Abstract
Purpose
To evaluate the prevalence of and factors associated with meniscal ramp lesions on magnetic resonance imaging (MRI) in patients with anterior cruciate ligament (ACL) injuries.
Methods
Data from the Natural Corollaries and Recovery after ACL injury multicentre longitudinal cohort study (NACOX) were analysed. Only patients who underwent MRI were included in this study. All MRI scans were reviewed by an orthopaedic knee surgeon and a musculoskeletal radiologist. The patients were divided into two groups, those with and without ramp lesions according to MRI findings. Univariable and stepwise forward multiple logistic regression analyses were used to evaluate patient characteristics (age, gender, body mass index, pre-injury Tegner activity level, activity at injury) and concomitant injuries on MRI (lateral meniscus, medial collateral ligament [MCL], isolated deep MCL, lateral collateral ligament, pivot-shift-type bone bruising, posteromedial tibial [PMT] bone bruising, medial femoral condyle bone bruising, lateral femoral condyle [LFC] impaction and a Segond fracture) associated with the presence of meniscal ramp lesions.
Results
A total of 253 patients (52.2% males) with a mean age of 25.4 ± 7.1 years were included. The overall prevalence of meniscal ramp lesions was 39.5% (100/253). Univariate analyses showed that contact sports at ACL injury, pivot-shift-type bone bruising, PMT bone bruising, LFC impaction and the presence of a Segond fracture increased the odds of having a meniscal ramp lesion. Stepwise forward multiple logistic regression analysis revealed that the presence of a meniscal ramp lesion was associated with contact sports at ACL injury [odds ratio (OR) 2.50; 95% confidence intervals (CI) 1.32–4.72; P = 0.005], pivot-shift-type bone bruising (OR 1.29; 95% CI 1.01–1.67; P = 0.04), PMT bone bruising (OR 4.62; 95% CI 2.61–8.19; P < 0.001) and the presence of a Segond fracture (OR 4.38; 95% CI 1.40–13.68; P = 0.001).
Conclusion
The overall prevalence of meniscal ramp lesions in patients with ACL injuries was high (39.5%). Contact sports at ACL injury, pivot-shift-type bone bruising, PMT bone bruising and the presence of a Segond fracture on MRI were associated with meniscal ramp lesions. Given their high prevalence, meniscal ramp lesions should be systematically searched for on MRI in patients with ACL injuries. Knowledge of the factors associated with meniscal ramp lesions may facilitate their diagnosis, raising surgeons’ and radiologists’ suspicion of these tears.
Level of evidence
III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meniscal ramp lesions are peripheral tears of the medial meniscus (MM) involving the meniscocapsular ligament, meniscotibial ligament and/or the red-red zone of the posterior horn, in the setting of an anterior cruciate ligament (ACL) tear [13, 14, 40]. Previous studies have shown that meniscal ramp lesions are associated with increased anterior and rotational knee laxity in the ACL-deficient knee [1, 12, 29, 30, 37] and that only their repair restores knee biomechanics [1, 12, 37]. Moreover, patients with meniscal ramp lesions exhibit accelerated cartilage degeneration in the medial compartment in comparison with controls [15]. It is therefore essential to recognise these injuries.
The literature is inconsistent regarding the prevalence of meniscal ramp lesions. Previous studies have reported a wide variation in the prevalence of meniscal ramp lesions diagnosed at the time of ACL reconstruction (ACLR) [6, 11, 26, 36].
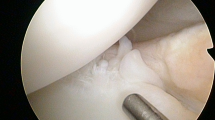
Even though direct arthroscopic visualisation is generally regarded as the gold standard for diagnosing ramp lesions [6, 26, 36], several ramp lesions might be missed intraoperatively due to their difficult visualisation and probing through the anterolateral and anteromedial portals [36].
Magnetic resonance imaging (MRI) is regarded as the best imaging modality to diagnose meniscal ramp lesions [10, 14, 43, 44], even though its accuracy has been questioned due to the varying sensitivity reported in previous studies [2, 11, 19, 41]. In their recent systematic review and meta-analysis, Koo et al. [21] reported that MRI has high specificity (94%) but moderate sensitivity (71%) for diagnosing ramp lesions. It should, however, be noted that studies evaluating MRI accuracy have used different and limited pathological signs to define ramp lesions [2, 11, 20, 25, 41, 43]. In some studies [6, 28, 40] MRI criteria were not even reported. In a recent study, using an extension of Thaunat’s classification [39], Greif et al. [14] described seven different types of meniscal ramp lesion together with their MRI appearance. Failure to consider the MRI appearance of the different types of meniscal ramp lesion may have been responsible for an underestimation of the true prevalence of these injuries and the reported reduced sensitivity of this imaging modality in the literature.
An awareness of the prevalence and appearance of the different types of meniscal ramp lesion on MRI may improve the diagnosis of these injuries and would alert the orthopaedic surgeon to focus particularly on the posteromedial ramp area during ACLR. In addition, detailed knowledge of potential epidemiological factors and injuries on MRI associated with the presence of meniscal ramp lesions may further facilitate the diagnosis of these injuries by raising surgeons’ and radiologists’ suspicion of these important tears.
The primary purpose of this study was to evaluate the prevalence of meniscal ramp lesions in patients with ACL injuries, using well-defined MRI pathological signs [14]. Another purpose was to investigate epidemiological factors and injuries on MRI associated with the presence of meniscal ramp lesions. It was hypothesised that the prevalence of meniscal ramp lesions was high and that younger age, contact sports at ACL injury and the presence of posteromedial tibial (PMT) bone bruising or a Segond fracture would be associated with the presence of meniscal ramp lesions.
Materials and methods
Data were extracted from a prospectively collected patient database. This study is part of the Natural Corollaries and Recovery after ACL injury study [NACOX] [24]. Ethical approval was obtained from the regional ethics committee in Linköping, Sweden (Dnr 2016/44-31 and 2017/221-32). All the patients signed informed consent to participate. Patients were recruited between 2016 and 2018, from six orthopaedic clinics in Sweden. The inclusion criteria were ACL injury sustained no more than six weeks prior to presentation and age between 15 and 40 years at the time of injury. Patients were excluded if they had had previous ACL injury/surgery on the same knee, fractures that required separate treatment, an inability to understand the written or spoken Swedish language, cognitive impairments, other illnesses or injuries that impaired function (e.g. fibromyalgia, rheumatic diseases and other diagnoses associated with chronic pain) [24]. ACL ruptures were diagnosed by an orthopaedic surgeon and were, if needed, verified by MRI. For the purposes of this study, only patients who underwent MRI scans were included.
Data collection
The assessed patient characteristics were age at injury, gender, body mass index (BMI), pre-injury Tegner activity level [38] and activity at ACL injury. Age at injury was dichotomised into unbiased classes close to the median (< 25 years or ≥ 25 years). The BMI was dichotomised at 25, as a BMI of ≥ 25 is regarded as overweight [42]. The pre-injury Tegner activity level was dichotomised as high (≥ 6) or low (< 6). Finally, the activity at ACL injury was dichotomised as contact sports or non-contact sports/other.
Radiological assessment
The majority (n = 209) of the patients underwent MRI scans at two institutions (Capio Artro Clinic, Stockholm, Sweden, and Linköping University Hospital, Linköping, Sweden). The remaining patients underwent MRI scans at the other institutions involved in the NACOX study [24]. The mean time from ACL injury to MRI was 19.6 ± 15.2 days. MRI examinations were performed using a 1.5 (n = 115) or 3.0 (n = 138) Tesla scanner. The images were acquired in three planes (sagittal, axial and coronal) using T1-weighted, T2-weighted and proton-density (PD) fat saturation sequences. The slice thickness was 3 mm with a 0.3 mm gap. All the MRI scans were independently analysed by an orthopaedic knee surgeon (RC) and a musculoskeletal radiologist (FvdB). In the event of inconsistencies, the examiners assessed the MRI scans together and reached a consensus in a second phase.
The presence of meniscal ramp lesions was best assessed on sagittal images on PD fat saturation or T2-weighted sequences and was defined according to the MRI appearance and classification described by Greif et al. [14]. Seven different subtypes of meniscal ramp lesion were evaluated: type 1, meniscocapsular ligament tear; type 2, partial superior peripheral meniscal horn tear; type 3A, partial inferior peripheral posterior horn meniscal tear; type 3B, meniscotibial ligament tear; type 4A, complete peripheral posterior horn meniscal tear; type 4B, complete meniscocapsular junction tear; type 5, peripheral posterior horn meniscal double tear.
Injuries to the lateral meniscus (LM), medial collateral ligament (MCL), lateral collateral ligament (LCL) and the presence of a Segond fracture were recorded. Injuries to the MCL or LCL were defined as partial rupture/discontinuity with some preserved fibres or complete disruption [31]. Isolated deep MCL injuries were defined as tears of the meniscofemoral and/or meniscotibial ligament with intact superficial MCL on axial images. The presence and location of bone bruising were also evaluated. Bone bruising in the posteromedial tibial (PMT) plateau and medial femoral condyle (MFC) was recorded. Pivot-shift-type bone bruising was defined as the presence of bone marrow oedema in the posterior aspect of the lateral tibial plateau and the midportion of the lateral femoral condyle (LFC) [32]. Finally, the presence of an LFC impaction was defined as an osteochondral depression with an intact or disrupted articular surface [31].
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences, SPSS (version 25.0; IBM Corp., Armonk, New York, USA). All the variables were summarised with standard descriptive statistics such as the mean, standard deviations or frequency. Univariable analyses were performed with age (< 25 years vs. ≥ 25 years), gender, BMI (< 25 vs. ≥ 25), pre-injury Tegner activity level (high ≥ 6 vs. low < 6), activity at injury (contact sports vs. non-contact sports/other), LM injury, MCL injury, isolated deep MCL injury, LCL injury, pivot-shift-type bone bruising, PMT bone bruising, MFC bone bruising, LFC impaction and a Segond fracture as independent variables and the presence of meniscal ramp lesions as a dependent variable. A stepwise forward multiple logistic regression analysis was used to identify variables independently associated with meniscal ramp lesions. The independent variables included in the analyses were chosen based on background knowledge and because they were regarded as being clinically relevant to the purpose of the study. The results were reported as odds ratios (OR) with 95% confidence intervals (CI). The level of significance in all analyses was 5% (two tailed).
Results
Prevalence of meniscal ramp lesions
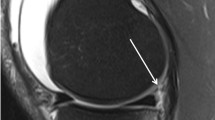
A total of 275 patients are included in the NACOX study. Eight patients had a clinical diagnosis of ACL injury and 14 MRIs were not available for the second analysis. Finally, MRIs from 253 patients were analysed in the present study. Overall, meniscal ramp lesions were identified in 100 (39.5%) patients. The subtype distribution was as follows: 13 (13%), type 1 (meniscocapsular ligament tear); 4 (4%), type 2 (partial superior peripheral meniscal horn tear); 7 (7%), type 3A (partial inferior peripheral posterior horn meniscal tear); 7 (7%), type 3B (meniscotibial ligament tear); 20 (20%), type 4A (complete peripheral posterior horn meniscal tear); 43 (43%), type 4B (complete meniscocapsular junction tear); 6 (6%), type 5 (peripheral- posterior horn meniscal double tear) (Fig. 1a–g).
Meniscal ramp lesion subtypes (red arrow) on sagittal proton density fat saturation MRI sequences. a Type 1; meniscocapsular ligament tear, as shown by the linear vertical fluid signal reaching the superior articular surface. b Type 2; partial superior peripheral meniscal horn tear, as shown by the linear vertical fluid signal reaching the superior articular surface. c Type 3A; partial inferior peripheral posterior horn meniscal tear, as shown by the linear oblique fluid signal reaching the inferior articular surface. d Type 3B; meniscotibial ligament tear, as shown by the disruption of the ligament with a fluid signal. e Type 4A; complete peripheral posterior horn meniscal tear, as shown by the fluid signal extending from the superior to the inferior articular surface. f Type 4B; complete meniscocapsular junction tear, as shown by the fluid intensity signal extending from the superior to the inferior articular surface. g Type 5; peripheral posterior horn meniscal double tear, as shown by two parallel linear fluid signals extending from the superior to the inferior articular surface. Note that PMT bone bruising is present in all images. MFC bone brusining is present in a and d. MFC medial femoral condyle, MRI magnetic resonance imaging, PMT posteromedial tibial
Patient characteristics for both the ramp (n = 100) and no-ramp (n = 153) groups are summarised in Table 1.
Univariable analyses
Univariable logistic regression analyses revealed that contact sports at ACL injury (OR 2.33; 95% CI 1.33–4.09; P = 0.003), pivot-shift-type bone bruising (OR 3.64; 95% CI 1.72–7.69; P = 0.0007), PMT bone bruising (OR 4.57; 95% CI 2.65–7.86; P < 0.001), LFC impaction (OR 1.77; 95% CI 1.06–2.94; P = 0.02) or a Segond fracture (OR 3.66; 95% CI 1.34–9.98; P = 0.01) were associated with the presence of meniscal ramp lesions. Age at an injury, gender, BMI, pre-injury Tegner activity level, LM injury, MCL injury, isolated deep MCL injury, LCL injury and MFC bone bruising were not associated with the presence of meniscal ramp lesions (Table 1).
Multivariable analysis
Stepwise forward multiple logistic regression analysis revealed that contact sports at ACL injury (OR 2.50; 95% CI 1.32–4.72; P = 0.005), pivot-shift-type bone bruising (OR 1.29; 95% CI 1.01–1.67; P = 0.04), PMT bone bruising (OR 4.62; 95% CI 2.61–8.19; P < 0.001) or a Segond fracture (OR 4.38; 95% CI 1.40–13.68; P = 0.001) were significantly associated with the presence of meniscal ramp lesions (Table 2).
Discussion
The most important finding in this study was that the prevalence of meniscal ramp lesions in patients with an ACL injury was high (39.5%). This study also revealed the prevalence of the different meniscal ramp lesion types. Finally, another important finding was that meniscal ramp lesions were associated with contact sports at ACL injury and the presence on MRI of pivot-shift-type bone bruising, PMT bone bruising and a Segond fracture.
Previous studies have reported a variable prevalence (9.3%–40%) of meniscal ramp lesions diagnosed arthroscopically at the time of ACLR [6, 11, 26, 36]. This wide variation in prevalence might depend on the different definitions of ramp lesions employed [2, 6, 11, 18, 26, 36], as well as, which is probably more important, the method employed for diagnosis. Although direct arthroscopic visualisation is regarded as the gold standard for diagnosing ramp lesions [6, 8, 26, 36], several studies have shown that the standard anteromedial and anterolateral portal have low sensitivity when diagnosing ramp lesions [19, 28, 36]. While inspection through the Gillquist view, the use of a 70-degree arthroscope (while viewing the posteromedial ramp area) and, more importantly, the creation of a posteromedial portal are more accurate in detecting ramp lesions [7, 19, 36, 39, 44], they are not routinely used. This may have led to an underestimation of the true incidence of meniscal ramp lesions in the literature. Sonnery-Cottet et al. [36] systematically explored the posterior horn of the MM in three sequential stages. In the first stage, exploration was performed through standard anterior visualisation via the anterolateral portal. In the second stage, the posterior horn of the MM was visualised through the Gillquist view. Finally, in the third stage, the posterior horn was probed through an additional posteromedial portal. The authors reported the highest (40%) prevalence of meniscal ramp lesions, diagnosed arthroscopically, in the literature. However, only 58% of meniscal ramp lesions were diagnosed at the second stage, with inspection through the Gillquist view. Forty-two per cent were only diagnosed at the third stage, after probing and debridement with a motorised shaver of a superficial tissue layer over the meniscocapsular junction covering the “hidden lesion”. Despite using MRI as a diagnostic method, the prevalence of meniscal ramp lesions in the present series is comparable to that of Sonnery-Cottet et al. [36] (39.5% vs. 40%, respectively). In their study, Balazs et al. [3] also utilised MRI to determine whether a meniscal ramp lesion was present. In line with our results, the overall prevalence of meniscal ramp lesions in their series was 42% [3]. As suggested by Sonnery-Cottet et al. [36], it might be argued that, without the creation of a posteromedial portal and superficial soft tissue dissection over the meniscocapsular junction, many meniscal ramp lesions may be missed. Studies investigating the prevalence of meniscal ramp lesions without the systematic creation of a posteromedial portal and tissue debridement at the meniscocapsular junction reported a prevalence between 15.5% and 24% [11, 25, 26, 34, 40, 41]. This corresponds roughly to the prevalence (23.2%) of meniscal ramp lesions identified by Sonnery-Cottet et al. [36] using only exploration through the Gillquist view. Previous literature may have missed a significant number of meniscal ramp lesions that Sonnery-Cottet et al. [36] identified after soft tissue debridement through the posteromedial portal and we, as well as Balazs et al. [3], identified with MRI. MRI has high (> 92%) specificity for diagnosing ramp lesions when compared with probing through the posteromedial portal [2].
The use of MRI for the diagnosis of meniscal ramp lesions has been criticised due to the moderate sensitivity reported in previous literature [2, 11, 16, 19, 28, 41]. However, studies evaluating the accuracy of MRI for diagnosing meniscal ramp lesions (with arthroscopy as a gold standard) used different pathological signs to define these tears [2, 11, 20, 25, 41, 43]. In several studies [2, 11, 20, 41] a meniscal ramp lesion on MRI was only defined by a tear in the peripheral attachment of the posterior horn of the medial meniscus at the meniscocapsular junction. Other studies did not even report which MRI criteria were used for the diagnosis of meniscal ramp lesions [6, 28, 40]. Failure to consider the different types of meniscal ramp lesion and their varied MRI appearance [14] may have been responsible for an underestimation of the real prevalence of these injuries as well as for the reported reduced sensitivity of this imaging modality.
Bollen et al. [6] found a meniscal ramp lesion in 17 of a series of 183 ACLRs. The MRI was performed on 11 patients with a meniscal ramp lesion, but it was unable to identify the injury in any case. However, the study did not report which MRI criteria were used for the diagnosis, who read the MRI scans and with which experience and which magnetic field strength and sequences were used. Moreover, the time from injury to MRI was not reported. The author attributed the poor sensitivity of MRI in diagnosing ramp lesions to the fact that this examination is performed with the knee in near full extension and, as a result, the meniscocapsular separation is probably reduced, leading to false negatives. This theory has subsequently been supported by other authors [11, 25, 41]. However, a short time from injury to MRI (19.6 ± 15.2 days, in the present study) may prevent oedema in the injured structures of the posteromedial ramp area to reabsorb, allowing the diagnosis of meniscal ramp lesions regardless of the position of the knee. MRI performed with appropriate magnetic field strength and spatial resolution allows the clear visualisation of the entire thickness of the meniscal ramp area [3].
Several studies have reported a higher prevalence of meniscal ramp lesions in the event of the ACL tear being caused by a contact injury [3, 27, 34, 35]. Even if information about the exact injury mechanism was not available in the present study, our findings are somewhat in line with previous literature, as meniscal ramp lesions were associated with contact sports at ACL injury. As suggested by Seil et al. [34], it might be hypothesised that meniscal ramp lesions are more common in the event of high-energy trauma. This might also support the association between meniscal ramp lesions and pivot-shift bone bruising found in this study. Bisson et al. [5] showed that contact injuries were associated with more severe bone bruising in the lateral tibial plateau and that the increased severity of lateral tibial plateau bone bruising was associated with medial meniscal tears.
In the present study, PMT bone bruising was the factor with the strongest association with meniscal ramp lesions (OR 4.62; 95% CI 2.61–8.19; P < 0.001). This MRI sign was present in 61% of the patients with meniscal ramp lesions in comparison with 25.5% without. These findings contrast with those of Song et al. [35] and Hatayama et al. [16] reporting that PMT bone bruising is not associated with meniscal ramp lesions. These differences might be related to the different timing of MRI. Hatayama et al. [16] reported a time interval between injury and MRI ranging from 1 day to 10 years, whereas Song et al. [35] did not report the delay from injury to MRI. In the present study, the short delay (19.6 ± 15.2 days) from injury to MRI may have prevented the PMT bone bruising to reabsorb and therefore increased its association with meniscal ramp lesions. Most of the literature suggests that PMT bone bruising is an important secondary MRI sign of meniscal ramp lesions [3, 4, 11, 20, 22, 23, 41]. The strong association between PMT bone bruising and meniscal ramp lesions might be due to one possible common injury mechanism. A contrecoup injury with impaction of the MFC and PMT plateau, due to a sudden tibial reduction with compensatory varus alignment and internal tibial rotation after the initial pivot-shift mechanism, might be the origin of both meniscal ramp lesions and PMT bone bruising [11, 17]. The same consideration might be applied to the Segond fracture, which is thought to occur as the result of internal rotation and varus stress [9, 33]. This injury was strongly associated (OR 4.38; 95% CI 1.40–13.68; P = 0.001) with the presence of meniscal ramp lesions.
Meniscal ramp lesions are more common than previously thought. The findings in the present study suggest that they are among the most frequent injuries associated with ACL tears. Surgeons who treat ACL tears are likely to encounter meniscal ramp lesions in daily practice. The recognition of meniscal ramp lesions is essential, as they are associated with increased anterior and rotational laxity, increased strain on both the native and ACL graft, as well as accelerated cartilage degeneration in the medial compartment [6, 12, 15, 29, 30, 37]. Only the repair of meniscal ramp lesions is able to restore anterior and rotational laxity [12, 37]. If meniscal ramp lesions are overlooked in patients with ACLR, anterior and rotational laxity persists [1, 7, 12, 37].
This study provides important information regarding the prevalence and appearance of MRI of meniscal ramp lesions in patients with ACL injuries. In addition, it identifies some factors (contact sport at ACL injury, pivot-shift-type bone bruising, PMT bone bruising and a Segond fracture) associated with meniscal ramp lesions. Their presence should further raise surgeons’ and radiologists’ suspicion of these important tears.
The main strength of this study was that the evaluation of meniscal ramp lesions was performed using standardised and well-defined MRI pathological signs [14]. An orthopaedic surgeon specialising in knee surgery and a musculoskeletal radiologist reviewed all the MRI scans. Moreover, a substantial number (54.5%) of MRI examinations were performed using a 3.0 Tesla scanner. These factors probably improved the diagnosis of meniscal ramp lesions [21]. The short delay (19.6 ± 15.2 days) from the injury to the MRI may have prevented oedema in the injured structures of the posteromedial ramp area to reabsorb and therefore increased the accuracy of MRI in diagnosing meniscal ramp lesions. In addition, it strengthened the association between bone bruises (that would otherwise have reabsorbed over time) and meniscal ramp lesions. Finally, the cohort studied and the number of patients with meniscal ramp lesions were relatively large (n = 253 and 100, respectively). This enabled the analysis of several factors potentially associated with the presence of meniscal ramp lesions in our logistic regression analysis.
There are some limitations. MRI examinations were performed at different institutions with different scanners. However, the imaging protocol was standardised and all the MRI scans were performed at 1.5 or 3.0 Tesla and were reviewed by the same orthopaedic surgeon and musculoskeletal radiologist. Other factors, such as medial posterior tibial slope, medial meniscal slope, gradual lateral tibial slope and varus alignment of more than three degrees, were not controlled for, although they were previously associated with the presence of meniscal ramp lesions [20, 35]. However, also analysing these factors would have required a much larger number of patients.
Conclusion
The overall prevalence of meniscal ramp lesions in patients with ACL injuries was high (39.5%). Contact sports at ACL injury, pivot-shift-type bone bruising, PMT bone bruising and the presence of a Segond fracture on MRI were associated with meniscal ramp lesions. Given their high prevalence, meniscal ramp lesions should be systematically searched for on MRI in patients with ACL injuries. Knowledge of the factors associated with meniscal ramp lesions may facilitate their diagnosis, raising surgeons’ and radiologists’ suspicion of these tears.
References
Ahn JH, Bae TS, Kang KS, Kang SY, Lee SH (2011) Longitudinal tear of the medial meniscus posterior horn in the anterior cruciate ligament-deficient knee significantly influences anterior stability. Am J Sports Med 39(10):2187–2193
Arner JW, Herbst E, Burnham JM, Soni A, Naendrup JH, Popchak A, Fu FH, Musahl V (2017) MRI can accurately detect meniscal ramp lesions of the knee. Knee Surg Sports Traumatol Arthrosc 25(12):3955–3960
Balazs GC, Greditzer HG 4th, Wang D, Marom N, Potter HG, Marx RG, Rodeo SA, Williams RJ 3rd (2019) Ramp lesions of the medial meniscus in patients undergoing primary and revision ACL reconstruction: prevalence and risk factors. Orthop J Sports Med 7(5):2325967119843509
Beel W, Mouton C, Tradati D, NührenbörgerSeil CR (2022) Ramp lesions are six times more likely to be observed in the presence of a posterior medial tibial bone bruise in ACL-injured patients. Knee Surg Sports Traumatol Arthrosc 30(1):184–191
Bisson LJ, Kluczynski MA, Hagstrom LS, Marzo JM (2013) A prospective study of the association between bone contusion and intra-articular injuries associated with acute anterior cruciate ligament tear. Am J Sports Med 41(8):1801–1807
Bollen SR (2010) Posteromedial meniscocapsular injury associated with rupture of the anterior cruciate ligament: a previously unrecognised association. J Bone Joint Surg Br 92(2):222–223
Brophy RH, Steinmetz RG, Smith MV, Matava MJ (2022) Meniscal ramp lesions: anatomy, epidemiology, diagnosis, and treatment. J Am Acad Orthop Surg 30(6):255–262
Bumberger A, Koller U, Hofbauer M, Tiefenboeck TM, Hajdu S, Windhager R, Waldstein W (2020) Ramp lesions are frequently missed in ACL-deficient knees and should be repaired in case of instability. Knee Surg Sports Traumatol Arthrosc 28(3):840–854
Campos JC, Chung CB, Lektrakul N, Pedowitz R, Trudell D, Yu J, Resnick D (2001) Pathogenesis of the Segond fracture: anatomic and MR imaging evidence of an iliotibial tract or anterior oblique band avulsion. Radiology 219(2):381–386
De Maeseneer M, Lenchik L, Starok M, Pedowitz R, Trudell D, Resnick D (1998) Normal and abnormal medial meniscocapsular structures: MR imaging and sonography in cadavers. AJR Am J Roentgenol 171(4):969–976
DePhillipo NN, Cinque ME, Chahla J, Geeslin AG, Engebretsen L, LaPrade RF (2017) Incidence and detection of meniscal ramp lesions on magnetic resonance imaging in patients with anterior cruciate ligament reconstruction. Am J Sports Med 45(10):2233–2237
DePhillipo NN, Moatshe G, Brady A, Chahla J, Aman ZS, Dornan GJ, Nakama GY, Engebretsen L, LaPrade RF (2018) Effect of meniscocapsular and meniscotibial lesions in ACL-deficient and ACL-reconstructed knees: a biomechanical study. Am J Sports Med 46(10):2422–2431
DePhillipo NN, Moatshe G, Chahla J, Aman ZS, Storaci HW, Morris ER, Robbins CM, Engebretsen L, LaPrade RF (2019) Quantitative and qualitative assessment of the posterior medial meniscus anatomy: defining meniscal ramp lesions. Am J Sports Med 47(2):372–378
Greif DN, Baraga MG, Rizzo MG, Mohile NV, Silva FD, Fox T, Jose J (2020) MRI appearance of the different meniscal ramp lesion types, with clinical and arthroscopic correlation. Skeletal Radiol 49(5):677–689
Guimaraes JB, Schwaiger BJ, Gersing AS, Neumann J, Facchetti L, Li X, Joseph GB, Link TM (2021) Meniscal ramp lesions: frequency, natural history, and the effect on knee cartilage over 2 years in subjects with anterior cruciate ligament tears. Skeletal Radiol 50(3):551–558
Hatayama K, Terauchi M, Saito K, Aoki J, Nonaka S, Higuchi H (2018) Magnetic resonance imaging diagnosis of medial meniscal ramp lesions in patients with anterior cruciate ligament injuries. Arthroscopy 34(5):1631–1637
Kaplan PA, Gehl RH, Dussault RG, Anderson MW, Diduch DR (1999) Bone contusions of the posterior lip of the medial tibial plateau (contrecoup injury) and associated internal derangements of the knee at MR imaging. Radiology 211(3):747–753
Keyhani S, Ahn JH, Verdonk R, Soleymanha M, Abbasian M (2017) Arthroscopic all-inside ramp lesion repair using the posterolateral transseptal portal view. Knee Surg Sports Traumatol Arthrosc 25(2):454–458
Kim SH, Lee SH, Kim KI, Yang JW (2018) Diagnostic accuracy of sequential arthroscopic approach for ramp lesions of the posterior horn of the medial meniscus in anterior cruciate ligament-deficient knee. Arthroscopy 34(5):1582–1589
Kim SH, Seo HJ, Seo DW, Kim KI, Lee SH (2020) Analysis of risk factors for ramp lesions associated with anterior cruciate ligament injury. Am J Sports Med 48(7):1673–1681
Koo B, Lee SH, Yun SJ, Song JG (2020) Diagnostic performance of magnetic resonance imaging for detecting meniscal ramp lesions in patients with anterior cruciate ligament tears: a systematic review and meta-analysis. Am J Sports Med 48(8):2051–2059
Kumar NS, Spencer T, Cote MP, Arciero RA, Edgar C (2018) Is edema at the posterior medial tibial plateau indicative of a ramp lesion? an examination of 307 patients with anterior cruciate ligament reconstruction and medial meniscal tears. Orthop J Sports Med 6(6):2325967118780089. https://doi.org/10.1177/2325967118780089
Kunze KN, Wright-Chisem J, Polce EM, DePhillipo NN, LaPrade RF, Chahla J (2021) Risk factors for ramp lesions of the medial meniscus: a systematic review and meta-analysis. Am J Sports Med 49(13):3749–3757
Kvist J, Gauffin H, Tigerstrand Grevnerts H, Ardern C, Hägglund M, Stålman A, Frobell R (2018) Natural corollaries and recovery after acute ACL injury: the NACOX cohort study protocol. BMJ Open 8(6):e020543
Laurens M, Cavaignac E, Fayolle H, Sylvie R, Lapègue F, Sans N, Faruch M (2022) The accuracy of MRI for the diagnosis of ramp lesions. Skeletal Radiol 51(3):525–533
Liu X, Feng H, Zhang H, Hong L, Wang XS, Zhang J (2011) Arthroscopic prevalence of ramp lesion in 868 patients with anterior cruciate ligament injury. Am J Sports Med 39(4):832–837
Magosch A, Mouton C, Nührenbörger C, Seil R (2021) Medial meniscus ramp and lateral meniscus posterior root lesions are present in more than a third of primary and revision ACL reconstructions. Knee Surg Sports Traumatol Arthrosc 29(9):3059–3067
Malatray M, Raux S, Peltier A, Pfirrmann C, Seil R, Chotel F (2018) Ramp lesions in ACL deficient knees in children and adolescent population: a high prevalence confirmed in intercondylar and posteromedial exploration. Knee Surg Sports Traumatol Arthrosc 26(4):1074–1079
Mouton C, Magosch A, Pape D, Hoffmann A, Nührenbörger C, Seil R (2020) Ramp lesions of the medial meniscus are associated with a higher grade of dynamic rotatory laxity in ACL-injured patients in comparison to patients with an isolated injury. Knee Surg Sports Traumatol Arthrosc 28(4):1023–1028
Peltier A, Lording T, Maubisson L, Ballis R, Neyret P, Lustig S (2015) The role of the meniscotibial ligament in posteromedial rotational knee stability. Knee Surg Sports Traumatol Arthrosc 23(10):2967–2973
Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A (2014) Anterior cruciate ligament osteoarthritis score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthr Cartil 22(5):668–682
Sanders TG, Medynski MA, Feller JF, Lawhorn KW (2000) Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury. Radiographics. https://doi.org/10.1148/radiographics.20.suppl_1.g00oc19s135
Segond P (1879) Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progres Med 7:340–341
Seil R, Mouton C, Coquay J, Hoffmann A, Nührenbörger C, Pape D, Theisen D (2018) Ramp lesions associated with ACL injuries are more likely to be present in contact injuries and complete ACL tears. Knee Surg Sports Traumatol Arthrosc 26(4):1080–1085
Song GY, Liu X, Zhang H, Wang QQ, Zhang J, Li Y, Feng H (2016) Increased medial meniscal slope is associated with greater risk of ramp lesion in noncontact anterior cruciate ligament injury. Am J Sports Med 44(8):2039–2046
Sonnery-Cottet B, Conteduca J, Thaunat M, Gunepin FX, Seil R (2014) Hidden lesions of the posterior horn of the medial meniscus: a systematic arthroscopic exploration of the concealed portion of the knee. Am J Sports Med 42(4):921–926
Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA (2016) Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med 44(2):400–408
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligaments injuries. Clin Orthop Relat Res 198:43–49
Thaunat M, Fayard JM, Guimaraes TM, Jan N, Murphy CG, Sonnery-Cottet B (2016) Classification and surgical repair of ramp lesions of the medial meniscus. Arthrosc Tech 5(4):e871–e875
Thaunat M, Ingale P, Penet A, Kacem S, Haidar I, Bauwens PH, Fayard JM (2021) Ramp lesion subtypes: prevalence, imaging, and arthroscopic findings in 2156 anterior cruciate ligament reconstructions. Am J Sports Med 49(7):1813–1821
Willinger L, Balendra G, Pai V, Lee J, Mitchell A, Jones M, Williams A (2021) Medial meniscal ramp lesions in ACL-injured elite athletes are strongly associated with medial collateral ligament injuries and medial tibial bone bruising on MRI. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-021-06671-z
World Health Organization (2000) Obesity: preventing and managing the global epidemic. World Health Organization, Geneva
Yeo Y, Ahn JM, Kim H, Kang Y, Lee E, Lee JW, Kang HS (2018) MR evaluation of the meniscal ramp lesion in patients with anterior cruciate ligament tear. Skeletal Radiol 47(12):1683–1689
Zappia M, Sconfienza LM, Guarino S, Tumminello M, Iannella G, Mariani PP (2021) Meniscal ramp lesions: diagnostic performance of MRI with arthroscopy as reference standard. Radiol Med 126(8):1106–1116
Acknowledgements
This study is part of the NACOX-cohort, a project investigating the natural corollaries and recovery after the acute ACL injury. We would like to thank all collaborators in the NACOX study and Henrik Hedevik for his contribution to data management.
Funding
The NACOX-cohort study is supported by the Swedish Research Council, the Swedish Research Council for Sport Science, the Medical Research Council of Southeast, and ALF Grants Region Östergötland. Open access funding provided by Karolinska Institute. There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
IRB
Ethical approval was obtained by the regional ethics committee in Linköping, Sweden (Dnr 2016/44-31 and 2017/221-32). All patients signed informed consent to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cristiani, R., van de Bunt, F., Kvist, J. et al. High prevalence of meniscal ramp lesions in anterior cruciate ligament injuries. Knee Surg Sports Traumatol Arthrosc 31, 316–324 (2023). https://doi.org/10.1007/s00167-022-07135-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-022-07135-8