Abstract
Introduction and hypothesis
Randomized controlled trials (RCTs) must comply with the strict rules of design and conduct and their reporting should reflect it. Our aim was to evaluate how the quality of RCT reporting in pelvic organ prolapse (POP) has evolved.
Methods
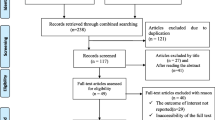
RCTs in POP published between 1997 and 2010 were retrieved through a PubMed search. The quality of reporting was assessed by applying the 2010 revised Consolidated Standards of Reporting Trials (CONSORT) statement. Appropriate statistical analysis was performed.
Results
Forty-one RCTs were identified for review. The implementation of randomization, recruitment, blinding, outcomes with effect size and precision, trial registration, and full protocol availability were reported in less than half of the trials. Comparing two periods (1997–2006 and 2007–2010), there was no improvement in the quality of reporting for any of the CONSORT criteria.
Conclusions
RCTs in POP are scarce. The quality of reporting is suboptimal in many aspects and has not improved in recent years.

Similar content being viewed by others
References
Begg C, Cho M, Eastwood S et al (1996) Improving the quality of reporting for randomized controlled trials, the CONSORT statement. JAMA 276:637–639
Altman DG, Schulz KF, Moher D et al (2001) The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134:663–694
Moher D, Hopewell S, Schulz KF et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. doi:10.1136/bmj.c869
Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG (2010) The quality of reports of randomised trials in 2000 and 2006: comparative study of articles indexed in Pubmed. BMJ. doi:10.1136/bmj.c723
Maher C, Feiner B, Baessler K, Adams EJ, Hagen S, Glazener CM (2010) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 4:CD004014
Karram MM (2009) “Evidence-based medicine” to support the surgical procedures we perform on patients with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 20:763–764
Chan AW, Altman DG (2005) Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 365:1159–1162
Kane RL, Wang J, Garrard J (2007) Reporting in randomized clinical trials improved after adoption of the CONSORT statement. J Clin Epidemiol 60:241–249
Plint AC, Moher D, Morrison A et al (2006) Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust 185:263–267
Schulz KF, Chalmers I, Grimes DA, Altman DG (1994) Assessing the quality of randomization from reports of controlled trials published in obstetrics and gynecology journals. JAMA 272:125–128
Scales CD Jr, Norris RD, Keitz SA et al (2007) A critical assessment of the quality of reporting of randomized, controlled trials in the urology literature. J Urol 177:1090–1095
Bump RC, Mattiasson A, Bø K et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Muir TW, Stepp KJ, Barber MD (2003) Adoption of pelvic organ prolapse classification system in peer-reviewed literature. Am J Obstet Gynecol 189:1632–1636
Treszezamsky AD, Rascoff L, Shahryarinejad A, Vardy MD (2010) Use of pelvic organ prolapse staging systems in published articles of selected specialized journals. Int Urogynecol J Pelvic Floor Dysfunct 21:359–363
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vítor Cavadas and Frederico Branco equally contributed to this article.
Rights and permissions
About this article
Cite this article
Cavadas, V., Branco, F., Carvalho, F.L. et al. The quality of reporting of randomized controlled trials in pelvic organ prolapse. Int Urogynecol J 22, 1117–1125 (2011). https://doi.org/10.1007/s00192-011-1426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-011-1426-z