Abstract
Purpose
The development of acute kidney injury (AKI) after the acute respiratory distress syndrome (ARDS) reduces the chance of organ recovery and survival. The purpose of this study was to examine the AKI rate and attributable mortality in ARDS patients.
Methods
We performed an individual patient-data analysis including 10 multicenter randomized controlled trials conducted over 20 years. We employed a Super Learner ensemble technique, including a time-dependent analysis, to estimate the adjusted risk of AKI. We calculated the mortality attributable to AKI using an inverse probability of treatment weighting estimator integrated with the Super Learner.
Results
There were 5148 patients included in this study. The overall incidence of AKI was 43.7% (n = 2251). The adjusted risk of AKI ranged from 38.8% (95% confidence interval [CI], 35.7 to 41.9%) in ARMA, to 55.8% in ROSE (95% CI, 51.9 to 59.6%). 37.1% recovered rapidly from AKI, with a significantly lower recovery rate in recent trials (P < 0.001). The 90-day excess in mortality attributable to AKI was 15.4% (95% CI, 12.8 to 17.9%). It decreased from 25.4% in ARMA (95% CI, 18.7 to 32%), to 11.8% in FACTT (95% CI, 5.5 to 18%) and then remained rather stable over time. The 90-day overall excess in mortality attributable to acute kidney disease was 28.4% (95% CI, 25.3 to 31.5%).
Conclusions
The incidence of AKI appears to be stable over time in patients with ARDS enrolled in randomized trials. The development of AKI remains a significant contributing factor to mortality. These estimates are essential for designing future clinical trials for AKI prevention or treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This individual-patient analysis of randomized controlled trials shows that the adjusted risk of acute kidney injury (AKI) in patients with acute respiratory distress syndrome ranged from 38.8% to 55.8%, with a significantly lower recovery rate in recent trials. The 90-day excess in mortality attributable to AKI was 15.4% and remained rather stable over time after. |
Introduction
Acute kidney injury (AKI) is a common complication of critical illness, including acute respiratory distress syndrome (ARDS) [1]. Mortality is higher in patients with ARDS who develop AKI [2, 3].
Mortality directly attributable to ARDS has been reported to be between 21% and 38% [4], suggesting an important contribution from other factors, most likely comorbidities, and extra-pulmonary organ injury. The attributable mortality from AKI in ARDS patients has however not been reported.
Strategies to prevent new episodes of AKI or improve recovery from AKI therefore carry the promise to decrease mortality in patients with ARDS [5]. Designing trials to prevent or treat ARDS-associated AKI requires a good understanding of both the incidence and attributable mortality of AKI in this population.
An accurate estimate of the attributable mortality of AKI is mandatory when calculating the effect size of an intervention targeting AKI. In other words, calculating a sample size on the overall mortality of ARDS with an intervention targeting AKI would likely overestimate the effect size of the intervention and decrease the power of the trial relative to the attributable mortality of AKI [6].
Therefore, the objective of this study is to assess the incidence of AKI and estimate the attributable mortality from AKI in the population of patients with ARDS, using prospectively collected data from clinical trials over 20 years.
Materials and methods
Study population
We performed a secondary individual patient-data analysis including subjects enrolled in 10 multicenter randomized controlled trials conducted by the ARDS Network and the Prevention and Early Treatment of Acute Lung Injury Network. These trials investigated various supportive and pharmacological interventions for ARDS patients, supplementary Table 1 [7,8,9,10,11,12,13,14,15,16].
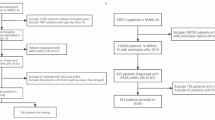
Because some trials employed a factorial design, this analysis incorporated participants from KARMA [8] and LARMA [9] into ARMA [7], and participants from OMEGA [13] into EDEN [14]. The LASRS trial [17] was excluded due to its enrollment of patients with persistent ARDS, which exceeded the timeline of our primary outcome. The patients with end-stage renal disease, defined as chronic maintenance dialysis requirements, were excluded.
Declarations
Each trial received approval from the local (ARDS Network) or central (PETAL) Institutional Review Board, and informed consent was obtained or waived.
Access to the data was authorized via the Biologic Specimen and Data Repository Information Coordinating Center, under the National Heart, Lung, and Blood Institute. Because the data was provided in a de-identified format and the observational nature of this investigation implied no risk exposure for the subjects, the Institutional Review Board at the University of California San Francisco deemed this study exempt from review (Certification #23–38490). Data from each of the included Randomized Controlled Trials is accessible by submitting an application to the Biologic Specimen and Data Repository Information Coordinating Center as per their data sharing policies.
Variables definitions
Demographic data
We report age, sex, comorbidities, and primary risk factors for ARDS at randomization. Malignancy was defined as the presence of a solid tumor, non-Hodgkin lymphoma, or leukemia. Baseline data were collected capturing the most recent values at enrollment.
Potential chronic kidney disease at baseline
Baseline serum creatinine was established as the lowest among the measurements obtained during the 24 h preceding randomization or, if not available, before initiating study procedures.
The glomerular filtration rate (GFR) was estimated per the 2021 CKD-EPI formula, without the race variable [18]. A patient was defined as having potential chronic kidney disease (CKD) at baseline when GFR < 60 ml/kg/1.73 m.2
Respiratory system and mechanical ventilation
We report tidal volume, positive end-expiratory pressure (PEEP), plateau end-inspiratory airway pressure (Pplat), inspired fraction of oxygen (FiO2), and arterial oxygen partial pressure (PaO2) at baseline. The driving pressure was calculated as the difference between Pplat and PEEP. The compliance of the respiratory system was calculated as the ratio of tidal volume to driving pressure. The ventilatory ratio was calculated as [minute ventilation (mL/min) x arterial CO2 partial pressure (mmHg)] / (predicted body weight × 100 × 37.5) [19].
ARDS severity was defined per the Berlin definition [20] and the New Global Definition [21]: mild ARDS when PaO2/FiO2 ratio ≤ 300 mmHg and PaO2/FiO2 ratio > 200 mmHg; moderate ARDS when PaO2/ FiO2 ratio ≤ 200 mmHg and PaO2/FiO2 ratio > 100 mmHg; severe ARDS when PaO2/FiO2 ratio ≤ 100 mmHg.
Rapidly-improving ARDS was defined as meeting one or both of the following conditions: a PaO2/FiO2 ratio > 300 on the first study day after enrollment and/or achieving unassisted breathing on the first study day following enrollment and maintaining independence from assisted breathing for a minimum of 48 h [22, 23].
Organ failure at baseline
Circulatory failure was defined as the use of vasopressor and/or inotropes at enrollment. Vasopressor use was defined as the administration of dopamine at ≥ 6 μg·kg−1·min−1, or norepinephrine, phenylephrine, or vasopressin at any dose. Inotropes use was defined as the administration of dobutamine or epinephrine at any dose.
Coagulation failure was defined as a platelet count < 100·103/µL at enrollment. Hepatic failure was defined as bilirubin ≥ 2 mg/dL at enrollment. Neurologic failure was defined as a Glasgow Coma Score ≤ 12 at enrollment.
Outcomes
The primary outcome was the incidence of AKI. AKI was defined by the creatinine criteria per Kidney Disease: Improving Global Outcomes (KDIGO) [24], characterized by either a minimum increase in serum creatinine of 0.3 mg/dL from baseline within the first 48 h of the study, or an increase in serum creatinine to at least 1.5 times the baseline, or the initiation of renal replacement therapy (RRT) within the first 7 days of the study. Baseline serum creatinine was established as the lowest among the measurements obtained during the 24 h preceding randomization or, if not available, before initiating study procedures.
When RRT data were missing, we defined AKI incidence according solely to the serum creatinine levels. Data regarding the use of RRT was not available for ARMA and ALVEOLI trials (27.2%, 1401 of 5148 patients).
Secondary outcomes included AKI stages, severe AKI, rapidly-improving AKI, acute kidney disease (AKD), mortality at 28 and 90 days, attributable mortality of AKI, attributable mortality of rapidly-improving AKI, attributable mortality of AKD, and population-attributable fraction of mortality from AKI (population-AFAKI).
Stages of AKI were defined according to the serum creatinine and RRT criteria per KDIGO definition [24], with severe AKI as AKI Stage 2 or 3.
Rapidly-improving AKI was defined as achieving a serum creatinine ≤ 1.5 times the baseline and not requiring RRT at 48 h after AKI onset. AKD was defined as not achieving a serum creatinine ≤ 1.5 times the baseline or requiring RRT at 7 days after AKI onset [25]. The closest available observed creatinine value to the 48 h or 7 days timepoint was used for this determination.
Statistical analysis
Continuous data are described as median values (interquartile range [IQR]) and categorical data as numbers and percentages. Continuous variables were compared using the Wilcoxon rank-sum test and categorical variables with Pearson’s chi-squared test or Fisher’s exact test, as appropriate.
To flexibly estimate the conditional trial-specific risks of AKI without relying on strict model assumptions, we used a Super Learner cross-validated stacked ensemble [26] using a library of logistic regression and Bayesian logistic regression, adjusting for both baseline and time-varying confounders. The marginal trial-specific risks were estimated by averaging the cumulative 7-day AKI risks over the observed distribution of a set of baseline covariates agreed upon by the investigators based on a priori clinical reasoning, supplementary Table 2, as well as time-varying covariates, supplementary Table 3, to account for the outcome dependency on discharge and mortality over the study period and for the variability related to gas exchanges, respiratory mechanics, fluid balance, and vasopressors use during the time on-study preceding AKI occurrence.
We defined mortality attributable to AKI as the difference between the mortality risk in a pseudo-population where all subjects developed AKI and the mortality risk in a pseudo-population where none of the subjects developed AKI and estimated this quantity using the Hájek inverse probability of treatment weighting (IPTW) estimator [27]. IPTW estimates this excess mortality by weighting each individual’s mortality status by the inverse of their probability of experiencing AKI, resulting in a pseudo-population in which baseline covariates are independent of AKI status [28].
We defined the population-AFAKI as the proportion of deaths attributable to AKI among all the deaths in the accessible population (patients with ARDS). We calculated the population-AFAKI as the difference between the observed mortality risk and the mortality risk in a pseudo-population where none of the subjects developed AKI, divided by the observed mortality risk.
The propensity score calculation was performed using the Super Learner method; adjusting for the same sets of baseline and time-varying covariates. To evaluate the Super Learner algorithm’s performance, we computed cross-validated negative log loss and cross-validated area under the receiver operating curve, supplementary Table 4.
95% confidence intervals were computed using standard errors estimated by non-parametric bootstrap. We utilized the Multiple Imputation by Chained Equations method to address missingness, supplementary Tables 2 and 3 display the percentage of data imputed in covariates used in the analysis.
For sensitivity analyses, we employed a multivariable logistic regression to assess the association between the development of AKI and 90-day mortality.
We used the IPTW approach previously described to calculate the mortality attributable to severe AKI and to AKD. The same method was applied to estimate trial-specific mortality attributable to AKI, and the trial-specific population-AFAKI. The distribution of the propensity scores is depicted in supplementary Figs. 2 and 3.
A data analysis and statistical plan was written and filed with the local Institutional Review Board and the Biologic Specimen and Data Repository Information Coordinating Center before data were accessed. All statistical analyses were performed using the R software (The R Foundation; R version 4.2.2). The sample size was determined solely based on data availability, without a pre-established effect size, and statistical power calculation. All tests were two-tailed and a p-value < 0.05 was considered statistically significant.
This study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.
Results
Patient characteristics
After merging single-patient observations and excluding patients with end-stage renal disease before enrollment (n = 132), this study included 5148 patients enrolled between 1996 and 2018. Baseline characteristics, including demographics, comorbidities, ARDS risk factors, respiratory system variables, and non-renal organ failure were statistically different among patients with and without AKI, and across trials, Table 1, and supplementary Tables 5, 6, and 7.
36.6% of patients were identified as having potential CKD at baseline. The prevalence of potential CKD at baseline differed across trials, supplementary Table 8.
Patients who developed AKI were significantly older, with higher body mass index, had a higher prevalence of comorbidities, and had more commonly ARDS related to pneumonia, Table 1. Moreover, patients who experienced AKI had worse oxygenation levels (PaO2/FiO2 ratio and ARDS severity), lower compliance of the respiratory system, and higher ventilatory pressures, Table 1, and a higher prevalence of organ failure at baseline, supplementary Table 6. Notably, the ROSE trial [16] included patients with more severe ARDS, distinguished by significantly older age, higher body mass index (BMI), and a more prevalent history of diabetes and organs failure, supplementary Tables 5 and 7.
The overall percentage of patients identified as having Rapidly-improving ARDS was 9.5% (490 out of 5148), supplementary Table 9.
Acute kidney injury
Overall, 2251 out of 5148 patients (43.7%) developed AKI, with an unadjusted incidence of AKI increasing from 37.9% (332 of 876) in the first trial, to 63.1% (587 of 930) in the latest trial, with statistically significant difference between the trials (P < 0.001, Table 2).
The unadjusted incidence of AKI and the median cumulative fluid balance at 72 h after enrollment were significantly different between the intervention and control groups in the FACTT trial, but not in other trials, supplementary Fig. 1 and supplementary Table 10.
The adjusted conditional risk of AKI ranged from 38.8% (95% CI, 35.7 to 41.9%) in ARMA, to 39.2% (95% CI, 35.7 to 42.7%) in SAILS, and was higher in ROSE (conditional risk of AKI 55.8%; 95% CI, 51.9 to 59.6%), Fig. 1.
Conditional risks for AKI and severe AKI. The bright red plot represents the adjusted trial-specific risks of AKI over time. The dark red plot represents the adjusted trial-specific risks of severe AKI over time. The adjusted trial-specific risks are estimated with Super Learner, by averaging the estimated conditional AKI risks over the observed distribution of the baseline and time-varying covariates included in the model, supplementary Tables 2 and 3. The dates on the x-axis refer to the enrollment period of each trial
1422 of 5148 patients (27.6%) developed Severe AKI. Out of the 2251 patients with AKI, 829 (36.8%) were classified as Stage 1, 357 (15.9%) as Stage 2, and 1065 (47.3%) as Stage 3, with statistically significant differences across the trials (P < 0.001), Table 2.
Across the 2251 patients with AKI, 834 (37.1%) met the criteria for rapidly-improving AKI, and 1041 (46.2%) met the criteria for AKD, with a significant difference in the recovery pattern among trials (P < 0.001), supplementary Table 11, Fig. 3. Rapidly-improving AKI was more common in patients with milder stages of AKI; while AKD was more common in patients with more severe stages of AKI, supplementary Table 12.
Attributable mortality of AKI
In the overall population, 1,331 of 5148 patients died by day 28 (25.9%) and 1,573 (30.6%) died by day 90, Table 2. The unadjusted 90-day mortality varied significantly over time across different trials, ranging from 34.5% in ARMA to 27.3% in SAILS (P < 0.001). Notably, we observed a higher 90-day mortality (42.6%) in ROSE, the more recent trial (P < 0.001, Table 2).
Multivariable logistic regression revealed a significant association between AKI occurrence and 90-day mortality (adjusted odds ratio [OR] 2.30, 95% confidence interval [CI] 2.00 to 2.65), with the risk increasing with the severity of AKI (adjusted OR for severe AKI 2.76, 95% CI 2.37 to 3.21), supplementary Tables 13 and 14.
The excess in 28-day mortality attributable to AKI was 14.1% (95% CI, 11.7 to 16.5%); the excess in 90-day mortality attributable to AKI was 15.4% (95% CI, 12.8 to 17.9%), Fig. 2A.
Excess in mortality attributable to AKI and Severe AKI and population-attributable mortality from AKI. In panels A-C, the depicted mortality risk difference is between the mortality risk if hypothetically all patients were to have experienced AKI compared to the mortality risk if hypothetically no patients were to have experienced AKI. panel A depicts the excess in mortality attributable to AKI at 28 and 90 days; panel B depicts the excess in mortality attributable to severe AKI at 28 and 90 days; panel C depicts the excess in mortality attributable to different patterns of renal recovery at 28 and 90 days; panel D depicts the excess in 90-day mortality attributable to AKI when calculated separately for each trial; panel E depicts the trial-specific population-attributable fraction of mortality from AKI (population-AFAKI) at 90 days. Given the model assumptions, population-AFAKI represents the proportion of deaths attributable to AKI among all deaths in the population of patients with ARDS that constitute the population of this study. The dates on the x-axis refer to the enrollment period of each trial
The excess in mortality attributable to severe AKI was 18.6% at 28 days (95% CI, 15.4 to 21.7%); and 20.3% at 90 days (95% CI, 17 to 23.6%), Fig. 2B.
The excess in mortality attributable to rapidly-improving AKI was 4.2% at 28 days (95% CI, 1.3 to 7%) and 4.7% at 90 days (95% CI, 1.4 to 7.8%); while the excess in mortality attributable to AKD was 25.3% at 28 days (95% CI, 22.3 to 28.3%) and 28.4% at 90 days (95% CI, 25.3 to 31.5%), Fig. 2C.
The excess in 90-day mortality varied from 25.4% in ARMA (95% CI, 18.7 to 32%), to 11.2% in FACTT (95% CI, 5.5 to 18%) and then remained stable over time, reaching 14.4% in the ROSE trial (95% CI, 7.7 to 21%), Fig. 2D. The overall population-AFAKI was 24.8% at 90 days (95% CI, 20.7 to 28.9%); Fig. 2E shows the population-AFAKI for each trial.
Discussion
This individual patient analysis among 5148 patients with ARDS, enrolled over 20 years in 10 multicenter randomized clinical trials, shows a 43.7% crude incidence of AKI. The risk of developing AKI remained stable over time across the trials, after adjusting for risk factors. The excess mortality attributable to AKI was estimated to be 15.4% at 90 days, highlighting a substantial impact on the clinical outcomes of ARDS patients. Of note, the timing of AKI onset aligns with previous studies, with AKI occurring predominantly during the first days of critical illness [29,30,31].
We observed a high incidence of AKI and higher attributable mortality compared to the overall critically ill patients. In patients with ARDS, AKI may result from a combination of mechanisms [32, 33] including systemic release of pro-inflammatory mediators and biotrauma [33], as well as endothelial injury and increased capillary permeability. Furthermore, hypoxemia may reduce renal blood flow and lead to altered renal function [34]. Elevation of central venous pressure under mechanical ventilation may translate into elevated renal venous pressure and reduced renal perfusion pressure [35, 36].
We estimated the conditional risk of AKI by adjusting for multiple baseline and time-dependent covariates associated with an increased risk of kidney injury. To account for differences between the studies in individual confounders, we used the Super Learner model to compute trial-specific AKI incidences, all marginalized over the full set of observed individual confounders, with different hypothetical trial assignments. We considered the chronological order of the trials to be a corse capture of the trend over time (Fig. 1). Our observation aligns with other large studies in high-resource countries showing a stable trend in the incidence of AKI in critically ill patients [37, 38]. While critical care processes and overall prognosis of patients admitted to intensive care units have improved over the last two decades [39], this does not appear to apply to the prevention of AKI. AKI incidence was found higher in the ROSE trial. The isolated higher risk of AKI in ROSE is likely due to the sicker population enrolled in the trial (ROSE included patients with a PaO2/FiO2 ratio ≤ 150 mmHg) with higher severity score. This is reflected by the higher mortality in the ROSE trial (42.6% 90-day mortality in ROSE, 27.3% in SAILS, for instance). Similar findings were observed in the ACURASYS trial [40] in which RRT was needed in 33% and 36% in the patients randomized in the neuromuscular agent and placebo groups, respectively.
We also observed no improvement in renal recovery (and a trend toward increased incidence of AKD, Fig. 3). This observation is alarming as non-recovery from AKI is associated with a higher risk of adverse outcomes such as death, cardiovascular diseases, or CKD [41, 42].
Observed renal recovery pattern across trials. A patient was considered to have rapidly-improving AKI when the serum creatinine level was ≤ 1.5 times the baseline value and the patient did not require renal replacement therapy (RRT) within 48 h after AKI onset. A patient was defined as having acute kidney disease (AKD) when the serum creatinine level was > 1.5 times the baseline value or required RRT after 7 days after AKI onset
In this line, the lack of interventions to prevent or treat AKI among critically ill patients has been, and continues to be, a major concern. Recent efforts have highlighted the need for changes to improve the chances of success in trials to prevent and treat AKI in the critically ill [5].
A crosstalk between the lung and kidney has long been reported, with lung injury being a risk factor for AKI, and AKI being a trigger for lung injury [43]. AKI could therefore drive mortality through remote organ injury [41]. It is believed that the occurrence of AKI in patients with lung injury contributes to a vicious circle of damage compromising the chances of recovery and survival [32]. In critically ill patients, the excess in 90-day mortality attributable to AKI has been estimated to be 8.6% (95% CI 2.6–17.6) [44]. Another prospective study estimated the excess 30-day mortality linked to early AKI in general intensive care unit (ICU) patients to be 8.1% (95% CI 4.1–12.1%) [45].
We observed an excess in 90-day mortality attributable to AKI of 15.4%, which increased to 20.3% when considering severe AKI (Fig. 2). The excess in 90 days mortality appeared to decrease after the ALVEOLI trial (i.e. lower or higher PEEP levels) and remained stable since then. Nonetheless, the multivariable logistic regression model used for sensitivity analysis showed that ALTA, SAILS, and EDEN trials were associated with a significantly lower risk of mortality when compared to ARMA. The reduction of mortality attributable to AKI after the implementation of the first interventions introduced by the ARDS network might be due to the use of protective ventilation, mediated by a reduction in venous congestion or systemic inflammation [46] (Fig. 2). The differences in AKI incidence between the intervention and control groups in the FACTT trial could be related to chance or to the intervention itself. The FACTT trial investigated the impact of a restrictive versus liberal fluid strategy in patients with ARDS. This resulted in more negative fluid balance in the intervention group. The intervention group also showed a higher incidence of AKI. This could be explained by hypovolemia or hemoconcentration in the intervention group. After adjustment of creatinine for fluid balance, Liu et al. reported that the incidence of AKI was greater in those managed with the liberal fluid protocol (66 versus 58%, P = 0.007) [1]. Cumulative fluid balance at 72 h after enrollment was mostly similar between the intervention and control groups in other trials.
The overall stable attributable mortality highlights the needs for developing preventive and curative interventions for AKI in ARDS patients.
The results of our study have several strengths and implications. We used Super Learner for AKI incidence estimation, accounting for variations in AKI rates between trials, across treatment groups and individual characteristics, which minimized the contribution of coufounding factors.
Furthermore, we introduced factors previously linked to mortality in patients with AKI and adjusted for them in a time-dependent analysis to account for covariates trajectories effect on the development of AKI. Death and ICU discharge within the first 7 days were treated as competing risks to avoid immortal time bias.
Finally we integrated the Super Learner with the IPTW analysis to estimate the attributable mortality during ARDS management.
The estimated attributable mortality will serve to design future trials with mortality as the primary endpoint. The observed attributable mortality of AKI could guide calculating the appropriate sample size with the effect size in future trials. Unrealistic effect size estimations are one of the reasons for the multiple so-called “negative trials” in critically ill patients [6, 47].
Our study has several limitations. First, we included only patients enrolled in randomized trials performed in the United States which may limit the generalizability of our findings, as the accessible population may not be representative of the broader population of ARDS patients. Of note, the trials included in the study were not originally designed for the purpose of evaluating the incidence and attributable mortality of AKI in ARDS patients. The limited amount of data available influenced the development of the most effective model for testing our hypothesis. The definition of ARDS varied during the timeline in which the trials we included were performed, supplementary Table 1. Nonetheless, all of them used the American-European Consensus Conference on ARDS (1994) [48], except for SAILS, which included only patients having sepsis-related ARDS, and ROSE, which only included patients with a PaO2/FiO2 ratio ≤ 150 mmHg.
However, this cohort is likely to be very close to the population eligible for many future trials in ARDS, although the New Global Definition of ARDS recommends including patients who are being treated with high flow nasal oxygen or continuous positive airway pressure [21].
Second, some unmeasured confounders (e.g. chronic cardiovascular, respiratory, and metabolic conditions) persist in the estimation of the risk of AKI and the attributable mortality to AKI. Inflammatory, immunologic, or genetic factors, measures of venous congestion (e.g. central venous pressure), and other surrogate markers of renal function (e.g. cystatin C) were not included in the models and should be considered for future studies.
The study did not explore the mechanisms of AKI in ARDS patients. AKI in ARDS is multifactorial, including inflammation, hemodynamic factors, hypoxia, drug toxicity or strategies of mechanical ventilation and it is likely that some episodes of AKI were not directly caused by ARDS. The cause of death was not available for the observations included in our original dataset. A direct cause of death is most often difficult to identify in ICU patients as several contributing factors are at play (e.g. cardiovascular dysfunction, sepsis), and respiratory failure is rarely the sole or primary contributor [49, 50].
AKI was defined by an increase in serum creatinine from baseline, as all positive delta in serum creatinine reflects a change in GFR and meets the definition of AKI. Therefore, patients who had an elevation of serum creatinine prior to admission and a decline before randomization were not considered to have developed AKI during their ICU stay for ARDS. Unavailability of serum creatinine prior to admission may have misclassified patients with underlying CKD or patients who had AKI with peak serum creatinine before admission and did not recover. We consider this scenario unlikely for the majority of patients. Then, we estimated potential CKD upon baseline serum creatinine levels and excluded patients with chronic dialysis. Nonetheless, using the acute variation of serum creatinine from baseline would identify patients with AKI, regardless of their CKD status, including de novo and acute-on-chronic injury [51]. Furthermore, we provided results of a sensitivity analysis on Severe AKI, to reduce the risk of misclassification.
Finally, urine output was unavailable in most trials. The specificity of urine output as a sole biomarker of AKI (i.e. without elevation of serum creatinine) has however been challenged given the multiple factors affecting urine output in mechanically ventilated patients.
In conclusion, the incidence of AKI appears to be stable over time in the population of patients with ARDS enrolled in randomized trials in the United States, and the development of AKI is a significant contributing factor to mortality. These findings can be used to inform the design of future clinical trials of AKI prevention or treatment in this high-risk subpopulation.
Data availability
Data from each of the included Randomized Controlled Trials is accessible by submitting an application to the Biologic Specimen and Data Repository Information Coordinating Center as per their data sharing policies.
References
Liu KD, Thompson BT, Ancukiewicz M et al (2011) Acute Kidney Injury in Patients with Acute Lung Injury: Impact of Fluid Accumulation on Classification of Acute Kidney Injury and Associated Outcomes. Crit Care Med 39:2665–2671. https://doi.org/10.1097/CCM.0b013e318228234b.Acute
McNicholas BA, Rezoagli E, Tài Pham M, Fabiana M, Elsa G, Vito FP, Giacomo B, Matthew DG, Marco Ranieri M et al (2019) Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit Care Med. https://doi.org/10.1097/CCM.0000000000003832
McNicholas BA, Rezoagli E, Simpkin AJ et al (2023) Epidemiology and outcomes of early-onset AKI in COVID-19-related ARDS in comparison with non-COVID-19-related ARDS: insights from two prospective global cohort studies. Crit Care 27:3. https://doi.org/10.1186/s13054-022-04294-5
Saha R, Pham T, Sinha P et al (2023) Estimating the attributable fraction of mortality from acute respiratory distress syndrome to inform enrichment in future randomised clinical trials. Thorax 78:990–1003. https://doi.org/10.1136/thorax-2023-220262
Legrand M, Bagshaw SM, Koyner JL et al (2022) Optimizing the Design and Analysis of Future AKI Trials. JASN 33:1459–1470. https://doi.org/10.1681/ASN.2021121605
Harhay MO, Wagner J, Ratcliffe SJ et al (2014) Outcomes and statistical power in adult critical care randomized trials. Am J Respir Crit Care Med 189:1469–1478. https://doi.org/10.1164/rccm.201401-0056CP
Network ARDS, Brower RG, Matthay MA et al (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308. https://doi.org/10.1097/00132586-200102000-00017
The ARDS Network (2000) Ketoconazole for Early Treatment of Acute Lung Injury and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA 283:1995–2002. https://doi.org/10.1001/jama.283.15.1995
The ARDS Clinical Trials Network (2002) Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome: Critical Care Medicine 30:1–6. https://doi.org/10.1097/00003246-200201000-00001
Brower RG, Lanken PN, MacIntyre N et al (2004) Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351:327–336. https://doi.org/10.1056/NEJMoa032193
Heart N, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network (ARDS) Clinical Trials, Bernard GR, Thompson BT, et al (2006) Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N Engl J Med 354:2564–2575. https://doi.org/10.1056/NEJMoa062200
Heart N, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Matthay MA, Brower RG, et al (2011) Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184:561–568. https://doi.org/10.1164/rccm.201012-2090OC
Rice TW, Wheeler AP, Thompson BT et al (2011) Enteral Omega-3 Fatty Acid, γ-Linolenic Acid, and Antioxidant Supplementation in Acute Lung Injury. JAMA 306:1574–1581. https://doi.org/10.1001/jama.2011.1435
Heart N, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Todd W Rice, Arthur P Wheeler, B Taylor Thompson, Jay Steingrub, R Duncan Hite, Marc Moss, Alan Morris, Ning Dong PR, (2012) Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 307:795–803. https://doi.org/10.1001/jama.2012.137.Initial
Heart N, Lung, and Blood Institute ARDS Clinical Trials Network, Truwit JD, Bernard GR, et al (2014) Rosuvastatin for Sepsis-Associated Acute Respiratory Distress Syndrome. N Engl J Med 370:2191–2200. https://doi.org/10.1056/NEJMoa1401520
Heart N, Lung, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, et al (2019) Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 380:1997–2008. https://doi.org/10.1056/NEJMoa1901686
Steinberg KP, Hudson LD, Goodman RB et al (2006) Efficacy and Safety of Corticosteroids for Persistent Acute Respiratory Distress Syndrome. N Engl J Med 35416:1671–1684. https://doi.org/10.1056/NEJMoa051693
Inker LA, Eneanya ND, Coresh J et al (2021) New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med 385:1737–1749. https://doi.org/10.1056/NEJMoa2102953
Sinha P, Calfee CS, Beitler JR et al (2019) Physiologic Analysis and Clinical Performance of the Ventilatory Ratio in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 199:333–341. https://doi.org/10.1164/rccm.201804-0692OC
The ARDS Definition Task Force, V Marco Ranieri, Gordon D Rubenfeld, B Taylor Thompson, Niall D Ferguson, Ellen Caldwell, Eddy Fan, Luigi Camporota ASS (2012) Acute Respiratory Distress Syndrome. The Berlin definition. JAMA. https://doi.org/10.1001/jama.2012.5669
Matthay MA, Arabi Y, Arroliga AC, et al (2023) A New Global Definition of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med rccm.202303–0558WS. https://doi.org/10.1164/rccm.202303-0558WS
Bellani G, Laffey JG, Pham T et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800. https://doi.org/10.1001/jama.2016.0291
Schenck EJ, Oromendia C, Torres LK et al (2019) Rapidly Improving ARDS in Therapeutic Randomized Controlled Trials. Chest 155:474–482. https://doi.org/10.1016/j.chest.2018.09.031
Khwaja A (2012) Kidney Diseases Improving Global Outcomes. KDIGO clinical practice guideline for acute kidney injury. Nephron Clin Pract 42:7–14. https://doi.org/10.3810/hp.2014.02.1086
Chawla LS, Bellomo R, Bihorac A, et al (2017) Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. In: Nat Rev Nephrol. Nature Publishing Group, pp 241–257
Van der Laan MJ, Polley EC, Hubbard AE (2007) Super learner. Stat Appl Genet Mol Biol. https://doi.org/10.2202/1544-6115.1309
Robins J (1986) A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model 7:1393–1512. https://doi.org/10.1016/0270-0255(86)90088-6
Horvitz DG, Thompson DJ (1952) A Generalization of Sampling Without Replacement from a Finite Universe. J Am Stat Assoc 47:663–685. https://doi.org/10.1080/01621459.1952.10483446
Hoste EAJ, Bagshaw SM, Cely CM et al (2015) Epidemiology of acute kidney injury in critically ill patients : the multinational AKI-EPI study Hospital mortality. Intensive Care Med. https://doi.org/10.1007/s00134-015-3934-7
White KC, Serpa-Neto A, Hurford R et al (2023) Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med 49:1079–1089. https://doi.org/10.1007/s00134-023-07138-0
Monard C, Bianchi N, Kelevina T et al (2024) Epidemiology and outcomes of early versus late septic acute kidney injury in critically ill patients: A retrospective cohort study. Anaesthesia Critical Care & Pain Medicine 43:101332. https://doi.org/10.1016/j.accpm.2023.101332
Joannidis M, Forni LG, Klein SJ et al (2020) Lung–kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med 46:654–672. https://doi.org/10.1007/s00134-019-05869-7
Liu KD, Glidden DV, Eisner MD et al (2007) Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med 35:2755–2761. https://doi.org/10.1097/01.CCM.0000291649.72238.6D
Panitchote A, Mehkri O, Hastings A et al (2019) Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care 9:74. https://doi.org/10.1186/s13613-019-0552-5
Legrand M, Dupuis C, Simon C et al (2013) Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care 17:R278. https://doi.org/10.1186/cc13133
Van Den Akker JP, Egal M, Groeneveld JA (2013) Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care 17:R98. https://doi.org/10.1186/cc12743
Bagshaw SM, George C, Bellomo R, Database A (2007) Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 11:1–9. https://doi.org/10.1186/cc5949
Hwang S, Park H, Kim Y et al (2019) Changes in acute kidney injury epidemiology in critically ill patients: a population-based cohort study in Korea. Ann Intensive Care 9:65. https://doi.org/10.1186/s13613-019-0534-7
Zimmerman JE, Kramer AA, Knaus WA (2013) Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care 17:R81. https://doi.org/10.1186/cc12695
Laurent P, Jean-Marie F, Arnaud G et al (2010) Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. N Engl J Med 363:1107–1116. https://doi.org/10.1056/NEJMoa1005372
Legrand M, Rossignol P (2020) Cardiovascular Consequences of Acute Kidney Injury. N Engl J Med 382:2238–2247. https://doi.org/10.1056/NEJMra1916393
Kellum JA, Sileanu FE, Bihorac A et al (2017) Recovery after Acute Kidney Injury. Am J Respir Crit Care Med 195:784–791. https://doi.org/10.1164/rccm.201604-0799OC
Rezoagli E, McNicholas B, Pham T et al (2020) Lung–kidney cross-talk in the critically ill: insights from the Lung Safe study. Intensive Care Med 46:1072–1073. https://doi.org/10.1007/s00134-020-05962-2
Vaara ST, Pettilä V, Kaukonen K-M et al (2014) The Attributable Mortality of Acute Kidney Injury: A Sequentially Matched Analysis*. Crit Care Med 42:878–885. https://doi.org/10.1097/CCM.0000000000000045
Jiang Y-J, Xi X-M, Jia H-M et al (2022) The attributable mortality of new-onset acute kidney injury among critically ill patients: a propensity-matched analysis based on a multicentre prospective cohort study. Int Urol Nephrol 54:1987–1994. https://doi.org/10.1007/s11255-021-03087-z
Matthay MA, Ware LB, Zimmerman GA (2012) The acute respiratory distress syndrome. J Clin Invest 122:2731–2740. https://doi.org/10.1172/JCI60331
Sidebotham D, Popovich I, Lumley T (2021) A Bayesian analysis of mortality outcomes in multicentre clinical trials in critical care. Br J Anaesth 127:487–494. https://doi.org/10.1016/j.bja.2021.06.026
Bernard GR, Artigas A, Brigham KL, et al (1994) The American-European Consensus Conference on ARDS. Definitions , Mechanisms , Relevant Outcomes , and Clinical Trial Coordination. Am J Respir Crit Care Med 149:818–824. https://doi.org/10.1164/ajrccm.149.3.7509706.
Stapleton RD, Wang BM, Hudson LD et al (2005) Causes and Timing of Death in Patients With ARDS. Chest 128:525–532. https://doi.org/10.1378/chest.128.2.525
Ketcham SW, Sedhai YR, Miller HC et al (2020) Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: a retrospective cohort study. Crit Care 24:391. https://doi.org/10.1186/s13054-020-03108-w
Rimes-Stigare C, Frumento P, Bottai M et al (2015) Long-term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care 19:383. https://doi.org/10.1186/s13054-015-1101-8
Author information
Authors and Affiliations
Contributions
Conceptualization: ML and EA; Methodology: EA and ML; Data curation: EA; Formal analysis and investigation: DC and EA; Writing—original draft preparation: EA; Writing—review and editing: ML, BG, MAM, KDL and DC; Supervision: ML.
Corresponding author
Ethics declarations
Conflicts of interest
ML is supported by grant number R01-GM151494-01 from NIGMS/NIH and received consulting fees form Alexion and La Jolla. The authors declare that they have no competing interests related to the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Antonucci, E., Garcia, B., Chen, D. et al. Incidence of acute kidney injury and attributive mortality in acute respiratory distress syndrome randomized trials. Intensive Care Med 50, 1240–1250 (2024). https://doi.org/10.1007/s00134-024-07485-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-024-07485-6