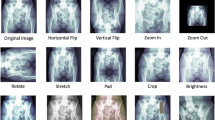
Abstract
Deep learning has a profound impact on daily life. As Orthopedics makes use of this rapid escalation in technology, Orthopedic surgeons will need to take leadership roles on deep learning projects. Moreover, surgeons must possess an understanding of what is necessary to design and implement deep learning-based project pipelines. This review provides a practical guide for the Orthopedic surgeon to understand the steps needed to design, develop, and deploy a deep learning pipeline for clinical applications. A detailed description of the processes involved in defining the problem, building the team, acquiring and curating the data, labeling the data, establishing the ground truth, pre-processing and augmenting the data, and selecting the required hardware is provided. In addition, an overview of unique considerations involved in the training and evaluation of deep learning models is provided. This review strives to provide surgeons with the groundwork needed to identify gaps in the clinical landscape that deep learning models may be able to fill and equips them with the knowledge needed to lead an interdisciplinary team through the process of creating novel deep-learning-based solutions to fill those gaps.



Similar content being viewed by others
References
3D Slicer Image Computing Platform. https://www.slicer.org. Accessed 10–10–2022.
Aryanto KY, Oudkerk M, van Ooijen PM (2015) Free DICOM de-identification tools in clinical research: functioning and safety of patient privacy. Eur Radiol 25:3685–3695
Chaudhari AS, Kogan F, Pedoia V, Majumdar S, Gold GE, Hargreaves BA (2020) Rapid knee MRI acquisition and analysis techniques for imaging osteoarthritis. J Magn Reson Imaging 52:1321–1339
de Mello RAF, Ma YJ, Ashir A, Jerban S, Hoenecke H, Carl M et al (2020) Three-dimensional zero echo time magnetic resonance imaging versus 3-dimensional computed tomography for glenoid bone assessment. Arthroscopy 36:2391–2400
DICOM Library. https://www.dicomlibrary.com/. Accessed 10–10–2022.
DICOM Standards Committee. DICOM PS3.15 2022c—Security and System Management Profiles. 2022; https://dicom.nema.org/medical/dicom/current/output/html/part15.html#chapter_E. Accessed 10–10–2022.
Goodfellow I, Bengio Y, Courville A. 2016 Deep Learning. MIT Press.
He K, Gkioxari G, Dollár P, Girshick R. Mask R-CNN. 2017; https://arxiv.org/abs/1703.06870;https://doi.org/10.48550/ARXIV.1703.06870.
Hill BG, Krogue JD, Jevsevar DS, Schilling PL (2022) Deep learning and imaging for the orthopaedic surgeon: how machines “read” radiographs. J Bone Joint Surg Am 104:1675–1686
Horng MH, Kuok CP, Fu MJ, Lin CJ, Sun YN (2019) Cobb Angle Measurement of spine from X-ray images using convolutional neural network. Comput Math Methods Med 2019:6357171
ImFusion Labels. https://www.imfusion.com/products/imfusion-labels. Accessed 10–10–2022.
itk SNAP. http://www.itksnap.org/pmwiki/pmwiki.php. Accessed 10–10–2022.
Karnuta JM, Haeberle HS, Luu BC, Roth AL, Molloy RM, Nystrom LM et al (2021) Artificial intelligence to identify arthroplasty implants from radiographs of the hip. J Arthroplasty 36:S290–S294
Kijowski R, Liu F, Caliva F, Pedoia V (2020) Deep learning for lesion detection, progression, and prediction of musculoskeletal disease. J Magn Reson Imaging 52:1607–1619
Ko S, Pareek A, Ro DH, Lu Y, Camp CL, Martin RK et al (2022) Artificial intelligence in orthopedics: three strategies for deep learning with orthopedic specific imaging. Knee Surg Sports Traumatol Arthrosc 30:758–761
Krogue JD, Cheng KV, Hwang KM, Toogood P, Meinberg EG, Geiger EJ et al (2020) Automatic hip fracture identification and functional subclassification with deep learning. Radiol Artif Intell 2:e190023
Lansdown DA, Cvetanovich GL, Verma NN, Cole BJ, Bach BR, Nicholson G et al (2019) Automated 3-dimensional magnetic resonance imaging allows for accurate evaluation of glenoid bone loss compared with 3-dimensional computed tomography. Arthroscopy 35:734–740
Lindsey R, Daluiski A, Chopra S, Lachapelle A, Mozer M, Sicular S et al (2018) Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci USA 115:11591–11596
Medical imaging resource center radiological society of North America association. https://mircwiki.rsna.org/index.php?title=Main_Page#MIRC_CTP. Accessed 10–10–2022.
Miotto R, Wang F, Wang S, Jiang X, Dudley JT (2018) Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform 19:1236–1246
Norman B, Pedoia V, Noworolski A, Link TM, Majumdar S (2019) Applying densely connected convolutional neural networks for staging osteoarthritis severity from plain radiographs. J Digit Imaging 32:471–477
Pesapane F, Volonté C, Codari M, Sardanelli F (2018) Artificial intelligence as a medical device in radiology: ethical and regulatory issues in Europe and the United States. Insights Imaging 9:745–753
Python Pydicom. https://pydicom.github.io/pydicom/stable/. Accessed 10–10–2022.
QuPath quantitative pathology & bioimage analysis. https://qupath.github.io. Accessed 10–10–2022.
Rouzrokh P, Khosravi B, Johnson QJ, Faghani S, Vera Garcia DV, Erickson BJ et al (2022) Applying deep learning to establish a total hip arthroplasty radiography registry: a stepwise approach. J Bone Joint Surg Am 104:1649–1658
Shah RF, Bini SA, Martinez AM, Pedoia V, Vail TP (2020) Incremental inputs improve the automated detection of implant loosening using machine-learning algorithms. Bone Joint J. 102-B:101–106
The medical imaging interaction toolkit (MITK). https://www.mitk.org/wiki/The_Medical_Imaging_Interaction_Toolkit_(MITK). Accessed 10–10–2022.
Wolf I, Vetter M, Wegner I, Böttger T, Nolden M, Schöbinger M et al (2005) The medical imaging interaction toolkit. Med Image Anal 9:594–604
Wu L, Yang F, Wu Y, Cui J, Shi H, Bin S (2022) A deep learning framework for diagnosing periprosthetic joint infections using X-ray images: a discovery and validation study. J Arthroplasty. https://doi.org/10.1016/j.arth.2022.08.037
Zheng Q, Shellikeri S, Huang H, Hwang M, Sze RW (2020) Deep learning measurement of leg length discrepancy in children based on radiographs. Radiology 296:152–158
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oeding, J.F., Williams, R.J., Nwachukwu, B.U. et al. A practical guide to the development and deployment of deep learning models for the Orthopedic surgeon: part I. Knee Surg Sports Traumatol Arthrosc 31, 382–389 (2023). https://doi.org/10.1007/s00167-022-07239-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-07239-1