Abstract.
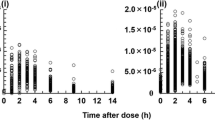
Neoral cyclosporine has better absorption characteristics than the original Sandimmun formulation. This has allowed Neoral to be administered orally in circumstances where Sandimmun had been ineffective, including the postoperative phase of liver transplantation. Sampling strategies, such as the measurement of drug concentration 2 h after oral administration, have been used in a variety of settings to estimate systemic exposure to Neoral (measured as the area under the blood concentration curve (AUC) of the drug) in blood. We conducted a pilot study to determine whether Neoral could be administered orally immediately after heart transplantation and to determine which pharmacokinetic parameters reflect systemic drug exposure in this setting. Eight male patients (mean age 50 years) undergoing a first heart transplant were studied. Neoral was administered orally before surgery and at 12-h intervals via a nasogastric tube after surgery. Twelve-hour pharmacokinetic profiles were obtained on postoperative days 1, 3 and 5. Cyclosporine concentrations were measured with the Dade Behring Emit assay, which is specific for the parent drug. Drug concentrations were dose-normalised and drug exposure was measured by the AUC. Drug exposure following administration (AUC0–12) was low on day 1 but increased by 99% between postoperative day 1 and day 5 (P<0.05), indicating more complete absorption of cyclosporine; exposure in the first 4 h post-dose (AUC0–4) increased by 126% (P<0.01), reflecting more rapid cyclosporine absorption, and the maximum blood concentration observed increased by 137% (P<0.05) during the same period. The correlation between the cyclosporine trough concentration and AUC0–12 was low on all days. Due to the changing pattern of cyclosporine absorption, concentration measurements at a single time point could not accurately predict 12-h exposure to the drug on all study days. However, the drug concentration at 2 h post-dose had a high correlation with drug exposure during the first 4 h (correlation of C2 to AUC0–4: r 2>0.93 on all days). Absorption of Neoral was low immediately after heart transplantation but improved substantially during the first 5 days after surgery. No single timed measurement of drug concentration reflected cyclosporine exposure; however, the 2-h concentration did provide an accurate measure of the early phase of drug absorption (AUC0–4). Oral administration of Neoral may result in inadequate immunosuppression immediately after heart transplantation unless it is supplemented either by intravenous cyclosporine or by the use of an induction agent.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
About this article
Cite this article
Banner, N.R., David, O.J., Leaver, N. et al. Pharmacokinetics of oral cyclosporine (Neoral) in heart transplant recipients during the immediate period after surgery. Transpl Int 15, 649–654 (2002). https://doi.org/10.1007/s00147-002-0491-0
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00147-002-0491-0