Abstract
Purpose
Patients receiving venoarterial extracorporeal membrane oxygenation (VA-ECMO) frequently develop arterial hyperoxaemia, which may be harmful. However, lower oxygen saturation targets may also lead to harmful episodes of hypoxaemia.
Methods
In this registry-embedded, multicentre trial, we randomly assigned adult patients receiving VA-ECMO in an intensive care unit (ICU) to either a conservative (target SaO2 92–96%) or to a liberal oxygen strategy (target SaO2 97–100%) through controlled oxygen administration via the ventilator and ECMO gas blender. The primary outcome was the number of ICU-free days to day 28. Secondary outcomes included ICU-free days to day 60, mortality, ECMO and ventilation duration, ICU and hospital lengths of stay, and functional outcomes at 6 months.
Results
From September 2019 through June 2023, 934 patients who received VA-ECMO were reported to the EXCEL registry, of whom 300 (192 cardiogenic shock, 108 refractory cardiac arrest) were recruited. We randomised 149 to a conservative and 151 to a liberal oxygen strategy. The median number of ICU-free days to day 28 was similar in both groups (conservative: 0 days [interquartile range (IQR) 0–13.7] versus liberal: 0 days [IQR 0–13.7], median treatment effect: 0 days [95% confidence interval (CI) – 3.1 to 3.1]). Mortality at day 28 (59/159 [39.6%] vs 59/151 [39.1%]) and at day 60 (64/149 [43%] vs 62/151 [41.1%] were similar in conservative and liberal groups, as were all other secondary outcomes and adverse events. The conservative group experienced 44 (29.5%) major protocol deviations compared to 2 (1.3%) in the liberal oxygen group (P < 0.001).
Conclusions
In adults receiving VA-ECMO in ICU, a conservative compared to a liberal oxygen strategy, did not affect the number of ICU-free days to day 28.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this multicentre registry-embedded, randomised controlled trial of 300 patients who received venoarterial extracorporeal membrane oxygenation in Australia, a conservative oxygen administration strategy (SaO2 aim 92–96%) which limited exposure to hyperoxaemia, lead to similar short-term (ICU-free days to day 28, in-hospital mortality) and long-term outcomes (mortality and disability at 6 months), when compared to a liberal oxygen strategy (SaO2 aim ≥ 97%). The conservative oxygen strategy did not result in more major hypoxic complications but was associated with more protocol deviations. |
Introduction
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is increasingly being used for treatment of cardiogenic shock and refractory cardiac arrest, however, it remains a high risk, resource-intensive intervention [1]. VA-ECMO treats cardiogenic shock by increasing both the blood flow to the native circulation and the oxygen content of blood. Arterial hyperoxaemia on ECMO is common [2]. Although readily modifiable by titration of the ECMO circuit and ventilator, there is uncertainty about the optimal blood oxygenation target while receiving VA-ECMO [3].
Severe hyperoxaemia increases cellular oxidative stress and free radical production, leading to DNA damage, direct lung toxicity, and coronary and cerebral vasoconstriction [4, 5]. Observational studies in patients receiving VA-ECMO suggest hyperoxaemia may be associated with increased risk of death and worse neurological outcomes [6, 7].
Many patients who receive VA-ECMO are also at risk of concurrent hypoxic respiratory failure, so that conservative oxygenation targets may reduce the oxygenation buffer provided by ECMO, leading to organ injury. Recently, several large trials of critically ill patients (not receiving ECMO) have suggested lower oxygenation targets may be associated with potential harm [8,9,10].
Current guidelines suggest targeting a post-oxygenator partial pressure of oxygen (PaO2) of 150 mm Hg and a systemic arterial oxygen saturation of 92–97% [1, 11]. However, data to guide these recommendations are limited and there is no uniform standard approach to oxygenation targets or to titration of inspired oxygen fraction to the ECMO oxygenator. The optimal target oxygen saturation while receiving VA-ECMO has never been evaluated in a randomised controlled trial. Accordingly, we conducted the Blend to Limit Oxygen in ECMO: A Randomised Controlled Registry (BLENDER) Trial to test the hypothesis that compared to a liberal oxygenation target, a conservative target might lead to a reduction in intensive care unit (ICU) free days to day 28, in patients receiving VA-ECMO for cardiogenic shock or refractory cardiac arrest.
Methods
Trial design
We performed a registry-embedded, open label, multicentre, parallel group, comparative effectiveness randomised trial, which compared conservative versus liberal oxygen strategies in adult patients receiving VA-ECMO in the ICU. The protocol was approved by ethics or governance committees at each site (HREC/50486/Alfred-2019, Local Reference: Project 88/19). The trial protocol and statistical analysis plan were published prior to the completion of enrolment [12, 13]. Because of the emergency nature of ECMO, informed consent prior to enrolment was waived. The patient or surrogate was contacted as soon as practicable after ECMO initiation to determine agreement to continue the trial protocol. An independent data and safety monitoring committee reviewed one prespecified, interim analysis after 150 patients had reached 60 days of follow-up, for the sole purpose of safety monitoring. Additional information about trial design and trial sites is provided in the electronic supplementary materials (ESM).
Patients
All adult patients (> 18 years) who were commenced on VA-ECMO, were eligible for enrolment, unless they could not be randomised within 6 h of ECMO initiation, had known pregnancy, were unwilling to receive blood products, were enrolled in another oxygen titration study or where clinicians stipulated a specific oxygen target.
Randomisation and blinding
We randomly assigned patients in a 1:1 ratio to receive a conservative or liberal oxygen strategy as soon as possible after VA-ECMO initiation. Randomisation was performed via a secure, web-based system using permuted block randomisation with variable block sizes (2 or 4), stratified by site and indication (cardiogenic shock or refractory cardiac arrest). Clinicians caring for patients were aware of the intervention allocation. Outcome assessors, statisticians conducting analyses and authors were unaware of allocation.
Trial intervention
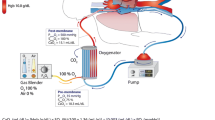
The intervention strategy involved titration of the inspired oxygen percentage delivered by the oxygen/air blender to the ECMO oxygenator, with sequential titration of inspired oxygen percentage delivered by the ventilator. The oxygen strategy was continued until ECMO support was ceased or until day 60 (whichever came first). Monitoring of systemic oxygenation was performed via pulse oximetry on the right hand and intermittent arterial blood sampling. Post-oxygenator saturations were measured at least twice daily or more frequently as required when titrating inspired oxygen to achieve the desired target.
Conservative oxygenation target
The inspired oxygen fraction delivered to the oxygenator (FbO2) was immediately reduced to 0.6. This was then titrated between a minimum FbO2 0.5 and maximum FbO2 1.0 to achieve post-oxygenator ECMO circuit blood saturation of 92–96%. The ventilator FiO2 was then also titrated (minimum FiO2 0.21) to achieve right arm arterial saturations of 92–96%. Patients who were not mechanically ventilated while receiving VA-ECMO, had nasal or facemask oxygen titrated to a flow rate which achieved right arm oxygen saturations 92–96% (ESM, Figs. S1 and S2).
Liberal oxygenation target
The oxygenator FbO2 was set at 1.0 at all times. The ventilator FiO2 was titrated to right arm saturations 97–100% (but not lower than 0.5). Patients who were not receiving mechanical ventilation, had nasal or facemask oxygen delivered at a flow rate which achieved right arm saturations 97–100% (ESM, Figs. S3 and S4).
Outcomes
The primary outcome of ICU-free days from randomisation to day 28 was defined as the total number of days free of ICU between randomisation and day 28, with all patients who died before day 28 allocated zero ICU-free days [12, 13].
Secondary outcomes included ICU and in-hospital mortality, mortality at 28, 60, 90 and 180 days, ICU-free days to day 60, ICU and hospital length of stay, duration of mechanical ventilation and ECMO. ICU-free days to day 60 was calculated in a similar manner to ICU-free days to day 28 (for all patients and for survivors). To account for the potential impact of ICU discharge delay, an additional secondary outcome considered was ICU-free days after accounting for time in ICU after being deemed ready to leave [14]. Functional assessments of quality of life and disability were assessed at 6 month following ICU admission in available survivors using the European Quality of Life Five Dimension Five Level (EQ5D5L) and World Health Organisation Disability Assessment Schedule (WHODAS 2.0).
Prespecified adverse events included episodes of critical hypoxia (SpO2 < 80% for more than 5 min requiring an emergency response), clinical or electro-encephalogram confirmed seizures and any need for cardiopulmonary resuscitation.
Data collection
Data were extracted from the EXCEL registry, a bi-national research registry coordinated by the Australian and New Zealand Intensive Care Research Center, Monash University which collects demographics, diagnostics, therapies, morbidity and mortality outcomes on patients requiring ECMO at major hospitals in Australia and New Zealand [15, 16]. Arterial blood gases are reported to the registry for the first seven days after ECMO initiation. Although blood gas sampling was performed whenever there was a change in inspired oxygen percentage, values were recorded in the EXCEL registry only every 12 h. Patients requiring ECMO for longer than seven days continued their allocated oxygen strategy, but no further arterial blood gas values were reported centrally to the registry.
Study-specific information collected directly by local staff (which was not part of the existing EXCEL registry dataset) related to screening, randomisation, intervention allocation, prespecified adverse events and protocol compliance. BLENDER-specific electronic case report forms were linked through encrypted patient identifiers to the EXCEL registry. Additional information was obtained through linkage to the Australian and New Zealand Intensive Care Society Adult Patient Database, the national clinical quality registry dataset [17, 18].
Statistical analysis
Sample size calculation was derived from pilot data, where the overall mean ± standard deviation in ICU-free days to day 28 was 11.8 ± 8.4 [19]. Using a type I error rate of 0.05 and a three-day difference between groups in ICU-free days, recruiting 124 patients per group facilitated 80% power. To account for non-normality and potential dropout, the sample size was inflated to 300.
Analysis was performed on an intention to treat basis. ICU-free days to day 28 was analysed using raw and adjusted median (quantile = 0.5) regression with results reported as difference of medians (95% confidence interval [CI]). Adjusted analysis accounted for the stratifying variables of site and VA-ECMO mode (cardiogenic shock or ECMO-cardiopulmonary resuscitation for refractory cardiac arrest [ECPR]), age, diagnosis, and baseline imbalance (time from ECMO initiation to randomisation). Sensitivity to discharge delay was conducted by replacing ICU discharge time with the time that each patient was deemed ready for ICU discharge.
Mortality outcomes were analysed using generalized linear mixed effects models with a binomial distribution and a log-link to facilitate relative risks (95% CI). Patient survival was analysed using Cox-proportional hazards regression with results reported as hazard ratios with 95% CI and presented as a Kaplan–Meier survival curve. Duration outcomes (time to ECMO decannulation/cessation, extubation from mechanical ventilation, and ICU and hospital discharge) were analyzed using Fine and Gray models to account for the competing risk of death. Results are reported as sub-distribution hazard ratios (sdHRs) with 95% CIs, reflecting the overall effect of different oxygen strategies on the cumulative incidence of achieving the outcomes (e.g., ECMO cessation, extubation, or discharge) in the presence of the competing risk of death. For all duration outcomes, sub-distribution hazard ratios greater than 1 indicate an increased cumulative incidence of achieving the outcome, suggesting a higher likelihood of a positive outcome in the presence of the competing risk of death [20]. All secondary outcomes are presented as raw and adjusted analyses adjusting for the previously described covariates with robust errors clustered at a site level. Quality of life and disability measures at 6 months (EQ5DL & WHODAS) were analysed using median regression as previously described. Longitudinal analyses of daily oxygenation levels were performed using linear mixed-effects models fitting main effect for treatment and time and an interaction between the two. Per-protocol analysis was performed excluding patients for whom there were major protocol deviation. Subgroup analyses were performed in prespecified cohorts defined by ECMO mode (cardiogenic shock or ECPR, in whom randomisation was also sub-stratified), age (≤ 50 or > 50 years), time to intervention (≤ 3 h or > 3 h) and illness severity categorised using the median Survival After VA-ECMO (SAVE) score [21]. Subgroup and per-protocol analyses are presented in a forest plot.
Statistical significance for the primary outcome was determined using a two-sided hypothesis test with an alpha of 0.05. In the absence of adjustment for multiplicity, subgroup analyses should be considered exploratory and width of confidence intervals for secondary outcome comparison should not be used in place of hypothesis testing. A detailed analysis plan was published prior to study completion [12, 13]. Further details about the analysis are provided in the ESM.
Results
Patients
From September 2019 through June 2023, 934 patients at 26 hospitals received VA-ECMO and were reported to the EXCEL registry, representing 58.2% of the total 1606 patients listed as having received ECMO of any mode throughout Australia in The Australian and New Zealand Intensive Care Society registry during the same period. There were 594 patients who received VA-ECMO at the 13 participating hospitals. Of these, 300 patients (192 cardiogenic shock, 108 ECPR) were recruited into the trial at 12 hospitals (ESM, Fig. S5). Their characteristics were similar to other patients who received VA-ECMO. However, trial participants had lower mortality (ESM, Table S1).
Overall, 149 patients were assigned to the conservative oxygen strategy and 151 to the liberal strategy. All patients or their surrogate agreed to continue the trial when approached after initially being randomised. Baseline characteristics of patients in each intervention group are reported in Table 1. Characteristics of the pre-specified sub-stratified subgroups of cardiogenic shock and ECPR patients are reported in ESM, Table S3 and S4.
Oxygen strategy intervention
Patient and post-oxygenator partial pressures, oxygen saturations (Fig. 1) and inspired oxygen percentages delivered to the patient/ventilator and to the ECMO oxygenator (ESM, Fig. S6) were all lower in the conservative group.
Partial pressures of arterial oxygen and oxygen saturations of the patient and post-oxygenator. Shown are the partial pressures (panels a., b.) and saturations (panels c.–e.) of oxygen recorded from the post-oxygenator (PbO2, SbO2), the radial arterial line (PaO2, SaO2) and pulse oximetry on the right hand (SpO2) of patients still on ECMO from day 1 to 7. Patients were assigned to either a conservative oxygen strategy (SaO2 92–96%) or to a liberal oxygen strategy (SaO2 97–100%) at the time of randomization. The intervention strategy was continued until cessation of ECMO. One patient in the conservative group who died on day 1, had no blood gas values recorded. All curves show the geometric mean and 95% confidence interval of the available values
The prevalence of hypoxaemia measured in the patient’s radial artery was similar in both groups. There were 135/149 (90.6%) patients in conservative group with at least one PaO2 < 60 mm Hg during the first seven days, similar to 132/151 (87.4%) in the liberal group. The prevalence of hypoxaemia in post-oxygenator blood gas samples was higher in the conservative group with 43/1013 (4.2%) of PaO2 values < 60 mm Hg compared to 3/909 (0.3%) in the liberal group. The conservative group experienced less hyperoxaemia, with 40/149 (26.8%) patients having at least one PaO2 measurement > 300 mm Hg during the first seven days, compared to 65/151 (43.1%) in the liberal group (ESM, Methods).
Haemoglobin, lactate levels, inotrope usage, ECMO blood flow rates, use of invasive ventilation and renal replacement therapy were similar in both groups. Conservative group patients experienced more major protocol deviations and were more commonly withdrawn from their allocated oxygen strategy than the liberal group (Table 2).
Primary outcome
The median number of ICU-free days to day 28 was 0 days (interquartile range [IQR] 0–13.7) in the conservative group and 0 days (IQR 0–13.3) in the liberal group (median difference 0 days (95% CI – 3.1 to 3.1). These results were consistent after adjusting for stratification (site and ECMO mode), age, diagnosis, and baseline imbalance (time from ECMO initiation to randomisation) (Table 3).
Outcomes in all subgroups were consistent with the primary analysis including VA-ECMO for cardiogenic shock, ECPR, the per-protocol group, ages above or below 50 years, SAVE scores above or below the median value of – 5, and in those randomised before or after 3 h from initiation of ECMO (Fig. 2, and ESM, Table S3 and S4).
Overall and subgroup analyses of difference in medians for intensive care unit (ICU)-free days to day 28. Shown are the results of analysis of difference in medians for ICU-free days to day 28 between the conservative oxygen strategy (SaO2 92–96%) group and the liberal oxygen strategy (SaO2 97–100%) group. Groups shown include the whole 300 patient cohort included in the ‘intention-to-treat’ analysis, 254 patients included in the per-protocol analysis, pre-specified subgroups of 184 patients aged above 50 years, 116 patients aged 50 years or less, 160 patients with a SAVE score of – 5 or less, 130 patients with a SAVE score of more than – 5, 176 patients randomised within three hours or less from VA-ECMO cannulation, 124 patients randomised more than three hours from VA-ECMO cannulation, 192 patients who underwent VA-ECMO for cardiogenic shock and 108 patients who received ECMO cardio-pulmonary resuscitation. VA-ECMO venoarterial extracorporeal membrane oxygenation, SAVE Survival After Venoarterial ECMO score
Secondary Outcomes
Secondary outcomes were similar in both groups including ICU-free days to day 28 accounting for discharge delay, ICU-free days to day 60 with or without accounting for discharge delay, patient survival up to 6 months, time to extubation, ECMO decannulation/cessation and discharge from ICU or hospital. Assessments of disability and quality of life at 6 months were similar in both groups (Table 3, Fig. 3, and ESM, Figs. S12–S15). In the subgroups of cardiogenic shock and ECPR patients, there were no differences in any secondary outcomes (ESM, Table S3 and S4).
Adverse events
The frequency of adverse events such as critical hypoxaemia episodes, cardiopulmonary resuscitation or seizures were similar (Table 2).
Discussion
In this registry-embedded, randomised trial, we compared a conservative oxygen strategy to a liberal oxygen strategy in 300 patients receiving VA-ECMO in the ICU. The number of ICU-free days to day 28 was similar in both groups. These findings remained consistent across all prespecified subgroups, including VA-ECMO for cardiogenic shock and ECPR for refractory cardiac arrest. Secondary outcomes including mortality up to 180 days and adverse events were also similar in both groups. Patients in the conservative oxygen group were more likely to deviate from the protocol or be withdrawn from their allocated oxygen strategy.
Patients receiving VA-ECMO are commonly exposed to hyperoxaemic blood returning to the body from the ECMO circuit and are thus liable to its potential adverse effects [2, 22]. Observational studies have identified an association between hyperoxaemia and adverse outcomes in a variety of critically ill patient groups [23,24,25]. This has been consistently reported in patients receiving VA-ECMO [2, 7, 22, 26, 27]. However, the degree to which this is driven by direct hyperoxaemic injury rather than underlying disease severity (such as a low native cardiac output) is uncertain [27].
The findings of other interventional oxygen target trials in the general ICU population have been inconsistent. Earlier trials suggested conservative oxygen targets may reduce mortality in critically ill patients [28, 29]. Subsequent trials have either failed to show any difference [30,31,32,33] or suggested potential harm from conservative oxygen targets in patients with cardiac arrest and acute respiratory distress syndrome [8, 10].
The lack of difference in the primary or secondary outcomes in our trial suggests that avoidance of hyperoxaemia while receiving VA-ECMO is unlikely to impact patient outcomes. It also implies that the previously demonstrated association between hyperoxaemia and adverse outcomes was likely primarily driven by underlying pathological processes.
Our trial has several strengths. Our findings are highly generalisable. Its design as a registry-embedded pragmatic trial allowed us to recruit patients from multiple hospitals and ensure participants were characteristic of all other patients receiving VA-ECMO throughout Australia. We examined both short-term survival outcomes and long-term functional status. Over one third of the recruited patients received ECPR, a group likely to be highly sensitive to the adverse effects of high or low oxygen levels. Our protocol mandated titration of both ventilator and oxygenator blender. This increased the chance of consistent oxygenation levels throughout the patient’s arterial system (i.e. less differential hypoxia). Although our intervention only targeted blood oxygenation, other markers of oxygen delivery such as haemoglobin, lactate, inotrope use and ECMO blood flow were similar in both groups.
Our study also had several limitations. Despite lower oxygenation parameters in the conservative group (Fig. 1), there were 40 (27%) patients in this group who experienced at least one episode of severe hyperoxaemia (PaO2 > 300 mm Hg). We cannot determine if a lower FbO2 target might have resulted in greater group separation but also a greater risk of hypoxaemia. Our choice of primary outcome was based on pilot data where the mean ICU-free days was 11.8 ± 8.4 days [19]. Our recruitment of more ECPR patients (over half of whom died) contributed to the finding of a median of zero ICU-free days overall and suggests that ICU-free days to day 28 may not be an informative primary outcome measure for this cohort. We cannot tell if other unmeasured outcome measures might be more sensitive to changes in oxygenation status. We found no evidence of an increase in adverse events due to hypoxia, but patients in the conservative group more commonly had hypoxaemic post-oxygenator samples and were more likely to discontinue their allocated oxygen intervention protocol. Potential reasons include local difficulties applying the protocol or the development of transient hypoxia, which prompted clinicians to remove the patient from the allocated oxygen strategy. However, there was no difference in outcomes between the two groups in the per-protocol analysis. As a registry-embedded trial, oxygenation values which were taken more frequently at the hospital, as well as details about the nature of the protocol deviations were unavailable in the registry dataset. There was also no reporting of vasopressor dose or liver function to the registry and no reporting of blood gas data to the registry after day seven, although monitoring of protocol compliance and local site-based measurements of oxygenation continued throughout the whole duration of ECMO support. Non-invasive monitoring of post-oxygenator saturations was not available. Although right hand pulse oximetry values were available for all patients, the proportion of arterial blood gases taken from the right (as opposed to left) radial artery was unknown. The impact of difficulty in measuring patients’ oxygen saturations related to skin perfusion, skin colour or limited native pulsatility is unknown. The applicability of our findings to patients who do not require VA ECMO and the possibility of heterogeneity in effect of oxygen strategies in different patient groups cannot be determined from our study [34, 35]. Clinicians and patients were unblinded. However, follow-up adjudicators were unaware of the allocated intervention strategy. Biochemical markers of inflammation and ‘oxygen stress’ were not available.
In adults receiving VA-ECMO in the ICU, a conservative compared to a liberal oxygen strategy did not affect the number of ICU-free days to day 28, nor any other measurable patient outcomes up to 6 months after ICU admission. A conservative oxygen strategy resulted in more frequent protocol deviations.
Data availability
Requests for data dictionary, analytic code or deidentified participant data will be considered by the management committee, and released only after approval with a signed data use agreement. Requests should be sent to anzicrc@monash.edu and the corresponding author, david.pilcher@monash.edu.
References
Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, Haft J, Vercaemst L, Pappalardo F, Bermudez C, Belohlavek J, Hou X, Boeken U, Castillo R, Donker DW, Abrams D, Ranucci M, Hryniewicz K, Chavez I, Chen YS, Salazar L, Whitman G (2021) ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J 67:827–844
Munshi L, Kiss A, Cypel M, Keshavjee S, Ferguson ND, Fan E (2017) Oxygen thresholds and mortality during extracorporeal life support in adult patients. Crit Care Med 45:1997–2005
Premraj L, Brown A, Fraser JF, Pellegrino V, Pilcher D, Burrell A (2023) Oxygenation during venoarterial extracorporeal membrane oxygenation: physiology, current evidence, and a pragmatic approach to oxygen titration. Crit Care Med 52(4):637–648
McDonald CI, Fraser JF, Coombes JS, Fung YL (2014) Oxidative stress during extracorporeal circulation. Eur J Cardiothorac Surg 46:937–943
Fujii Y, Tatsumi E, Nakamura F, Oite T (2020) PaO2 greater than 300 mmHg promotes an inflammatory response during extracorporeal circulation in a rat extracorporeal membrane oxygenation model. J Thorac Dis 12:749–757
Jentzer JC, Miller PE, Tonna JE (2023) Response by Jentzer et al to letters regarding article, “Exposure to Arterial Hyperoxia During Extracorporeal Membrane Oxygenator Support and Mortality in Patients With Cardiogenic Shock.” Circ Heart Fail 16:e010732
Tigano S, Caruso A, Liotta C, LaVia L, Vargas M, Romagnoli S, Landoni G, Sanfilippo F (2024) Exposure to severe hyperoxemia worsens survival and neurological outcome in patients supported by veno-arterial extracorporeal membrane oxygenation: a meta-analysis. Resuscitation 194:110071
Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, Souweine B, Collange O, Pottecher J, Levy B, Puyraveau M, Vettoretti L, Constantin JM, Capellier G, Network LIaRR (2020) Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med 382:999–1008
Klitgaard TL, Schjørring OL, Nielsen FM, Meyhoff CS, Perner A, Wetterslev J, Rasmussen BS, Barbateskovic M (2023) Higher versus lower fractions of inspired oxygen or targets of arterial oxygenation for adults admitted to the intensive care unit. Cochrane Database Syst Rev 9:CD012631
Bernard SA, Bray JE, Smith K, Stephenson M, Finn J, Grantham H, Hein C, Masters S, Stub D, Perkins GD, Dodge N, Martin C, Hopkins S, Cameron P, Investigators E (2022) Effect of lower vs higher oxygen saturation targets on survival to hospital discharge among patients resuscitated after out-of-hospital cardiac arrest: the EXACT randomized clinical trial. JAMA 328:1818–1826
Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, Bernard S, Finney SJ, Grunau B, Youngquist ST, McKellar SH, Shinar Z, Bartos JA, Becker LB, Yannopoulos D, Bˇelohlávek J, Lamhaut L, Pellegrino V (2021) Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J 67:221–228
Burrell A, Ng S, Ottosen K, Bailey M, Buscher H, Fraser J, Udy A, Gattas D, Totaro R, Bellomo R, Forrest P, Martin E, Reid L, Ziegenfuss M, Eastwood G, Higgins A, Hodgson C, Litton E, Nair P, Orford N, Pellegrino V, Shekar K, Trapani T, Pilcher D (2023) Blend to limit OxygEN in ECMO: a RanDomised ControllEd Registry (BLENDER) trial: study protocol and statistical analysis plan. Crit Care Resusc 25:118–125
Burrell A, Ng S, Ottosen K, Bailey M, Buscher H, Fraser J, Udy A, Gattas D, Totaro R, Bellomo R, Forrest P, Martin E, Reid L, Ziegenfuss M, Eastwood G, Higgins A, Hodgson C, Litton E, Nair P, Orford N, Pellegrino V, Shekar K, Trapani T, Pilcher D (2024) Corrigendum to “Blend to Limit OxygEN in ECMO: a RanDomised ControllEd Registry (BLENDER) trial: study protocol and statistical analysis plan.” Crit Care Resusc 26(1):60
Forster GM, Bihari S, Tiruvoipati R, Bailey M, Pilcher D (2020) The association between discharge delay from intensive care and patient outcomes. Am J Respir Crit Care Med 202:1399–1406
Hodgson CL, Fulcher B, Mariajoseph FP, Burrell AJC, Pellegrino V, Brodie D, Fan E, Network SSIobotIE (2021) A core outcome set for research in patients on extracorporeal membrane oxygenation. Crit Care Med 49:e1252–e1254
Hodgson CL, Higgins AM, Bailey MJ, Anderson S, Bernard S, Fulcher BJ, Koe D, Linke NJ, Board JV, Brodie D, Buhr H, Burrell AJC, Cooper DJ, Fan E, Fraser JF, Gattas DJ, Hopper IK, Huckson S, Litton E, McGuinness SP, Nair P, Orford N, Parke RL, Pellegrino VA, Pilcher DV, Sheldrake J, Reddi BAJ, Stub D, Trapani TV, Udy AA, SerpaNeto A, Group ESIobotIENatAaNZICSCT (2022) Incidence of death or disability at 6 months after extracorporeal membrane oxygenation in Australia: a prospective, multicentre, registry-embedded cohort study. Lancet Respir Med 10:1038–1048
Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resources Evaluation (2022) Adult Patient Database Data Dictionary Version 6.1. https://anzics.org/wp-content/uploads/2021/03/ANZICS-APD-Dictionary-Version-6.1.pdf. Accessed 11 June 2024
Secombe P, Millar J, Litton E, Chavan S, Hensman T, Hart GK, Slater A, Herkes R, Huckson S, Pilcher DV (2023) Thirty years of ANZICS CORE: a clinical quality success story. Crit Care Resusc 25:43–46
Burrell AJ, Pellegrino VA, Wolfe R, Wong WK, Cooper DJ, Kaye DM, Pilcher DV (2015) Long-term survival of adults with cardiogenic shock after venoarterial extracorporeal membrane oxygenation. J Crit Care 30(5):949–956
Lau B, Cole SR, Gange SJ (2009) Competing risk regression models for epidemiologic data. Am J Epidemiol 170:244–256
Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D (2015) Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 36:2246–2256
Halter M, Jouffroy R, Saade A, Philippe P, Carli P, Vivien B (2020) Association between hyperoxemia and mortality in patients treated by eCPR after out-of-hospital cardiac arrest. Am J Emerg Med 38:900–905
Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, Parrillo JE, Trzeciak S, Investigators EMSRNE (2010) Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 303:2165–2171
Boyle AJ, Holmes DN, Hackett J, Gilliland S, McCloskey M, O’Kane CM, Young P, Di Gangi S, McAuley DF (2021) Hyperoxaemia and hypoxaemia are associated with harm in patients with ARDS. BMC Pulm Med 21:285
Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schünemann HJ, Neary JD, Alhazzani W (2018) Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 391:1693–1705
Bonnemain J, Rusca M, Ltaief Z, Roumy A, Tozzi P, Oddo M, Kirsch M, Liaudet L (2021) Hyperoxia during extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest is associated with severe circulatory failure and increased mortality. BMC Cardiovasc Disord 21:542
Moussa MD, Beyls C, Lamer A, Roksic S, Juthier F, Leroy G, Petitgand V, Rousse N, Decoene C, Dupré C, Caus T, Huette P, Guilbart M, Guinot PG, Besserve P, Mahjoub Y, Dupont H, Robin E, Meynier J, Vincentelli A, Abou-Arab O (2022) Early hyperoxia and 28-day mortality in patients on venoarterial ECMO support for refractory cardiogenic shock: a bicenter retrospective propensity score-weighted analysis. Crit Care 26:257
Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M (2016) Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA 316:1583–1589
Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, Grimaldi D, Grelon F, Anguel N, Lasocki S, Henry-Lagarrigue M, Gonzalez F, Legay F, Guitton C, Schenck M, Doise JM, Devaquet J, Van Der Linden T, Chatellier D, Rigaud JP, Dellamonica J, Tamion F, Meziani F, Mercat A, Dreyfuss D, Seegers V, Radermacher P, Investigators HS, network Rr (2017) Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med 5:180–190
Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R, Young P, Group I-RIatAaNZICSCT, Group I-RItAaNZICSCT (2020) Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med 382:989–998
Schmidt H, Kjaergaard J, Hassager C, Mølstrøm S, Grand J, Borregaard B, RoelsgaardObling LE, Venø S, Sarkisian L, Mamaev D, Jensen LO, Nyholm B, Høfsten DE, Josiassen J, Thomsen JH, Thune JJ, Lindholm MG, Stengaard Meyer MA, Winther-Jensen M, Sørensen M, Frydland M, Beske RP, Frikke-Schmidt R, Wiberg S, Boesgaard S, Lind Jørgensen V, Møller JE (2022) Oxygen targets in comatose survivors of cardiac arrest. N Engl J Med 387:1467–1476
Semler MW, Casey JD, Lloyd BD, Hastings PG, Hays MA, Stollings JL, Buell KG, Brems JH, Qian ET, Seitz KP, Wang L, Lindsell CJ, Freundlich RE, Wanderer JP, Han JH, Bernard GR, Self WH, Rice TW, Group PIatPCCR (2022) Oxygen-saturation targets for critically ill adults receiving mechanical ventilation. N Engl J Med 387:1759–1769
Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, Bäcklund M, Keus F, Laake JH, Morgan M, Thormar KM, Rosborg SA, Bisgaard J, Erntgaard AES, Lynnerup AH, Pedersen RL, Crescioli E, Gielstrup TC, Behzadi MT, Poulsen LM, Estrup S, Laigaard JP, Andersen C, Mortensen CB, Brand BA, White J, Jarnvig IL, Møller MH, Quist L, Bestle MH, Schønemann-Lund M, Kamper MK, Hindborg M, Hollinger A, Gebhard CE, Zellweger N, Meyhoff CS, Hjort M, Bech LK, Grøfte T, Bundgaard H, Østergaard LHM, Thyø MA, Hildebrandt T, Uslu B, Sølling CG, Møller-Nielsen N, Brøchner AC, Borup M, Okkonen M, Dieperink W, Pedersen UG, Andreasen AS, Buus L, Aslam TN, Winding RR, Schefold JC, Thorup SB, Iversen SA, Engstrøm J, Kjær MN, Rasmussen BS, Investigators H-I (2021) Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med 384:1301–1311
Dumas G, Morris IS, Hensman T, Bagshaw SM, Demoule A, Ferreyro BL, Kouatchet A, Lemiale V, Mokart D, Pene F, Mehta S, Azoulay E, Munshi L, the AnzicstCC, the G-OHSG (2024) Association between arterial oxygen and mortality across critically ill patients with hematologic malignancies: results from an international collaborative network. Intensive Care Med 50:697–711
Buell KG, Spicer AB, Casey JD, Seitz KP, Qian ET, Graham Linck EJ, Self WH, Rice TW, Sinha P, Young PJ, Semler MW, Churpek MM (2024) Individualized treatment effects of oxygen targets in mechanically ventilated critically ill adults. JAMA 331:1195–1204
Acknowledgements
BLENDER Trial Investigators: Writing Committee: Aidan Burrell, Michael J. Bailey, Rinaldo Bellomo, Hergen Buscher, Glenn Eastwood, Paul Forrest, John F. Fraser, Bentley Fulcher, David Gattas, Alisa M. Higgins, Carol L. Hodgson, Edward Litton, Emma-Leah Martin, Priya Nair, Sze J. Ng, Neil Orford, Kelly Ottosen, Eldho Paul, Vincent Pellegrino, Liadain Reid, Kiran Shekar, Richard J. Totaro, Tony Trapani, Andrew Udy, Marc Ziegenfuss, David Pilcher.
BLENDER Management Committee: David Pilcher, Aidan Burrell, Michael Bailey, Rinaldo Bellomo, Hergen Buscher, Amanda Corley (until 2021), Glenn Eastwood, Paul Forrest, John Fraser, David Gattas, Alisa Higgins, Andrew Hilton (deceased 2022), Carol Hodgson, Edward Litton, Emma-Leah Martin, Priya Nair, Sze Ng, Neil Orford, Kelly Ottosen, Vincent Pellegrino, Liadain Reid, Kiran Shekar, Richard Totaro, Tony Trapani, Andrew Udy, Marc Ziegenfuss.
BLENDER Participating Sites: Alfred Hospital, Melbourne, Victoria: Aidan Burrell, Emma-Leah Martin, Meredith Young, Jasmin Board, Annalie Jones, Phoebe McCracken, David Pilcher, Andrew Udy, Vincent Pellegrino, Alastair Brown.
Austin Health, Heidelberg, Victoria: Rinaldo Bellomo, Glenn Eastwood, Helen Young, Leah Peck, Andrew Hilton. Box Hill Hospital, Box Hill, Victoria: John Dyett, Stephanie Hunter, Cheelim Liew, Kym Gellie, Nicole Robertson.
Fiona Stanley Hospital, Murdoch, Western Australia: Edward Litton, Anne-Marie Palermo, Chris Allen. Flinders Medical Centre, Adelaide, South Australia: Ubbo Wiersema, Joanne McIntyre, Shailesh Bihari. Geelong University Hospital, Geelong, Victoria: Joe McCaffrey, Neil Orford, Matthew Maiden, Nima Kakho, Allison Bone, Tania Salerno, Michelle Horton, Jemma Trickey, Samantha Breguet, Lucy Range, Meg Gallagher.
Gold Coast University Hospital, Gold Coast, Queensland: James Winearls, Mandy Tallott, Maimoonbe Gough, Julie Pitman, James McCullough, Maree Houbert.
John Hunter Hospital, Newcastle, New South Wales: Lewis McLean, Amber-Louise Poulter, Sarah Dalton, Jorge Brieva, Lucas Webb, Daniel de Wit.
Princess Alexandra Hospital, Woolloongabba, Queensland: James Walsham, Jason Meyer, Meg Harward, Anand Krishnan, Cassie Jones, Josephine Mackay.
Royal Adelaide Hospital, Adelaide, South Australia: Benjamin Reddi, Stephanie O'Connor, Kathleen Glasby, Nerissa Brown, Sarah Doherty, Justine Rivett, Fiona McDonald, Sophie Dohnt, Mahni Foster.
Royal Prince Alfred Hospital, Camperdown, New South Wales: Richard Totaro, Heidi Buhr, Jennifer Coles, Ruaidhri Carey.
St Vincent’s Hospital Sydney, Darlinghurst, New South Wales: Hergen Buscher, Sally Newman, Claire Reynolds. The Prince Charles Hospital, Chermside, Queensland: John Fraser, Andrew Thomas, Rachel Bushell, Dawn Lockwood, Oystein Tronstad, Jiville Latu, India Pearse.
Data Safety Monitoring Committee: Niall D. Ferguson, MD (Chair), University Health Network & Mount Sinai Hospital, Toronto General Research Institute, Toronto, ON M5G 2C4, Canada; Lehana Thabane, PhD, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON L8S 4L8, Canada; Matthieu Schmidt, PhD, Hospital La Pitie Salpetriere, Paris, France.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funded by The Medical Research Futures Fund of Australia. ClinicalTrials.gov number: NCT03841084.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The corresponding author (David Pilcher) states on behalf of all authors, that there are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The BLENDER Trial Investigators are listed in the Acknowledgements section of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Burrell, A., Bailey, M.J., Bellomo, R. et al. Conservative or liberal oxygen targets in patients on venoarterial extracorporeal membrane oxygenation. Intensive Care Med (2024). https://doi.org/10.1007/s00134-024-07564-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00134-024-07564-8