epidemiological studies.
Interpretation
This patient self-reported large register study with prospective data showed that evaluation of the risk of incontinence after hysterectomy in an individual patient needs to take into account uterine size and the presence of LUTS before surgery. A large Swedish epidemiological study with a mean follow-up time of 12 years has shown that hysterectomy increases the risk of subsequent surgery for stress urinary incontinence with a hazard ratio of 2.4 [18]. However, it did not include data on preoperative incontinence. The authors of that study concluded that the most plausible explanation for the increased hazard ratio was the effect of the surgical trauma inflicted during hysterectomy. The fact that several studies have failed to show differences in LUTS between patients with total and subtotal hysterectomy reduces the plausibility of the explanation [16, 23]. Moreover, neuroanatomical studies have shown that a simple hysterectomy is not likely to injure important neuroanatomical structures, e.g. bladder innervation [30]. Other studies have compared the outcomes after hysterectomy and hysteroscopy for LUTS. One randomized controlled study showed no differences in LUTS up to 2 years after surgery [26]. A retrospective study with a mean follow-up between 6 and 10 years with no preoperative data showed an increased risk of incontinence surgery amongst the women who had a hysterectomy than among those who had a hysteroscopy for heavy menstrual bleeding [14]. None of the above studies investigated the association between the size of the uterus and the risk of LUTS.
Conclusion
The present study used data from a large existing register, the GynOp register, to study the risk of LUTS before and after hysterectomy in comparison with that after hysteroscopic procedures. LUTS was found to improve after hysterectomy if the symptoms had been present before surgery especially in women with a large uterus. De novo LUTS was also found to occur after hysterectomy in women with a normal-sized uterus.
References
Hanstede MM, Burger MJ, Timmermans A, Burger MP. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012;91(2):220–225.
Brummer TH, Jalkanen J, Fraser J, Heikkinen A-M, Kauko M, Mäkinen J, et al. FINHYST 2006--national prospective 1-year survey of 5,279 hysterectomies. Hum Reprod. 2009;24(10):2515–2522.
Milsom I, Altman D, Lapitan M, Nelson R, Sillen U, Thom D. Epidemiology of urinary (UI) and faecal (FI) incontinence and pelvic organ prolapse (POP). In: Abrams P, Cardozo L, Khoury S, Wein A, editors. 4th International Consultation on Incontinence. Paris: Health Publications; 2009. p. 35–111.
Desforges JF, Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856–860.
Kjerulff KH, Rhodes JC, Langenberg PW, Harvey LA. Patient satisfaction with results of hysterectomy. Am J Obstet Gynecol. 2000;183(6):1440–1447.
Brummer THI, Jalkanen J, Fraser J, Heikkinen AM, Kauko M, Mäkinen J, et al. FINHYST, a prospective study of 5279 hysterectomies: complications and their risk factors. Hum Reprod. 2011;26(7):1741–1751.
Mäkinen J, Johansson J, Tomás C, Tomás E, Heinonen PK, Laatikainen T, et al. Morbidity of 10 110 hysterectomies by type of approach. Hum Reprod. 2001;16(7):1473–1478.
Wingo PA, Huezo CM, Rubin GL, Ory HW, Peterson HB. The mortality risk associated with hysterectomy. Am J Obstet Gynecol. 1985;152(7 Pt 1):803–808.
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53(11):1150–1157.
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well‐being in men and women: results from the EPIC study. BJU Int. 2008;101(11):1388–1395.
Milsom I. The prevalence of urinary incontinence. Acta Obstet Gynecol Scand. 2000;79(12):1056–1059.
Brown JS, Sawaya G, Thom DH, Grady D. Hysterectomy and urinary incontinence: a systematic review. Lancet. 2000;356(9229):535–539.
Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194(2):339–345.
Cooper K, Lee A, Chien P, Raja E, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG. 2011;118(10):1171–1179.
Kjerulff KH, Langenberg PW, Greenaway L, Uman J, Harvey LA. Urinary incontinence and hysterectomy in a large prospective cohort study in American women. J Urol. 2002;167(5):2088–2092.
Gimbel H, Zobbe V, Andersen BM, Filtenborg T, Gluud C, Tabor A. Randomised controlled trial of total compared with subtotal hysterectomy with one‐year follow up results. BJOG. 2004;110(12):1088–1098.
Gustafsson C, Ekström Å, Brismar S, Altman D. Urinary incontinence after hysterectomy – three-year observational study. Urology. 2006;68(4):769–774.
Altman D, Granath F, Cnattingius S, Falconer C. Hysterectomy and risk of stress-urinary-incontinence surgery: nationwide cohort study. Lancet. 2007;370(9597):1494–1499.
Hurskainen R, Teperi J, Rissanen P, Aalto A-M, Grenman S, Kivelä A, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357(9252):273–277.
Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420.
Ladfors MB, Lofgren ME, Gabriel B, Olsson JH. Patient accept questionnaires integrated in clinical routine: a study by the Swedish National Register for Gynecological Surgery. Acta Obstet Gynecol Scand. 2002;81(5):437–442.
Wolters U, Wolf T, Stützer H, Schröder T. ASA classification and perioperative variables as predictors of postoperative outcome. Br J Anaesth. 1996;77(2):217–222.
Thakar R, Ayers S, Clarkson P, Stanton S, Manyonda I. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347(17):1318–1325.
Vaart C, Bom J, Leeuw J, Roovers J, Heintz A. The contribution of hysterectomy to the occurrence of urge and stress urinary incontinence symptoms. BJOG. 2002;109(2):149–154.
Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79(1):120–127.
Bhattacharya S, Mollison J, Pinion S, Parkin DE, Abramovich DR, Terry P, et al. A comparison of bladder and ovarian function two years following hysterectomy or endometrial ablation. BJOG. 1996;103(9):898–903.
Hahn I, Fall M. Objective quantification of stress urinary incontinence: a short, reproducible, provocative pad‐test. Neurourol Urodyn. 1991;10(5):475–481.
Viktrup L. The risk of lower urinary tract symptoms five years after the first delivery. Neurourol Urodyn. 2002;21(1):2–29.
Kulseng‐Hanssen S, Borstad E. The development of a questionnaire to measure the severity of symptoms and the quality of life before and after surgery for stress incontinence. BJOG. 2003;110(11):983–988.
Smith P, Ballantyne B. The neuroanatomical basis for denervation of the urinary bladder following major pelvic surgery. Br J Surg. 2005;55(12):929–933.
Acknowledgements
We thank Prof. Ian Milsom, Department of Obstetrics and Gynaecology, Sahlgrenska Academy at Gothenburg University, Gothenburg, Sweden, for editing the manuscript and Håkan Lindkvist, Department of Mathematics and Mathematical Statistics, Umeå University, Umeå, Sweden, for initial statistical analyses and advice. We also thank the office of The Swedish National Quality Register for Gynecological Surgery (GynOp) for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by the Swedish Association of Local Authorities and Regions.
Conflicts of interest
None.
Ethical approval
Ethical approval was obtained from the Regional Ethics Review Board (the Ethics Committee at Sahlgrenska Academy, Gothenburg University, ref. no. 322-12, 18 June 2012).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pålsson, M., Stjerndahl, JH., Granåsen, G. et al. Patient-reported lower urinary tract symptoms after hysterectomy or hysteroscopy: a study from the Swedish Quality Register for Gynecological Surgery. Int Urogynecol J 28, 1341–1349 (2017). https://doi.org/10.1007/s00192-017-3268-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3268-9