Abstract
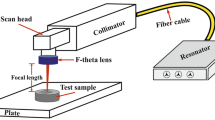
The surface of the commercial CoCr28Mo alloy was textured using a 20W pulsed fiber laser according to different beam scan strategies. Surface roughness and drop contact angle measurements made on the textured surface were used to evaluate the effects of process parameters. The surface topography was highly affected by both scan direction and beam overlaps named pulse-to-pulse overlap and scan overlap. As the beam overlap ratio increased, the amount of heat transferred to the unit area also increased, which caused more metal to evaporate in the interaction zone and form a more considerable amount of molten and re-solidified metal layer, resulting in a chaotic texture formation on the surface. The mean surface roughness values of textured surfaces for eighteen test samples ranged from 1 to 7 μm. These surface roughness values were in the micro-scale roughness value range. According to the results of the drop contact angles, the surface topography significantly changed the wettability. The test results were also analyzed by the Taguchi method considering signal-to-noise ratios and evaluated by analysis of variance. Considering the highest S/N values for each parameter, the optimum parameters combination for the laser engraving process was 45°/-45° for scan direction, 70 % for power, 1000 mm/s for scan speed, 50 kHz for frequency, 0.01 mm for hatch distance, and 75 ns for pulse duration.













Similar content being viewed by others
References
Sanan A, Haines SJ (1997) Repairing holes in the head: a history of cranioplasty. Neurosurgery 40(3):588–603. https://doi.org/10.1097/00006123-199703000-00033
Ben-Nissan B, Pezzotti G Bioceramics: an introduction. In: Eng Mater Biomed Appl pp 6-1–6-36. https://doi.org/10.1142/9789812562227_0006
Li Y, Yang C, Zhao H, Qu S, Li X, Li Y (2014) New developments of Ti-based alloys for biomedical applications. Materials Basel 7(3):1709–1800. https://doi.org/10.3390/ma7031709
Ahmad FN, Zuhailawati HJIJEM (2020) A brief review on the properties of titanium as a metallic biomaterials. Int J Electroactive Mater 8:63–67
Niinomi M, Nakai M, Hieda J (2012) Development of new metallic alloys for biomedical applications. Acta Biomater 8(11):3888–3903. https://doi.org/10.1016/j.actbio.2012.06.037
Gilbert JL (2017) 1.2 Electrochemical behavior of metals in the biological milieu. In: Ducheyne P (ed) Comprehensive Biomaterials II. Elsevier, Oxford, pp 19–49. https://doi.org/10.1016/B978-0-08-100691-7.00228-7
Narushima T, Ueda K, Alfirano (2015) Co-Cr alloys as effective metallic biomaterials. In: Niinomi M, Narushima T, Nakai M (eds) Advances in metallic biomaterials: tissues, materials and biological reactions. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 157–178. https://doi.org/10.1007/978-3-662-46836-4_7
Kaivosoja E, Tiainen VM, Takakubo Y, Rajchel B, Sobiecki J, Konttinen YT, Takagi M (2013) 7 - Materials used for hip and knee implants. In: Affatato S (ed) Wear of orthopaedic implants and artificial joints. Woodhead Publishing, pp 178–218. https://doi.org/10.1533/9780857096128.1.178
Goharian A, Abdullah MR (2017) Bioinert metals (stainless steel, titanium, cobalt chromium). In: Goharian A (ed) Trauma Plating Systems. Elsevier, pp 115–142. https://doi.org/10.1016/B978-0-12-804634-0.00007-0
Hailer NP, Garellick G, Kärrholm J (2010) Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop 81(1):34–41. https://doi.org/10.3109/17453671003685400
Naudie DDR, Ammeen DJ, Engh GA, Rorabeck CH (2007) Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg 15 (1). https://doi.org/10.5435/00124635-200701000-00006
Boyle KK, Nodzo SR, Ferraro JT, Augenblick DJ, Pavlesen S, Phillips MJ (2018) Uncemented vs cemented cruciate retaining total knee arthroplasty in patients with body mass index greater than 30. J Arthroplasty 33(4):1082–1088. https://doi.org/10.1016/j.arth.2017.11.043
Carlsson L, Röstlund T, Albrektsson B, Albrektsson T, Brånemark P-I (1986) Osseointegration of titanium implants. Acta Orthop Scand 57(4):285–289. https://doi.org/10.3109/17453678608994393
Liu X, Chu PK, Ding C (2004) Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng R Rep 47(3):49–121. https://doi.org/10.1016/j.mser.2004.11.001
Stenlund P, Kurosu S, Koizumi Y, Suska F, Matsumoto H, Chiba A, Palmquist A (2015) Osseointegration enhancement by Zr doping of Co-Cr-Mo implants fabricated by electron beam melting. Addit Manuf 6:6–15. https://doi.org/10.1016/j.addma.2015.02.002
Menci G, Demir AG, Waugh DG, Lawrence J, Previtali B (2019) Laser surface texturing of β-Ti alloy for orthopaedics: effect of different wavelengths and pulse durations. Appl Surf Sci 489:175-186. https://doi.org/10.1016/j.apsusc.2019.05.111
Hao L, Lawrence J, Li L (2005) Manipulation of the osteoblast response to a Ti–6Al–4V titanium alloy using a high power diode laser. Appl Surf Sci 247(1):602–606. https://doi.org/10.1016/j.apsusc.2005.01.165
Tiainen L, Abreu P, Buciumeanu M, Silva F, Gasik M, Serna Guerrero R, Carvalho O (2019) Novel laser surface texturing for improved primary stability of titanium implants. J Mech Behav Biomed Mater 98:26–39. https://doi.org/10.1016/j.jmbbm.2019.04.052
Kuroda K, Okido M (2017) Osteoconductivity of protein adsorbed titanium implants using hydrothermal treatment. Mater Sci Forum 879:1049–1052. https://doi.org/10.4028/www.scientific.net/MSF.879.1049
Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, Boyan BD (2014) A review on the wettability of dental implant surfaces II: biological and clinical aspects. Acta Biomater 10(7):2907–2918. https://doi.org/10.1016/j.actbio.2014.03.032
Brånemark R, Emanuelsson L, Palmquist A, Thomsen P (2011) Bone response to laser-induced micro- and nano-size titanium surface features. Nanomedicine 7(2):220–227. https://doi.org/10.1016/j.nano.2010.10.006
Coathup MJ, Blunn GW, Mirhosseini N, Erskine K, Liu Z, Garrod DR, Li L (2017) Controlled laser texturing of titanium results in reliable osteointegration. J Orthop Res 35(4):820–828. https://doi.org/10.1002/jor.23340
Wang Y, Zhang M, Li K, Hu J (2021) Study on the surface properties and biocompatibility of nanosecond laser patterned titanium alloy. Opt Laser Technol 139:106987. https://doi.org/10.1016/j.optlastec.2021.106987
Eghbali N, Naffakh-Moosavy H, Sadeghi Mohammadi S, Naderi-Manesh H (2021) The influence of laser frequency and groove distance on cell adhesion, cell viability, and antibacterial characteristics of Ti-6Al-4V dental implants treated by modern fiber engraving laser. Dent Mater 37(3):547–558. https://doi.org/10.1016/j.dental.2020.12.007
Wang Y, Yu Z, Li K, Hu J (2020) Effects of surface properties of titanium alloys modified by grinding, sandblasting and acidizing and nanosecond laser on cell proliferation and cytoskeleton. Appl Surf Sci 501:144279. https://doi.org/10.1016/j.apsusc.2019.144279
Jiao Y, Brousseau E, Nishio Ayre W, Gait-Carr E, Shen X, Wang X, Bigot S, Zhu H, He W (2021) In vitro cytocompatibility of a Zr-based metallic glass modified by laser surface texturing for potential implant applications. Appl Surf Sci 547:149194. https://doi.org/10.1016/j.apsusc.2021.149194
Cho S-A, Jung S-K (2003) A removal torque of the laser-treated titanium implants in rabbit tibia. Biomaterials 24(26):4859–4863. https://doi.org/10.1016/S0142-9612(03)00377-6
Shah FA, Johansson ML, Omar O, Simonsson H, Palmquist A, Thomsen P (2016) Laser-modified surface enhances osseointegration and biomechanical anchorage of commercially pure titanium implants for bone-anchored hearing systems. PloS One 11(6):e0157504. https://doi.org/10.1371/journal.pone.0157504
ISO 4287 (1998) Geometrical product specifications (GPS) – surface texture: profile method – terms, definitions and surface texture parameters. International Organisation for Standardization, Geneva
Zhao Z, Wan Y, Yu M, Wang H, Cai Y, Liu C, Zhang D (2021) Biocompability evaluation of micro textures coated with zinc oxide on Ti-6Al-4V treated by nanosecond laser. Surf Coat Technol 422:127453. https://doi.org/10.1016/j.surfcoat.2021.127453
Bizi-bandoki P, Valette S, Audouard E, Benayoun S (2013) Time dependency of the hydrophilicity and hydrophobicity of metallic alloys subjected to femtosecond laser irradiations. Appl Surf Sci 273:399–407. https://doi.org/10.1016/j.apsusc.2013.02.054
Boinovich LB, Emelyanenko AM, Emelyanenko KA, Domantovsky AG, Shiryaev AA (2016) Comment on “Nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications” by Duong V. Ta, Andrew Dunn, Thomas J. Wasley, Robert W. Kay, Jonathan Stringer, Patrick J. Smith, Colm Connaughton, Jonathan D. Shephard (Appl Surf Sci 357 (2015) 248–254). Appl Surf Sci 379:111–113. https://doi.org/10.1016/j.apsusc.2016.04.056
Drelich J, Chibowski E, Meng DD, Terpilowski K (2011) Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 7(21):9804–9828. https://doi.org/10.1039/C1SM05849E
Ijaola AO, Bamidele EA, Akisin CJ, Bello IT, Oyatobo AT, Abdulkareem A, Farayibi PK, Asmatulu E (2020) Wettability transition for laser textured surfaces: a comprehensive review. Surf Interfaces 21:100802. https://doi.org/10.1016/j.surfin.2020.100802
Samanta A, Wang Q, Shaw SK, Ding H (2020) Roles of chemistry modification for laser textured metal alloys to achieve extreme surface wetting behaviors. Mater Des 192:108744. https://doi.org/10.1016/j.matdes.2020.108744
Trdan U, Hočevar M, Gregorčič P (2017) Transition from superhydrophilic to superhydrophobic state of laser textured stainless steel surface and its effect on corrosion resistance. Corros Sci 123:21–26. https://doi.org/10.1016/j.corsci.2017.04.005
Teixidor D, Grzenda M, Bustillo A, Ciurana J (2015) Modeling pulsed laser micromachining of micro geometries using machine-learning techniques. J Intell Manuf 26(4):801–814. https://doi.org/10.1007/s10845-013-0835-x
Hribar L, Gregorčič P, Senegačnik M, Jezeršek M (2022) The influence of the processing parameters on the laser-ablation of stainless steel and brass during the engraving by nanosecond fiber laser. Nanomaterials 12(2):232. https://doi.org/10.3390/nano12020232
Banat D, Ganguly S, Meco S, Harrison P (2020) Application of high power pulsed nanosecond fibre lasers in processing ultra-thin aluminium foils. Opt Lasers Eng 129:106075. https://doi.org/10.1016/j.optlaseng.2020.106075
Assuncao E, Williams S (2013) Comparison of continuous wave and pulsed wave laser welding effects. Opt Lasers Eng 51(6):674–680. https://doi.org/10.1016/j.optlaseng.2013.01.007
Pardal G, Meco S, Dunn A, Williams S, Ganguly S, Hand DP, Wlodarczyk KL (2017) Laser spot welding of laser textured steel to aluminium. J Mater Process Technol 241:24–35. https://doi.org/10.1016/j.jmatprotec.2016.10.025
Zhou L, Pan M, Zhang Z, Diao Z, Peng X (2021) Enhancing osseointegration of TC4 alloy by surficial activation through biomineralization method. Front Bioeng Biotechnol 9:639835. https://doi.org/10.3389/fbioe.2021.639835
Wang Q, Zhou P, Liu S, Attarilar S, Ma RL-W, Zhong Y, Wang L (2020) Multi-scale surface treatments of titanium implants for rapid osseointegration: a review. Nanomaterials 10 (6):1244. https://doi.org/10.3390/nano10061244
Sarraf M, Rezvani Ghomi E, Alipour S, Ramakrishna S, Liana Sukiman N (2022) A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-des Manuf 5(2):371–395. https://doi.org/10.1007/s42242-021-00170-3
Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R (2016) Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater 31:425–434. https://doi.org/10.1016/j.actbio.2015.12.003
Gittens RA, Olivares-Navarrete R, Schwartz Z, Boyan BD (2014) Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater 10(8):3363–3371. https://doi.org/10.1016/j.actbio.2014.03.037
Bizi-Bandoki P, Benayoun S, Valette S, Beaugiraud B, Audouard E (2011) Modifications of roughness and wettability properties of metals induced by femtosecond laser treatment. Appl Surf Sci 257(12):5213–5218. https://doi.org/10.1016/j.apsusc.2010.12.089
Riveiro A, Maçon ALB, del Val J, Comesaña R, Pou J (2018) Laser surface texturing of polymers for biomedical applications. Front Phys 6:16. https://doi.org/10.3389/fphy.2018.00016
Kaiser JP, Bruinink A (2004) Investigating cell–material interactions by monitoring and analysing cell migration. J Mater Sci Mater Med 15(4):429–435. https://doi.org/10.1023/B:JMSM.0000021115.55254.a8
Pramanik D, Das S, Sarkar S, Debnath SK, Kuar AS, Mitra S (2019) Experimental investigation of fiber laser micro-marking on aluminum 6061 alloy. In: Sahoo P, Davim JP (eds) Advances in Mater Mech Ind Eng Sel Contrib First Int Conf Mech Eng Jadavpur University, Kolkata, India. Springer International Publishing, Cham, pp 273–294
Manninen M, Hirvimäki M, Poutiainen I, Salminen A (2015) Effect of pulse length on engraving efficiency in nanosecond pulsed laser engraving of stainless steel. Metall Mater Trans A Phys Metall Mater Sci 46(5):2129–2136. https://doi.org/10.1007/s11663-015-0415-x
Jiao Y, Brousseau E, Han Q, Zhu H, Bigot S (2019) Investigations in nanosecond laser micromachining on the Zr52.8Cu17.6Ni14.8Al9.9Ti4.9 bulk metallic glass: experimental and theoretical study. J Mater Process Technol 273:116232. https://doi.org/10.1016/j.jmatprotec.2019.05.013
Sandoval-Robles JA, Rodríguez CA, García-López E (2020) Laser surface texturing and electropolishing of CoCr and Ti6Al4V-ELI alloys for biomedical applications. Materials 13(22). https://doi.org/10.3390/ma13225203
Mineta S, Namba S, Yoneda T, Ueda K, Narushima T (2010) Carbide formation and dissolution in biomedical Co-Cr-Mo alloys with different carbon contents during solution treatment. Metall Mater Trans A Phys Metall Mater Sci 41(8):2129–2138. https://doi.org/10.1007/s11661-010-0227-1
Qiao J, Zhu L-n, Yue W, Fu Z-q, Kang J-j, Wang C-b (2018) The effect of attributes of micro-shapes of laser surface texture on the wettability of WC-CrCo metal ceramic coatings. Surf Coat Technol 334:429–437. https://doi.org/10.1016/j.surfcoat.2017.12.001
Fadeeva E, Schlie-Wolter S, Chichkov BN, Paasche G, Lenarz T (2016) 5 - Structuring of biomaterial surfaces with ultrashort pulsed laser radiation. In: Vilar R (ed) Laser Surface Modification of Biomaterials. Woodhead Publishing, pp 145–172. https://doi.org/10.1016/B978-0-08-100883-6.00005-8
Gunenthiram V, Peyre P, Schneider M, Dal M, Coste F, Koutiri I, Fabbro R (2018) Experimental analysis of spatter generation and melt-pool behavior during the powder bed laser beam melting process. J Mater Process Technol 251:376–386. https://doi.org/10.1016/j.jmatprotec.2017.08.012
Strickstrock M, Rothe H, Grohmann S, Hildebrand G, Zylla IM, Liefeith K (2017) Influence of surface roughness of dental zirconia implants on their mechanical stability, cell behavior and osseointegration. BioNanoMat 18:1–2. https://doi.org/10.1515/bnm-2016-0013
Wennerberg A, Albrektsson T (2009) Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res 20:172–184. https://doi.org/10.1111/j.1600-0501.2009.01775.x
Cruz MB, Silva N, Marques JF, Mata A, Silva FS, Carames J (2022) Biomimetic implant surfaces and their role in biological integration-a concise review. Biomimetics (Basel) 7(2):74. https://doi.org/10.3390/biomimetics7020074
Elias CN, Oshida Y, Lima JHC, Muller CA (2008) Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J Mech Behav Biomed Mater 1(3):234–242. https://doi.org/10.1016/j.jmbbm.2007.12.002
Acknowledgements
The present study was supported by the Dokuz Eylul University under project no. 2021.KB.FEN.043. The authors would like to acknowledge this financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No other journals are presently reviewing the manuscript, nor has it been submitted to any other journals. The provided content is unique and has not been published anywhere, regardless of the format or language used.
Consent to participate
Every author has made a conscious decision to freely take part in this research project.
Consent for publication
All authors consent willingly to the paper’s publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kasman, Ş., Uçar, İ.C. & Ozan, S. Investigation of laser surface texturing parameters of biomedical grade Co-Cr-Mo alloy. Int J Adv Manuf Technol 125, 4271–4291 (2023). https://doi.org/10.1007/s00170-023-10959-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00170-023-10959-4