Abstract
Worldwide interest in artificial intelligence (AI) applications is growing rapidly. In medicine, devices based on machine/deep learning have proliferated, especially for image analysis, presaging new significant challenges for the utility of AI in healthcare. This inevitably raises numerous legal and ethical questions. In this paper we analyse the state of AI regulation in the context of medical device development, and strategies to make AI applications safe and useful in the future. We analyse the legal framework regulating medical devices and data protection in Europe and in the United States, assessing developments that are currently taking place. The European Union (EU) is reforming these fields with new legislation (General Data Protection Regulation [GDPR], Cybersecurity Directive, Medical Devices Regulation, In Vitro Diagnostic Medical Device Regulation). This reform is gradual, but it has now made its first impact, with the GDPR and the Cybersecurity Directive having taken effect in May, 2018. As regards the United States (U.S.), the regulatory scene is predominantly controlled by the Food and Drug Administration. This paper considers issues of accountability, both legal and ethical. The processes of medical device decision-making are largely unpredictable, therefore holding the creators accountable for it clearly raises concerns. There is a lot that can be done in order to regulate AI applications. If this is done properly and timely, the potentiality of AI based technology, in radiology as well as in other fields, will be invaluable.
Teaching Points
• AI applications are medical devices supporting detection/diagnosis, work-flow, cost-effectiveness.
• Regulations for safety, privacy protection, and ethical use of sensitive information are needed.
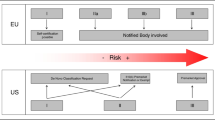
• EU and U.S. have different approaches for approving and regulating new medical devices.
• EU laws consider cyberattacks, incidents (notification and minimisation), and service continuity.
• U.S. laws ask for opt-in data processing and use as well as for clear consumer consent.
Similar content being viewed by others
Introduction
Artificial intelligence (AI) is a branch of computer science dedicated to the creation of systems that perform tasks that usually require human intelligence, with different technical approaches [1]. The term AI is used to describe computer systems that mimic cognitive functions, such as learning and problem-solving [2]. These systems are currently based on artificial neural networks, which are flexible mathematical models using multiple algorithms to identify complex nonlinear relationships within large datasets [3], nowadays known as big data. Huge amounts of information can be retrieved from electronic archives, which could hardly be analysed, searched, or interpreted using traditional data-processing methods. Big data includes data from mobile phone applications, wearable technology, social media, environmental and lifestyle-related factors, socio-demographics, omic data (e.g., genomics, metabolomics, proteomics, radiomics), and data from standardised electronic health records or precision medicine platforms [4].
Among the health data, medical images such as those obtained through x-ray, computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound examinations constitute one of the most interesting types of data, with high potential for research and clinical applications. Among other things, these data could help in improving the automatic detection of diseases (while minimising human errors) [5], creating study protocols [6], improving image quality and decreasing radiation dose [7], decreasing MRI scanner time [8], optimising staffing and scanner utilisation, thereby reducing costs [9], and offering the possibility of performing expensive and time-consuming screening programs in countries that otherwise cannot afford them [10, 11].
Machine learning is a term introduced by Arthur Samuel in 1959 [12] to define a field of AI where computers learn automatically from data accumulation; it has been extensively applied for big data analysis [13]. Within the machine learning domain, deep learning has emerged as a highly promising approach for image processing [14]. Unlike software, which requires specific instructions to complete a task, deep learning allows the system to recognise patterns independently and make predictions [15].
The deep learning modelling of big data exerts major influences on modern society, from web searching to financial technology banking, from facial recognition to medical decision support [16, 17]. The application of AI will change the working methodologies of many professionals, including physicians, and this will happen in radiology more quickly than in other medical fields.
Radiologists, having been pioneers of the digital era in medicine, can now accept AI as a new partner in their profession, along with a potential for a higher role of radiology in healthcare, as we have shown in a previous article [18]. However, there are challenges to AI applications in medicine and specifically in radiology that depend not on physicians, but on regulatory institutions and governments [19]. In this paper we analyse the issues related to recent policy initiatives to regulate AI in the context of medical device development, the accountability of AI under the law, and the implications of data protection and cybersecurity. The main differences between the policy of the European Union (EU) and that of the United States (U.S.) will be considered.
Challenges of AI in medicine and radiology
The advancement in algorithm development combined with the ease of accessing computational resources currently allows AI applications to be used in medical decision-making tasks with promising results [14, 17]. The utilization of AI techniques in radiology represents a relevant topic for research teams. Deep learning algorithms are currently used in mammography for breast cancer detection [20], in CT for colon cancer diagnosis [21], in chest radiographs for the detection of pulmonary nodules [22], in MRI for brain tumour segmentation [23] and for diagnosis of neurologic disorders, such as Alzheimer disease [24].
The widespread enthusiasm and dynamism regarding the development of software based on AI in radiology is shown by the highly positive trend of publications in the literature in the last 10 years: from 100 to 150 to 700–800 yearly [18].
However, some ethical challenges are straightforward and need to be guarded against. Notably, one of them is the concern that algorithms may mirror human biases in decision making. Since healthcare delivery already varies by ethnicity, it’s possible that some ethnical biases could inadvertently be built into medical algorithms. AI applications introduced in nonmedical fields have already been shown to make problematic decisions that reflect biases inherent in the data used to train them [25]. Recently, a program designed to aid judges in sentencing by predicting an offender’s risk of recidivism have shown an unnerving propensity for discrimination [26]. Similarly, an algorithm designed to predict outcomes from genetic findings may be biased if there are no genetic studies in certain populations [25].
The intent behind the design of AI also needs to be considered, because some devices can be programmed to perform in unethical ways. For example, Uber’s algorithm tool ‘Greyball’ was designed to predict which ride hailers might be undercover law-enforcement officers, thereby allowing the company to identify and circumvent local laws [25]. Also, Volkswagen’s algorithm allowed vehicles to pass emissions tests by reducing their nitrogen oxide emissions when they were being tested [25]. Analogously, private-sector designers who create AI algorithms for clinical use could be subject to similar temptations, programming AI systems to guide users toward clinical actions that would generate increased profits for their purchasers (such as by recommending drugs, tests, or medical devices in which they hold a stake or by altering referral patterns) but not necessarily reflect better care [25]. These examples show the urgency for serious regulations and policy initiatives about the use of AI, especially in complicated care practices in which the correct diagnosis of a disease and the best management of a patient can be controversial.
Regulatory issues and policy initiatives
AI systems do more than process information and assist officials to make decisions of consequence. Many systems — such as the software that controls an airplane on autopilot or a fully driverless car — exert direct and physical control over objects in the human environment [27]. Other systems, including medical and radiological devices, provide sensitive services that, when performed by physicians, require training and certification [15, 19, 27,27,29]. These applications raise additional questions concerning the standards to which AI systems are held and the procedures and techniques available to ensure those standards are being met [30]. What about technology under development today, such as autonomous imaging readers, whose very value turns on bringing skills into an environment where no one has them? And how do we think about systems that purport to dispense health advice, which requires adherence to complex fiduciary and other duties pegged to human judgement [31]?
Since unambiguity is one of the pillars of any legislation [32], the first aspect policymakers need to address when regulating any new field is definitions. This aspect becomes an actual issue with AI, a term that itself contains ambiguities: what is intelligence? What human abilities are to be considered the milestones that the AI systems need to accomplish? The AI’s lack of a stable, unanimous definition or instantiation complicates efforts to develop an appropriate policy infrastructure. Moreover, we might question the utility of the word policy in describing societal efforts to channel AI in the public interest. There are other terms in circulation. For instance, governance instead of policy: a new initiative anchored by the Media Lab of the Massachsetts Institute of Technology and the Berkman Klein Center for Internet and Society of the Harvard University refers to itself as the ‘Ethics and Governance of Artificial Intelligence Fund’ [33]. Anyway, we need a policy for the governance of AI in medicine (and radiology).
The second issue for policymakers is whether to consider AI software used in healthcare as a medical device for legislave purposes. Both the EU and the U.S. have their own criteria for identifying healthcare and medical devices, although both definitions share a purpose-based approach.
We will consider below what medical devices are and how legislation differs in these two geopolitical areas. However, it is interesting to point out that not all AI programs used in healthcare will be deemed to be medical devices. For example, as Tsang et al. [34] pointed out, “programs that analyse large amounts of data to develop knowledge about a disease or condition, rather than to decide on treatment options for an individual patient, may not necessarily be considered as having a medical purpose, and hence as a medical device”. It is the basic distinction between those research programs that enhance medical knowledge from those that promote changes in healthcare. How will the latter systems be regulated?
According to Thierer et al. [35] there are two main approaches that policymakers can use when regulating AI systems. One is the precautionary principle approach, which imposes some limits or sometimes outright bans on certain applications due to their potential risks. This means that these applications are never tested because of what could happen in the worst-case scenarios. The other approach is the so-called permissionless innovation approach, which allows experimentation to proceed freely, and the issues that do arise are addressed as they emerge. In 2016, Scherer [36] distinguished, instead, between ex ante and ex post regulation. Ex ante regulation is pre-emptive and tries to foresee the risks, similarly to the precautionary principle [35], while ex post regulation is retrospective and focuses more on providing a remedy to harm that actually occurred [37], and it is similar to the permissionless innovation approach mentioned above [35].
On the one hand, ex post regulation is hindered by the autonomous nature of AI systems, which evolve and change constantly according to their experiences and learning, in an unforeseeable way [38]. On the other hand, ex ante regulation is obstructed by AI applications discreetness (their development requires little physical infrastructure), discreteness (their components can be designed by different subjects, for different purposes and without actual coordination), diffuseness (these subjects can be dispersed geographically and yet collaborate on the same project), and opacity (it can be difficult for outside observers to identify and understand all the features of an AI system) [36].
Finally, a further issue for policymakers is time. Nowadays, companies understand the potential of machine/deep learning and are continuously collecting new types of data to analyse and exploit [39]. In an environment like the technology world and AI, which changes quickly and unpredictably, regulations need to be timely to be relevant.
These premises explain why regulation of medical devices is so controversial and subject to the vagaries of guidelines and subjective interpretations by the authorities. We consider below the regulatory minefield and the circumstances in which a software is regulated as a medical device in the EU and in the U.S.
Generally, while in the U.S. AI the technology sector prospered in a permissionless innovation policy environment, in the EU decision-makers adopted a different policy for this revolutionary technological branch [35]. Certainly, swifter approval of AI medical devices helps generate revenue for manufacturers, and physicians may benefit from having more tools at their disposal. But the final goal of bringing new devices to market should be to improve prevention, diagnosis, treatment, prognosis of diseases with a potential positive impact on patient outcome. Therefore, systems for approving new medical devices must provide pathways to market for important innovations while also ensuring that patients are adequately protected. To achieve these goals, the EU and the U.S. use different approaches [40].
The EU approach
In the EU, the definition of medical device is provided by Article 1(2) of Directive 93/42/EEC [41]: the term medical device is applied to any instrument or other tool, including any kind of software, intended by the manufacturer to be used for human beings for the purpose, among others, of diagnosis, prevention, monitoring, treatment, or alleviation of disease. This definition has been endorsed by the MEDDEVs, non-legally binding guidelines drafted by the European Commission to guide stakeholders in complying with legislation related to medical devices [42].
The European regulatory regime currently in force flows from three directives on medical devices [41, 43, 44] and it requires manufacturers to ensure that the devices they produce are fit for their intended purpose. This means that they must comply with a number of essential requirements set out by the directives themselves. Depending on the risk classification of the device, whether the essential requirements have been met can be assessed either by the manufacturer or by a notified body, which is an independent accredited certification organisation appointed by the competent authorities of EU Member States.
This regulatory framework has been reformed by the new Medical Devices Regulation (MDR) [45] and the new In Vitro Diagnostic Medical Device Regulation (IVDR) [46] (Table 1). Both came into force on 25 May 2017, however the MDR will apply from 26 May 2020 while the IVDR will apply from 26 May 2022. Because they are regulations, as opposed to directives, once they apply they do so directly, without the need for the governments of EU member states to pass legislation to implement their scope [47] (Table 2). This reform originated from the awareness that the existing directives, created in the 1990s [41, 43, 44], are not fit to deal with new, evolving technologies, including AI systems, and from the identification of some flaws of this regulatory system, for example lack of control on notified bodies.
Some of the main characteristics of this reform will be: extended scope to include a wider range of products, extended liability in relation to defective products, strengthening of requirements for clinical data and traceability of the devices, more rigorous monitoring of notified bodies, and improved transparency through making information relating to medical devices available to the public [48].
The U.S. approach
In the U.S., regulatory approval allowing machines to do the work of trained radiologists is a major obstacle still unsolved. The amount of testing and effort necessary to secure clearance from the U.S. Food and Drug Administration (FDA) for allowing machines to provide primary interpretations of imaging studies without a radiologist would be overwhelming.
At the end of 2016, the 21st Century Cures Act [49] clarified the scope of FDA regulatory jurisdiction over software used in healthcare, specifying that a medical device is an instrument or other tool “intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or intended to affect the structure or any function of the body of man or other animals” [50]. There are some other factors narrowing the definition, but this is the most important for the purposes of this paper.
Every AI system falling within this definition will be regulated by the FDA, as provided by the Federal Food, Drug and Cosmetic Act [34] (Table 3). The FDA categorises the medical devices into three classes, according to their uses and risks, and regulates them accordingly. The higher the risk, the stricter the control. Class III is the category which includes the devices involving the greatest risk.
The black box nature and the rapid growth of machine/deep learning applications will make it difficult for the FDA to approve in a timely fashion all the new medical devices that are continuously being developed, given the volume and the complex nature of testing and verification involved. An example was the introduction of computer-assisted detection software for mammography in 1998 [51], which took many years and extensive lobbying to obtain clearance from the FDA to be used as a second screening reader [52]. Compared to computer-aided detection systems, FDA clearance is even harder to be obtained for an AI system that does not need radiologist’s supervision and cannot be compared to predicated medical devices used as replacements for radiologists. For this reason, developers nowadays present AI systems as aids tools for radiologists rather than as tools that substitutes for them [53]. This is not only a legal issue: it implies a relevant discussion about ethical responsibility, as we will see below.
Data protection and cybersecurity implications
After the recent ferment about the Cambridge Analytica/Facebook scandal with personal data misuse [54], an ongoing debate about balance between privacy and better user experience (achieved via usage of personal data) is developing, especially when it comes to sensitive data such as medical information. The concept of circulating enormous amounts of confidential information in vast numbers of copies between many unregulated companies is increasingly insane and risky. Therefore, in the last decade personal data regulation is increasing and privacy concerns are growing [55].
However, we still need data as an integral part of technology development, especially for AI. As we discussed above, deep learning algorithms require a huge amount of data and powerful computers, and they usually take a long time to be trained because of the many parameters under consideration [19, 28]. The lack of well-annotated big datasets for training AI algorithms is a key obstacle to a large introduction of these systems in radiology [3, 13, 56]. Access to big data of medical images is needed to provide training material to AI devices, so that they can learn to recognise imaging abnormalities [13, 57]. One of the problems is that sensitive data might either be harvested illicitly or collected from unknown sources [58] because of the lack of unique and clear regulations [59].
In the era of electronic medical records, AI complicates an already complex cybersecurity landscape [60]: the concept of confidentiality requires that a physician withholds information from the medical record in order to keep it truly confidential [25]. Once a clinical decision based on AI is integrated into clinical care, withholding information from electronic records will become increasingly difficult, since patients whose data are not recorded cannot benefit from AI analyses [25]. The implementation of deep learning systems will therefore require a reimagining of confidentiality and other core tenets of medical ethics. Before using government over-regulation, we need to face the data protection and cybersecurity implications technologically. Data protection can no longer rely on current technologies that allow spreading of personal data and require data sharing at a large and uncontrolled scale [61].
In radiology, and in medicine in general, AI medical devices should use deep learning to provide data about patients without requiring their personally identifiable information in exchange. Currently, the use of AI raises two issues relating to the data collected by the devices. On the one hand, data must be protected from the same bodies collecting them. On the other hand, the same data are threatened by cyberattacks to these bodies as well as to the devices themselves.
Data protection in the EU
Because of these threats, European regulators have decided to update the legislation concerning data protection and cybersecurity (Table 4). The General Data Protection Regulation (GDPR) applies from 24 May 2018, substituting the European legal framework for data protection as set out by Directive 95/46/EC [62]. According to the GDPR, all data processing and use should be opt-in, and consumer consent for data use should be clear. In general, the GDPR completely prohibits current data marketing based on third-party non opt-in personal data. The GDPR is a more suitable instrument to regulate AI because it has an extended territorial scope and wider rights for data subjects. For instance, enhanced notification requirements (under Article 33, personal data breaches must be notified to the supervising authority within 72 h), and rights to compensation for material or non-material damage and additional liability for data controllers and processors (Article 88):Footnote 1 [63].
Again, because it is a regulation, the GDPR applies directly, and institutions have to comply with it starting from 24 May 2018 (which is why 2 years have been allowed for implementation since its enforcement on 24 May 2016).
In addition, the EU adopted the Cybersecurity Directive [64], which had to be implemented by the member states by 10 May 2018. This sets out a number of requirements for EU member states which aim to prevent cyberattacks and keep their consequences under control. Among other things, member states are required to ensure that operators of essential services take appropriate measures to prevent and minimise the impact of incidents and to preserve service continuity (Articles 14(2) and 16(2)), and to ensure that supervisory authorities are notified of incidents without undue delay (Articles 14(3) and 16(3)) [64].
Data protection in the U.S.
While in the U.S. government regulation is less strict, cases like Cambridge Analytica/Facebook should remind the government that actions should be taken, and that the behaviour of companies needs changes.
The Health Insurance Portability and Accountability Act (HIPAA) is a compliance focus for health information concerns [34]. This act elaborates rules requiring, among other things, the formulation of policies and the setup of training systems for those who have access to sensitive data [34] (Table 5). Moreover, HIPAA does not hinder the action of individual states, where it protects further the individual’s right to privacy.
Cybersecurity is dealt with by the FDA, which requires manufacturers to report only a limited number of risks their devices present and actions taken to minimise the vulnerability [34] (Table 5). The black box features of deep learning and the lack of transparency regarding how results are obtained have thorny legal implications [58]. In some cases, even the designers do not completely understand how AI algorithms process the data [58], so that the lack of transparency is, basically, a lack of knowledge. Considering the current amount of data collected, and that with an increased presence of AI applications this can only grow, regulatory actions regarding cybersecurity will face continuous challenges.
Accountability and responsibility: an “internal evidence” from AI in the frame of evidence-based medicine?
Alongside the actual regulation and data protection, there are other legal implications of AI and its use, whether in healthcare or elsewhere. One of these is accountability. As soon as AI starts making autonomous decisions about diagnoses and treatments, moving beyond its role as merely a support tool, a problem arises as to whether its developer can be held accountable for its decisions. The first question is: who will be sued if an AI-based device makes a mistake?
Errors in AI appear mainly when confounding factors are correlated with pathologic entities in the training datasets rather than actual signs of disease. When AI devices decide, their decision is based on the collected data, the algorithms they are based on, and what they learnt since their creation. The reason their decisions are unpredictable is twofold [36]. On the one hand, AI devices, even if they mimic human brain neural networks, think differently than humans. Better, they think more quickly and accurately [38, 56, 58]. There is a huge number of possibilities in every given situation, and humans are unable to process all of these and consider them in order to make a decision. We consider only what is more obvious for our brains, while AI systems can consider every potential scenario and every consideration [2, 3, 15, 29, 39, 56, 65]. Because of this, where faced with a decision to make, we do not share a common basis with AI devices. Therefore, we are unable to predict what they will decide in a given set of circumstances. On the other hand, AI systems are designed to learn from their genuine experiences, and these are by their very nature unpredictable. Because it is not possible to foresee what experiences a system will encounter, neither is it possible to foresee how the system will develop. For these reasons, it is worth considering, when something ‘goes wrong’ following a decision made by an AI application, whether the device itself or its designer/builder is to be considered at fault. Will the designer be deemed negligent for not having foreseen what we have described as unforeseeable? Or for having left the possibility for development of the AI-device that would bring it to that decision?
AI will play an increasingly important part over the next years in the relationship between doctors and patients [15, 19, 35, 53, 66] and it will need to be bound by core ethical principles, such as beneficence and respect for patients, which have guided clinicians during the history of medicine [25]. We should remember, indeed, that the radiologist, as a physician, is much more than simply an interpreter of images. The duties of a practicing radiologist also include communication of findings, quality assurance, quality improvement, education, interventional radiology procedures, policy-making, and many more tasks that cannot be performed by computer programs [2]. The ability to provide a nuanced interpretation for complex findings, medical judgement, and wisdom of an experienced radiologist is difficult to quantify and even more difficult to simulate with AI systems [15].
Given the evolving complexity of AI technology, it is almost inevitable that some of its inner workings will appear to be a ‘black box’ to all but the very few who can comprehend how they work [58]. But that does not mean accountability is out of the question.
Importantly, in a broader theoretical framework, we should distinguish between data analysis (in this case, the AI-device output) and decision making. In the context of evidence-based medicine [67], the best external evidence (data from high-quality studies and meta-analysis, guidelines from governmental bodies and medical societies) has to be combined with patients’ preferences and values by using our personal medical/radiological expertise. We could say that, introducing AI in radiology, we have also a kind of internal evidence coming from the application of AI to the patients’ imaging procedure(s). One can argue that AI might, at least in the future, be able to do this job instead of physicians. However, when patients’ preferences and values play a non-negligible role (and hopefully this factor will not reduce its weight in the future), human interaction and empathy by a physician/radiologist and a patient remain a fundamental dimension. Indeed, AI will allow radiologists to have more time to communicate with patients and to confer with colleagues in multidisciplinary teams, as they will be less busy doing routine and monotonous tasks that can be effectively performed by computers [53].
Accountability for an AI output may be also a simple matter for insurance purposes. Ethical and legal responsibility for decision making will remain in the hand (better, in the mind) of the natural intelligence of physicians. From this viewpoint, it’s probable that multidisciplinary boards will take the responsibility in difficult cases, considering the information AI provides as relevant but not always conclusive.
Conclusions
While the application of AI in medicine and radiology has several challenges to face, we should accept the fact that we need it. We need all these technologies and devices that rely on sensitive data to improve patient care and treatment. We believe that challenges such as the new policy initiatives, the regulation of data protection and cybersecurity, the debate about the unusual accountability and responsibility issues, the questions about the fiduciary relationship between patients and AI medical systems will have to be addressed as soon as possible.
AI-based devices could be built to reflect the ethical standards that have guided other actors in healthcare and could be held to those standards. A key step will be determining how to ensure that they are, whether by means of combination of policy enactment and programming approaches.
A good application of AI may be powerful, helpful, and valuable. On the contrary, a bad or unethical use of this cutting-edge technology may be dangerous, and patients, physicians (radiologists), and regulatory authorities must work together to prevent this [57]. In a spirit of good cooperation, we must find a balance that provides security, privacy protection, and ethical use of sensitive information to ensure both humane and regulated (and, therefore, responsible) management of the patients.
Notes
Data controllers are people who determine the purposes for which and the way any personal data are processed, while a data processor is the person who actually processes the data for a data controller.
Abbreviations
- CT:
-
Computed tomography
- EU:
-
European Union
- FDA:
-
Food and drug administration
- GDPR:
-
General data protection regulation
- HIPAA:
-
Health insurance portability and accountability act
- IVDR:
-
In vitro diagnostic medical device regulation
- MDR:
-
Medical devices regulation
- MRI:
-
Magnetic resonance imaging
- U.S.:
-
United States
References
Chartrand G, Cheng PM, Vorontsov E et al (2017) Deep learning: a primer for radiologists. Radiographics 37:2113–2131
Russell S, Bohannon J (2015) Artificial intelligence. Fears of an AI pioneer. Science 349:252
Miller DD, Brown EW (2018) Artificial intelligence in medical practice: the question to the answer? Am J Med 131:129–133
Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T (2017) Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol 69:2657–2664
Jha S (2016) Will computers replace radiologists? Available via https://www.medscape.com/viewarticle/863127
Sachs PB, Gassert G, Cain M, Rubinstein D, Davey M, Decoteau D (2013) Imaging study protocol selection in the electronic medical record. J Am Coll Radiol 10:220–222
Chen H, Zhang Y, Zhang W et al (2017) Low-dose CT via convolutional neural network. Biomed Opt Express 8:679–694
Golkov V, Dosovitskiy A, Sperl JI et al (2016) Q-space deep learning: twelve-fold shorter and model-free diffusion MRI scans. IEEE Trans Med Imaging 35:1344–1351
Lakhani P, Prater AB, Hutson RK et al (2018) Machine learning in radiology: applications beyond image interpretation. J Am Coll Radiol 15:350–359
Mayo RC, Leung J (2017) Artificial intelligence and deep learning - Radiology's next frontier? Clin Imaging 49:87–88
Jha S, Topol EJ (2016) Adapting to artificial intelligence: radiologists and pathologists as information specialists. JAMA 316:2353–2354
Samuel AL (1959) Some studies in machine learning using the game of checkers. IBM J Res Dev 3:210–229
Lee JG, Jun S, Cho YW et al (2017) Deep learning in medical imaging: general overview. Korean J Radiol 18:570–584
King BF Jr (2017) Guest editorial: discovery and artificial intelligence. AJR Am J Roentgenol 209:1189–1190
King BF Jr (2018) Artificial intelligence and radiology: what will the future hold? J Am Coll Radiol 15:501–503
Obermeyer Z, Emanuel EJ (2016) Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med 375:1216–1219
LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521:436–444
Pesapane F, Codari M, Sardanelli F (2018) Artificial intelligence in medical imaging: threat or opportunity? Radiologists again on the wavefront of innovation in medicine. Eur Radiol Exp. https://doi.org/10.1186/s41747-018-0061-6
Yi PH, Hui FK, Ting DSW (2018) Artificial intelligence and radiology: collaboration is key. J Am Coll Radiol. https://doi.org/10.1016/j.jacr.2017.12.037
Dheeba J, Albert Singh N, Tamil Selvi S (2014) Computer-aided detection of breast cancer on mammograms: a swarm intelligence optimized wavelet neural network approach. J Biomed Inform 49:45–52
Summers RM, Beaulieu CF, Pusanik LM et al (2000) Automated polyp detector for CT colonography: feasibility study. Radiology 216:284–290
Chen S, Suzuki K, MacMahon H (2011) Development and evaluation of a computer-aided diagnostic scheme for lung nodule detection in chest radiographs by means of two-stage nodule enhancement with support vector classification. Med Phys 38:1844–1858
Perez-Ramirez U, Arana E, Moratal D (2014) Computer-aided detection of brain metastases using a three-dimensional template-based matching algorithm. Conf Proc IEEE Eng Med Biol Soc 2014:2384–2387
Erickson BJ, Korfiatis P, Akkus Z, Kline TL (2017) Machine learning for medical imaging. Radiographics 37:505–515
Char DS, Shah NH, Magnus D (2018) Implementing machine learning in health care - addressing ethical challenges. N Engl J Med 378:981–983
Angwin J, Larson J, Mattu S, Kirchner L (2016) Machine Bias. Available via http://www.propublica.org/article/machine-bias-risk-assessments-in-criminal-sentencing
Calo R (2017) Artificial Intelligence policy: a primer and roadmap. Social Science Research Network. Available via https://lawreview.law.ucdavis.edu/issues/51/2/Symposium/51-2_Calo.pdf
Thrall JH, Li X, Li Q et al (2018) Artificial intelligence and machine learning in radiology: opportunities, challenges, pitfalls, and criteria for success. J Am Coll Radiol 15:504–508
Ravi D, Wong C, Deligianni F et al (2017) Deep learning for health informatics. IEEE J Biomed Health Inform 21:4–21
Christenses HI, Carnegie C, Krovi V, Smart B (2016) From Internet to Robotics. Stone et al. Supra note. Available via http://jacobsschool.ucsd.edu/contextualrobotics/docs/rm3-final-rs.pdf. Accessed 42 7
Molteni M (2017) Wellness apps evade the FDA, only to land in court. WIRED. Available via https://www.wired.com/2017/04/wellness-apps-evade-fda-land-court/
Majambere E (2011) Clarity, precision and unambiguity: aspects for effective legislative drafting. Commonwealth Law Bull 37:417–426
Zittrain JIJ (2017) Ethics and governance of artificial intelligence. Available via https://www.media.mit.edu/groups/ethics-and-governance/overview/
Tsang L, Mulryne J, Strom L et al (2017) The impact of artificial intelligence on medical innovation in the european union and United States. Available via https://www.arnoldporter.com/~/media/files/perspectives/publications/2017/08/the-impact-of-artificial-inteelligence-on-medical-innovation.pdf
Thierer A, O'Sullivan A, Russel R (2017) Artificial intelligence and public policy. Mercatus Research Paper. Available via https://www.mercatus.org/system/files/thierer-artificial-intelligence-policy-mr-mercatus-v1.pdf
Scherer MU (2016) Regulating artificial intelligence systems: risks, challenges, competencies, and strategies. Harv JL Tech 29:354–400
Law J (2009) A dictionary of law, Seventh edn. Oxford University Press, Oxford
Kohli M, Prevedello LM, Filice RW, Geis JR (2017) Implementing machine learning in radiology practice and research. AJR Am J Roentgenol 208:754–760
Mitchell T, Brynjolfsson E (2017) Track how technology is transforming work. Nature 544:290–292
Kramer DB, Xu S, Kesselheim AS (2012) Regulation of medical devices in the United States and European Union. N Engl J Med 366:848–855
European Economic Community (1993) 93/42/EEC - Council Directive concerning Medical Devices. Official Journal of the European Communities. Available via http://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/medical-devices_en
European Commission (2018) MDCG 2018–2 Future EU medical device nomenclature – Description of requirements. Available via https://ec.europa.eu/docsroom/documents/28668
European Economic Community (1990) 90/385/EEC - Council Directive on the approximation of the laws of the Member States relating to active implantable medical devices. Council Directive. Available via https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/implantable-medical-devices_en
European Commission (1998) Directive 98/79/EC of the European Parliament and of the Council on in vitro diagnostic medical devices. Official Journal of the European Communities. Available via https://ec.europa.eu/growth/single-market/european-standards/harmonised-standards/iv-diagnostic-medical-devices_en
The European Parliament and the Council of The European Union (2017) Regulation (EU) 2017/745 of the European Parliament and of the Council on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Official Journal of the European Communities. Available via https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745
The European Parliament and the Council of The European Union (2017) Regulation (EU) 2017/746 of the European Parliament and of the Council on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Official Journal of the European Communities. Available via https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0746
European Union (2018) Regulations, Directives and other acts. Available via https://europa.eu/european-union/eu-law/legal-acts_en
Crossley S, Eversheds LLP (2016) EU regulation of health information technology, software and mobile apps. Practical Law Global Guide 2016(17):1–14
114th Congress (2015–2016) (2016) H.R.34 - 21st Century Cures Act. Available via https://www.congress.gov/bill/114th-congress/house-bill/34
U.S. Food & Drug Administration (2018) Is The Product A Medical Device? U.S. Department of Health and Human Services. Available via https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/ClassifyYourDevice/ucm051512.htm
Méndez AJ, Tahoces PG, Lado MJ, Souto M, Vidal JJ (1998) Computer-aided diagnosis: automatic detection of malignant masses in digitized mammograms. Med Phys 25:957–964
Azavedo E, Zackrisson S, Mejàre I, Heibert Arnlind M (2012) Is single reading with computer-aided detection (CAD) as good as double reading in mammography screening? A systematic review. BMC Med Imaging 12:22
Recht M, Bryan RN (2017) Artificial intelligence: threat or boon to radiologists? J Am Coll Radiol 14:1476–1480
Stark L (2018) Algorithmic psychometrics and the scalable subject. Soc Stud Sci
Statista (2018) Online privacy - statistics & facts. Statistics Portal. Available via https://www.statista.com/topics/2476/online-privacy
Krittanawong C (2018) The rise of artificial intelligence and the uncertain future for physicians. Eur J Intern Med 48:e13–e14
Kruskal JB, Berkowitz S, Geis JR, Kim W, Nagy P, Dreyer K (2017) Big data and machine learning-strategies for driving this bus: a summary of the 2016 intersociety summer conference. J Am Coll Radiol 14:811–817
Castelvecchi D (2016) Can we open the black box of AI? Nature 538:20–23
Dilsizian SE, Siegel EL (2014) Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep 16:441
Allen G, Chan T (2017) Artificial intelligence and national security. Available via https://www.belfercenter.org/publication/artificial-intelligence-and-national-security
Helbing D, Frey B, Gigerenzer G et al (2017) Will democracy survive big data and artificial intelligence?. Scientific American. Available via https://www.scientificamerican.com/article/will-democracy-survive-big-data-and-artificial-intelligence/. Accessed Feb 25
The European Parliament and the Council of The European Union (1995) Directive 95/46/EC of the European Parliament and of the Council on the protection of individuals with regard to the processing of personal data and on the free movement of such data. Off J Eur Communities. Available via https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A31995L0046
Powles J, Hodson H (2017) Google DeepMind and healthcare in an age of algorithms. Health Technol (Berl) 7:351–367
The European Parliament and the Council of The European Union (2016) Directive (EU) 2016/1148 of the European Parliament and of the Council concerning measures for a high common level of security of network and information systems across the Union. Off J Eur Communities. Available via https://eur-lex.europa.eu/legal-content/EN/TXT/?toc=OJ:L:2016:194:TOC&uri=uriserv:OJ.L_.2016.194.01.0001.01.ENG
Mnih V, Kavukcuoglu K, Silver D et al (2015) Human-level control through deep reinforcement learning. Nature 518:529–533
Verghese A, Shah NH, Harrington RA (2018) What this computer needs is a physician: humanism and artificial intelligence. JAMA 319:19–20
Sardanelli F, Hunink MG, Gilbert FJ, Di Leo G, Krestin GP (2010) Evidence-based radiology: why and how? Eur Radiol 20:1–15
Author information
Authors and Affiliations
Contributions
F.P. and C.V. equally contributed in this work and share the first author role. Study concept: F.P. Literature research: F.P., C.V. Data acquisition: F.P., C.V.. Data analysis/interpretation: all authors. Manuscript drafting and revision: all authors. Manuscript editing: all authors. Approval of final version: all authors.
Corresponding author
Ethics declarations
Guarantor(s) of the study
Prof. Francesco Sardanelli.
Competing interests
All the authors have no competing interests related to the present paper to disclose.
Availability of data and materials
Data originating the Tables are available on request sent to the corresponding author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pesapane, F., Volonté, C., Codari, M. et al. Artificial intelligence as a medical device in radiology: ethical and regulatory issues in Europe and the United States. Insights Imaging 9, 745–753 (2018). https://doi.org/10.1007/s13244-018-0645-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13244-018-0645-y