Abstract
Purpose
The 2021 guidelines endorsed by the European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM) recommend using highly malignant electroencephalogram (EEG) patterns (HMEP; suppression or burst-suppression) at > 24 h after cardiac arrest (CA) in combination with at least one other concordant predictor to prognosticate poor neurological outcome. We evaluated the prognostic accuracy of HMEP in a large multicentre cohort and investigated the added value of absent EEG reactivity.
Methods
This is a pre-planned prognostic substudy of the Targeted Temperature Management trial 2. The presence of HMEP and background reactivity to external stimuli on EEG recorded > 24 h after CA was prospectively reported. Poor outcome was measured at 6 months and defined as a modified Rankin Scale score of 4–6. Prognostication was multimodal, and withdrawal of life-sustaining therapy (WLST) was not allowed before 96 h after CA.
Results
845 patients at 59 sites were included. Of these, 579 (69%) had poor outcome, including 304 (36%) with WLST due to poor neurological prognosis. EEG was recorded at a median of 71 h (interquartile range [IQR] 52–93) after CA. HMEP at > 24 h from CA had 50% [95% confidence interval [CI] 46–54] sensitivity and 93% [90–96] specificity to predict poor outcome. Specificity was similar (93%) in 541 patients without WLST. When HMEP were unreactive, specificity improved to 97% [94–99] (p = 0.008).
Conclusion
The specificity of the ERC-ESICM-recommended EEG patterns for predicting poor outcome after CA exceeds 90% but is lower than in previous studies, suggesting that large-scale implementation may reduce their accuracy. Combining HMEP with an unreactive EEG background significantly improved specificity. As in other prognostication studies, a self-fulfilling prophecy bias may have contributed to observed results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In patients who are comatose after resuscitation from cardiac arrest, the highly malignant electroencephalogram (EEG) patterns recommended in the 2021 guidelines from the European Resuscitation Council and the European Society of Intensive Care Medicine predict poor outcome with 93% specificity beyond 24 h after arrest. The combination with an unreactive EEG background significantly improves prognostic performance. |
Introduction
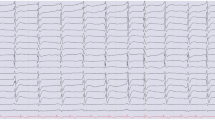
Hypoxic–ischaemic brain injury is a leading cause of intensive care unit (ICU) admission after resuscitation from out-of-hospital cardiac arrest (CA) [1, 2]. In patients who are comatose after resuscitation, about two-thirds of deaths occurring after ICU admission are due to neurological causes and generally occur after withdrawal of life-sustaining therapy (WLST) following a predicted poor neurological outcome [3, 4]. Electroencephalography (EEG) is the most widely used and available method to assess prognosis after CA [5] and is included in the multimodal prognostication algorithm of the 2021 guidelines endorsed by the European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM) for post-resuscitation care [6]. These guidelines recommend using suppression or burst-suppression, with or without discharges (electronic supplemental material, ESM, Fig. E1), at 24 h or later after CA, combined with at least one other concordant predictor, to predict poor outcome. These EEG patterns are usually referred to as “highly malignant EEG patterns” (HMEP) and are defined according to the standardised EEG terminology of the American Clinical Neurophysiology Society (ACNS) [7, 8]. The 2021 ERC-ESICM guidelines were based on an extensive systematic review showing that HMEP almost invariably predict poor neurological outcome, especially if they are recorded beyond 24 h after CA [9]. However, the certainty of the supporting evidence is low [6]. Knowledge gaps regarding the prognostic accuracy of EEG include the potential interference of hypothermia or sedation [10] and the value of EEG reactivity, defined as a change in the EEG background to external stimuli, for instance, sound and pain. Results of the systematic review informing the 2021 ERC-ESICM guidelines suggest that an unreactive EEG background is less accurate for predicting poor neurological outcome than HMEP [9]. However, it is unclear what the added value of absent EEG reactivity is in combination with HMEP.
The present study aimed to evaluate the prognostic ability of HMEP recommended by the 2021 ERC-ESICM guidelines in a large patient cohort and to investigate the added value of the absence of reactivity combined with HMEP. The secondary aim of this study was to assess whether sedation, hypothermia treatment or time point affected the prognostic reliability of EEG.
Methods
This is a pre-planned substudy of the international “Targeted Hypothermia versus Targeted Normothermia after Out-of-hospital Cardiac Arrest. A Randomised Clinical Trial”, TTM2-trial, in which adult comatose patients resuscitated after out-of-hospital CA of presumed cardiac cause were randomised to temperature control to 33 °C versus early treatment of fever (\(\ge\) 37.8 °C) [11]. The trial randomised 1900 patients between November 2017 and January 2020.
The ethics committees in participating countries approved the trial protocol (ClinicalTrials.gov NCT02908308) [12]. Consent was obtained from a legal representative and each patient regaining mental capacity.
Participants allocated to TTM at 33° C were rapidly cooled with a device and maintained at the targeted temperature until 28 h after randomisation, and then they were rewarmed for 12 h. In the normothermia arm, the aim was early treatment of fever (≥ 37.8 °C). Sedation was mandatory until 40 h after randomisation in both allocation arms; afterwards, it was stopped or tapered to minimal levels. There was no defined protocol for sedation and analgesia, but short-acting drugs or volatile anaesthesia were recommended, and the Richmond Agitation–Sedation Scale (RASS) of minus four was targeted. If the sedation was stopped before prognostication, the time point was reported in the electronic case report form (eCRF). This information was used to determine whether sedation was ongoing during the EEG. The cumulative dose and type of sedatives up to 72 h after CA were reported, but data regarding the dosage at the exact time of the EEG were unavailable.
Based on the TTM2 protocol, EEG recording was mandatory in patients who were still unconscious (not obeying commands) between 48 h and 96 h after CA, corresponding to a time interval beyond the intervention period when sedation was stopped or kept as low as possible. If this recommended time interval coincided with a weekend, the EEG was performed immediately afterwards. Instructions for performing and interpreting EEG, either routine EEG or continuous EEG monitoring, were prespecified (ESM) and included in the TTM2 protocol. The EEG recordings were assessed by local reviewers who were not blinded to clinical data in the EEG referral. The treating team was not blinded to the local EEG report. EEG results and time points were prospectively reported in the eCRF by the investigator team, and the sites were instructed to use the following classification defined according to the ACNS [7]:
• HMEP (yes/no):
-
Suppressed background (< 10 µV the entirety of the record) with or without superimposed periodic discharges.
-
Burst-suppression pattern with or without superimposed discharges with suppression periods (< 10 µV) constituting ≥ 50% of the recording.
• EEG background reactive to any external stimuli (yes/no):
-
Sound stimuli (calling the patient’s name and clapping hands) repeated at least two times.
-
Painful stimuli (both distal and proximal) repeated at least two times.
At 96 h or later after randomisation, a physician blinded to the target temperature intervention performed a multimodal prognostication. Comatose patients with Glasgow Coma Scale (GCS) motor scores 1–2 without confounding factors, such as severe metabolic derangement or lingering sedation, were eligible for prognostication. The criteria for predicting a poor prognosis according to the trial protocol were fulfilled if at least two of the following predictors were present: bilateral absence of pupillary and corneal reflexes, status myoclonus, unreactive HMEP, computed tomography (CT) or magnetic resonance imaging (MRI) of the brain with signs of global ischaemic injury, high neuron specific enolase (NSE) levels and bilaterally absent cortical somatosensory evoked potentials (SSEP) N20 (negative peak at 20 ms)-responses. Details are reported in the trial protocol [12]. The result of multimodal prognostication was prospectively reported; “likely poor prognosis” (Yes/No). The blinded physician who performed the prognostication could share the information relating to the neurological prognosis with the treating physicians but was not allowed to recommend WLST; this latter decision rested with the treating clinical team. If the prognosis was uncertain, active intensive care was continued, and patients were re-evaluated daily.
Follow-up was performed face-to-face or by telephone interview 180 days after CA. Poor outcome was defined as a modified Rankin Scale (mRS) score of 4–6 (moderate-severe disability, severe disability, or death) [13].
We used SPSS version 28 for the statistical analysis. We included the first EEG performed between 24 h and 14 days after CA. We calculated the ability of the HMEP to predict poor outcome (specificity, sensitivity, positive predictive value, and negative predictive value). To investigate the added value of unreactive background in combination with HMEP, we used the McNemar test. We performed Chi-square tests to assess whether the predictive ability of HMEP was similar between patients with or without ongoing sedation and between patients in the hypothermia vs the normothermia group. We used McNemar’s and Fisher’s tests when comparing the prognostic ability of HMEP at various time-windows (0-24 h, 24-48 h, 48-72 h, 72-96 h, 96-120 h, and > 120 h). We used logistic regression to calculate odds ratios (OR). We calculated 95% confidence intervals according to Wilson’s method.
Results
Patients
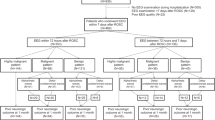
Among 1900 patients randomised in the TTM2-trial, 1029 were still comatose and alive at the time point of prognostication and eligible for an EEG according to protocol. EEG was not performed in 110 of these patients (Fig. 1, ESM Table E1). Among the remaining 919 patients, 14 were excluded because the EEG results were missing in the eCRF. In addition, 60 patients were excluded from the primary analysis (reported separately in the ESM Table E2) because their EEG was recorded before 24 h from CA. Thus, 845 patients (678 [80%] males; mean age 65 years) at 59 trial sites were included in the primary analysis. Their baseline characteristics are summarised in Table 1.
EEG was recorded at a median of 71 h after CA (interquartile range [IQR] 52-93 h; range 24 h–13.3 days) (ESM Fig. E2). Prognostication was performed in 611 (72%) patients, of whom 412 underwent WLST. In 304 of these patients, the reason for WLST was a presumed poor neurological prognosis. Prognostication and WLST approach per country is presented in ESM Table E3. At 6-month follow-up, 579 (69%) patients had poor neurological outcome.
The 110 missing patients without an EEG had a shorter time to ROSC (p = 0.003), fewer clinical seizures (p < 0.001), fewer rates of WLST (p = 0.011), and a better long-term outcome (p < 0.001) (ESM Table E1).
Predictive value of highly malignant EEG patterns
Of 845 patients, 307 (36%) had HMEP (Table 2). HMEP beyond 24 h predicted poor outcome with 50% (confidence interval [CI] 46–54%) sensitivity and 93% (CI 90–96%) specificity.
Eighteen patients with HMEP on EEG had a good outcome at 6 months. Their characteristics are described in Table 3. The distribution of false-positive patients was similar across the participating countries (ESM Table E3).
For the subgroup of patients in whom WLST for neurological reasons was not performed the sensitivity and specificity to predict poor outcome were 40% (CI 34–46%) and 93% (CI 90–96%), respectively (ESM Table E4).
Detailed follow-up data (mRS score) is presented in ESM Table E5.
Background reactivity
Results of reactivity testing to external stimuli were available in 821 (97%) patients, of whom 298 (36%) had HMEP. Among these, 268 (90%) had an unreactive EEG background. The combination of HMEP and unreactive EEG was significantly more specific (97% vs 93%; p = 0.008) and less sensitive (46% vs 50%; p < 0.001) than HMEP regardless of reactivity (Table 2).
Unreactive background per se, without considering whether the EEG fulfilled criteria for an HMEP, had 60% specificity to predict poor outcome.
Thirty patients with HMEP had a reactive EEG background. Of these, eight (27%) had a good outcome.
Sedation
In the study cohort, 730 (86%) patients received propofol and 395 (47%) midazolam during the first 72 h in the ICU. Data on whether sedation was ongoing during the EEG recording were available in 600 (71%) patients, of whom 402 (67%) were sedated. No difference in the specificity of the HMEP was observed comparing patients with and without ongoing sedation (p = 1.000).
Eleven (65%) of the false-positive patients (Table 3) received midazolam. The cumulative dose of midazolam up to 72 h was higher in these 11 patients (median 386 mg, IQR 16–648) compared to the remaining 384 patients who received the drug (median 150 mg, IQR 27–341).
Level of targeted temperature management
Among 845 patients, 442 (52%) were randomised to hypothermia. There were no differences between the hypothermia and normothermia groups regarding the prevalence (p = 1.000) and predictive ability (sensitivity p = 0.934; specificity p = 0.149) of HMEP. Since the intervention period lasted until 40 h after randomisation, only 39 (9%) patients had ongoing hypothermia during the EEG recording.
Time point of electroencephalogram
The prognostic accuracy of HMEP recorded after 24 h did not change across the various time points, while it was significantly lower when the EEG was recorded before 24 h (ESM Table E2).
Multimodal prognostication and HMEP in relation to WLST
Prognostication was performed in 611 (72%) patients, of whom 244 fulfilled the multimodal trial criteria for a “likely poor prognosis” at the time point of prognostication (96 h) (Table 1). The odds ratio of performing WLST in the patients with an HMEP compared to those without an HMEP was 4.6 (CI 3.4–6.3), and the odds ratio of performing WLST for the patients with and without a “likely poor prognosis” was 14.3 (CI 9.7–21.5).
Discussion
To the best of our knowledge, this prospective international multicentre study on the accuracy of EEG after CA is one of the largest ever conducted and the one with the broadest geographical representation, involving 59 sites in Europe, USA, Australia, and New Zealand. Its results show that the false-positive rate of HMEP recorded after 24 h from CA is 7%, which is higher than that reported in previous smaller studies. In addition, it showed that combining HMEP with the absence of reactivity significantly improved specificity.
The 2021 ERC-ESICM guidelines [6] and the recently published guidelines from the American Neurocritical Care Society [14] recommended HMEP as a predictor of poor outcome in patients with hypoxic–ischaemic brain injury based on previous investigations showing a specificity close to 100% [9] and substantial agreement among raters [15]. However, this evidence was based on smaller studies where EEG assessment was often centralised and carried out by a limited number of experts [8, 9, 16,17,18,19]. Conversely, in our multicentre study, local reviewers with diverse backgrounds and experiences assessed the EEG. Although we provided the local investigator teams with instructions (ESM Appendix) on how to record and classify the EEG according to ACNS [7] the EEG assessment by these teams may have been suboptimal, which may explain the lower specificity we observed. Nevertheless, even if the pragmatic design of our study may have been associated with reduced accuracy, it improves its generalisability, showing the results of real-life implementation of the ERC-ESICM guidelines in a wide geographical area. The reduced accuracy of the HMEP shown by the present study underlines the importance of adopting a multimodal approach to prognostication after CA.
The representativeness of our sample is confirmed by the 11% rate of patients with missing EEGs, which is in line with previous literature [20,21,22]. Even though the reasons for missing EEGs were not documented, the patients without EEG had more benign baseline characteristics and better outcome compared to the study cohort. This suggests that our included sample is representative of patients with more uncertain neurological outcome, eligible for prognostication. Although this may potentially represent selection bias, the patients with missing EEGs underwent multimodal prognostication based on other tests (ESM Table E1).
In our study, lack of EEG background reactivity per se was only inconsistently associated with poor outcome after CA, in line with current evidence [9, 23]. However, the presence of an unreactive EEG background significantly increased the specificity of HMEP, with only a 3% false-positive rate. Conversely, 8/30 (27%) patients with HMEP and a reactive background achieved neurological recovery in our cohort. This finding aligns with a previous study [24] and suggests that, although reactivity is a rare occurrence with HMEP [25, 26], it may have an added value to reduce the risk of falsely pessimistic predictions and should be actively searched for.
Sedation affects EEG in a dose-dependent manner [27]. In a previous study from our group, the only patient with good outcome and burst-suppression had EEG recorded during sedation [16]. A recent study investigated the impact of sedation and found that a sub-type of burst-suppression, with so-called identical bursts predicted poor outcome also during ongoing sedation [28]. However, this pattern is typically transient and disappears in median 36 h after CA, thus before most of the EEGs in our study. In the present study, the false-positive patients received twice as high cumulative dose of midazolam during the first 72 h compared to the rest of the study population (Table 3). This may suggest a potential interference from midazolam on the prognostic accuracy of EEG. However, the small size (n = 11) of this subgroup prevents a meaningful statistical analysis. Specifically designed studies will be needed to address this point.
In the present study, treatment of clinical or electrographic seizures was not protocolised. We note, however, that in the recent TELSTAR trial [29] that investigated effects of antiseizure treatment, all patients with a highly malignant EEG background, e.g. periodic discharges over a suppressed background, had a poor outcome regardless of intervention arm.
Hypothermia affects neurotransmitter release and may cause depression of the EEG background [30], depending on temperature levels. Our results showed that the prognostic ability of HMEP was not affected in patients managed at 33 °C, in line with a previous study from our group [8]. Evidence informing the 2021 ERC-ESICM guidelines shows that the specificity of HMEP for predicting poor outcome is greater when they are recorded after 24 h from CA[9], which aligns with the data from the early EEG (< 24 h) subgroup in our study (ESM Table E2). However, there is limited available evidence on the prognostic accuracy of EEG recorded later than 96 h after CA [9, 19]. Results of our study showed similar prognostic ability of the HMEP between 24 h and 14 days after CA (ESM Table E2). Interestingly, the sensitivity of HMEP remained high over time, in contrast with other studies showing a rapid decrease of their prevalence after 24–36 h from arrest [9, 31]. The reasons for this may include differences in case mix or sedation protocol across studies. These results should be interpreted with caution since the cohort gradually changed over time due to awakening and deaths.
An important limitation of our study was that clinicians were not blinded to the EEG. HMEP was part of our multimodal prognostication protocol and associated with higher probability for WLST compared to patients without HMEP indicating a potential self-fulfilling prophecy bias which may have overestimated the specificity of HMEP [32, 33]. However, studies conducted in communities where WLST was uncommon reported higher HMEP specificities than our study [34, 35]. We further note that in the large subgroup of patients (n = 541) who did not undergo WLST due to a presumed poor neurological prognosis, the specificity of the HMEP to predict poor outcome was equal to that of the whole study cohort (ESM Table E4). Self-fulfilling prophecy bias is difficult to avoid in prognostication studies, especially for predictors like the EEG that cannot be concealed from the treating team because they are essential for clinical management (e.g. to detect and treat nonconvulsive seizures) [6].
To limit the risk of an inappropriate WLST, our trial protocol stated that no decision on treatment withdrawal could be based on a single predictor, as recommended by the current ERC-ESICM guidelines, and that the time point of prognostication should be no earlier than 96 h. In patients with HMEP, prognostication was based on a median of three prognostic tests, and WLST was performed after a median of 47 h after EEG. Importantly, meeting the trial criteria for poor prognosis was a significantly stronger predictor of WLST than HMEP alone. These findings suggest that the trial sites used multiple prognostic tools in addition to HMEP during multimodal prognostication, but do not rule out a self-fulfilling prophecy bias for EEG. We emphasise the importance of a conservative approach after cardiac arrest, avoiding early WLST. We note that patients with falsely pessimistic test results in our study recovered consciousness at a median time of one week. In a French observational study [36], awakening from post-anoxic coma took up to 12 days after CA. Finally, although WLST tends to occur earlier in patients with prolonged disorders of consciousness due to anoxic brain injury compared with other causes of acquired brain injury [37] recent evidence shows that some of patients with anoxic brain injury may eventually recover at long-term follow-up [38].
A second limitation of our study was that the EEG results were dichotomised into presence or absence of HMEP in the eCRF. Consequently, the relative proportions of the HMEP subtypes (suppression vs burst-suppression) and their respective accuracies are unknown. Third, although the type and the cumulative dose of sedatives up to 72 h after CA were reported, their exact dose at the time of EEG recording was not available. Finally, this study is focussed on the ability of EEG patterns only to predict poor outcome. The specific EEG features predicting good outcome [39] were not investigated. Data concerning the predictive power of EEG in combination with other prognostic predictors are under investigation and will be reported in future studies.
Conclusions
In our study, the HMEP recommended in the 2021 ERC-ESICM guidelines for post-resuscitation care predicted poor outcome after CA with 93% specificity when assessed by local EEG reviewers. This is lower than that reported in studies with centralised EEG review by experts, suggesting that the large-scale implementation of these guidelines may be associated with reduced accuracy. The combination of the HMEP with an unreactive EEG background significantly improves prognostic performance. As with most prognostic studies, a self-fulfilling prophecy bias likely contributed to our results. However, the specificity of HMEP was similar in the two subpopulations of patients in whom WLST did and did not occur.
Data availability
The data set of the present study could be available from the corresponding author on a reasonable request to the TTM2 steering group.
References
Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A (2013) Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med 39:1972–1980
Sandroni C, Cronberg T, Sekhon M (2021) Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med 47:1393–1414
Laver S, Farrow C, Turner D, Nolan J (2004) Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 30:2126–2128
Dragancea I, Wise MP, Al-Subaie N, Cranshaw J, Friberg H, Glover G, Pellis T, Rylance R, Walden A, Nielsen N, Cronberg T, investigators TTMt, (2017) Protocol-driven neurological prognostication and withdrawal of life-sustaining therapy after cardiac arrest and targeted temperature management. Resuscitation 117:50–57
Friberg H, Cronberg T, Dunser MW, Duranteau J, Horn J, Oddo M (2015) Survey on current practices for neurological prognostication after cardiac arrest. Resuscitation 90:158–162
Nolan JP, Sandroni C, Bottiger BW, Cariou A, Cronberg T, Friberg H, Genbrugge C, Haywood K, Lilja G, Moulaert VRM, Nikolaou N, Olasveengen TM, Skrifvars MB, Taccone F, Soar J (2021) European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med 47:369–421
Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, Lee JW, Wusthoff CJ, Hahn CD, Westover MB, Gerard EE, Herman ST, Haider HA, Osman G, Rodriguez-Ruiz A, Maciel CB, Gilmore EJ, Fernandez A, Rosenthal ES, Claassen J, Husain AM, Yoo JY, So EL, Kaplan PW, Nuwer MR, van Putten M, Sutter R, Drislane FW, Trinka E, Gaspard N (2021) American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol 38:1–29
Westhall E, Rossetti AO, van Rootselaar AF, Wesenberg Kjaer T, Horn J, Ullen S, Friberg H, Nielsen N, Rosen I, Aneman A, Erlinge D, Gasche Y, Hassager C, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wetterslev J, Wise MP, Cronberg T, investigators TT-t, (2016) Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 86:1482–1490
Sandroni C, D’Arrigo S, Cacciola S, Hoedemaekers CWE, Kamps MJA, Oddo M, Taccone FS, Di Rocco A, Meijer FJA, Westhall E, Antonelli M, Soar J, Nolan JP, Cronberg T (2020) Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med 46:1803–1851
Soar J, Berg KM, Andersen LW, Bottiger BW, Cacciola S, Callaway CW, Couper K, Cronberg T, D’Arrigo S, Deakin CD, Donnino MW, Drennan IR, Granfeldt A, Hoedemaekers CWE, Holmberg MJ, Hsu CH, Kamps M, Musiol S, Nation KJ, Neumar RW, Nicholson T, O’Neil BJ, Otto Q, de Paiva EF, Parr MJA, Reynolds JC, Sandroni C, Scholefield BR, Skrifvars MB, Wang TL, Wetsch WA, Yeung J, Morley PT, Morrison LJ, Welsford M, Hazinski MF, Nolan JP, Adult Advanced Life Support C (2020) Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 156:A80–A119
Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullen S, Rylander C, Wise MP, Oddo M, Cariou A, Belohlavek J, Hovdenes J, Saxena M, Kirkegaard H, Young PJ, Pelosi P, Storm C, Taccone FS, Joannidis M, Callaway C, Eastwood GM, Morgan MPG, Nordberg P, Erlinge D, Nichol AD, Chew MS, Hollenberg J, Thomas M, Bewley J, Sweet K, Grejs AM, Christensen S, Haenggi M, Levis A, Lundin A, During J, Schmidbauer S, Keeble TR, Karamasis GV, Schrag C, Faessler E, Smid O, Otahal M, Maggiorini M, Wendel Garcia PD, Jaubert P, Cole JM, Solar M, Borgquist O, Leithner C, Abed-Maillard S, Navarra L, Annborn M, Unden J, Brunetti I, Awad A, McGuigan P, Bjorkholt Olsen R, Cassina T, Vignon P, Langeland H, Lange T, Friberg H, Nielsen N, Investigators TTMT (2021) Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med 384:2283–2294
Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Belohlavek J, Callaway C, Cariou A, Eastwood G, Erlinge D, Hovdenes J, Joannidis M, Kirkegaard H, Kuiper M, Levin H, Morgan MPG, Nichol AD, Nordberg P, Oddo M, Pelosi P, Rylander C, Saxena M, Storm C, Taccone F, Ullen S, Wise MP, Young P, Friberg H, Nielsen N (2019) Targeted hypothermia versus targeted Normothermia after out-of-hospital cardiac arrest (TTM2): A randomized clinical trial-Rationale and design. Am Heart J 217:23–31
Lilja G, Nielsen N, Ullen S, Blennow Nordstrom E, Dankiewicz J, Friberg H, Heimburg K, Jakobsen JC, Levin H, Callaway C, Cariou A, Eastwood GM, Helbok R, Hovdenes J, Kirkegaard H, Leithner C, Morgan MPG, Nordberg P, Oddo M, Pelosi P, Rylander C, Saxena M, Taccone FS, Siranec M, Wise MP, Young PJ, Cronberg T (2020) Protocol for outcome reporting and follow-up in the Targeted Hypothermia versus Targeted Normothermia after Out-of-Hospital Cardiac Arrest trial (TTM2). Resuscitation 150:104–112
Rajajee V, Muehlschlegel S, Wartenberg KE, Alexander SA, Busl KM, Chou SHY, Creutzfeldt CJ, Fontaine GV, Fried H, Hocker SE, Hwang DY, Kim KS, Madzar D, Mahanes D, Mainali S, Meixensberger J, Montellano F, Sakowitz OW, Weimar C, Westermaier T, Varelas PN (2023) Guidelines for neuroprognostication in comatose adult survivors of cardiac arrest. Neurocrit Care 38:533–563
Westhall E, Rosen I, Rossetti AO, van Rootselaar AF, Wesenberg Kjaer T, Friberg H, Horn J, Nielsen N, Ullen S, Cronberg T (2015) Interrater variability of EEG interpretation in comatose cardiac arrest patients. Clin Neurophysiol 126:2397–2404
Backman S, Cronberg T, Friberg H, Ullen S, Horn J, Kjaergaard J, Hassager C, Wanscher M, Nielsen N, Westhall E (2018) Highly malignant routine EEG predicts poor prognosis after cardiac arrest in the Target Temperature Management trial. Resuscitation 131:24–28
Rossetti AO, Tovar Quiroga DF, Juan E, Novy J, White RD, Ben-Hamouda N, Britton JW, Oddo M, Rabinstein AA (2017) Electroencephalography predicts poor and good outcomes after cardiac arrest: a two-center study. Crit Care Med 45:e674–e682
Duez CHV, Johnsen B, Ebbesen MQ, Kvaloy MB, Grejs AM, Jeppesen AN, Soreide E, Nielsen JF, Kirkegaard H (2019) Post resuscitation prognostication by EEG in 24 vs 48 h of targeted temperature management. Resuscitation 135:145–152
Doerrfuss JI, Kowski AB, Holtkamp M, Thinius M, Leithner C, Storm C (2021) Prognostic value of “late” electroencephalography recordings in patients with cardiopulmonal resuscitation after cardiac arrest. J Neurol 268:4248–4257
Bongiovanni F, Romagnosi F, Barbella G, Di Rocco A, Rossetti AO, Taccone FS, Sandroni C, Oddo M (2020) Standardized EEG analysis to reduce the uncertainty of outcome prognostication after cardiac arrest. Intensive Care Med 46:963–972
Scarpino M, Lolli F, Lanzo G, Carrai R, Spalletti M, Valzania F, Lombardi M, Audenino D, Celani MG, Marrelli A, Contardi S, Peris A, Amantini A, Sandroni C, Grippo A, ProNe CASG (2019) Neurophysiology and neuroimaging accurately predict poor neurological outcome within 24 hours after cardiac arrest: The ProNeCA prospective multicentre prognostication study. Resuscitation 143:115–123
Elmer J, Rittenberger JC (2019) Quantitative EEG after cardiac arrest: New insights from an old technology. Resuscitation 142:184–185
Admiraal MM, van Rootselaar AF, Hofmeijer J, Hoedemaekers CWE, van Kaam CR, Keijzer HM, van Putten M, Schultz MJ, Horn J (2019) Electroencephalographic reactivity as predictor of neurological outcome in postanoxic coma: A multicenter prospective cohort study. Ann Neurol 86:17–27
Caporro M, Rossetti AO, Seiler A, Kustermann T, Nguepnjo Nguissi NA, Pfeiffer C, Zimmermann R, Haenggi M, Oddo M, De Lucia M, Zubler F (2019) Electromyographic reactivity measured with scalp-EEG contributes to prognostication after cardiac arrest. Resuscitation 138:146–152
Broman NJ, Backman S, Westhall E (2021) Stimulus-induced EEG-patterns and outcome after cardiac arrest. Clin Neurophysiol Pract 6:219–224
Admiraal MM, Horn J, Hofmeijer J, Hoedemaekers CWE, van Kaam CR, Keijzer HM, van Putten M, Schultz MJ, van Rootselaar AF (2020) EEG reactivity testing for prediction of good outcome in patients after cardiac arrest. Neurology 95:e653–e661
Drohan CM, Cardi AI, Rittenberger JC, Popescu A, Callaway CW, Baldwin ME, Elmer J (2018) Effect of sedation on quantitative electroencephalography after cardiac arrest. Resuscitation 124:132–137
Ruijter BJ, van Putten M, van den Bergh WM, Tromp SC, Hofmeijer J (2019) Propofol does not affect the reliability of early EEG for outcome prediction of comatose patients after cardiac arrest. Clin Neurophysiol 130:1263–1270
Ruijter BJ, Keijzer HM, Tjepkema-Cloostermans MC, Blans MJ, Beishuizen A, Tromp SC, Scholten E, Horn J, van Rootselaar AF, Admiraal MM, van den Bergh WM, Elting JJ, Foudraine NA, Kornips FHM, van Kranen-Mastenbroek V, Rouhl RPW, Thomeer EC, Moudrous W, Nijhuis FAP, Booij SJ, Hoedemaekers CWE, Doorduin J, Taccone FS, van der Palen J, van Putten M, Hofmeijer J, Investigators T (2022) Treating rhythmic and periodic EEG patterns in comatose survivors of cardiac arrest. N Engl J Med 386:724–734
Stecker MM, Cheung AT, Pochettino A, Kent GP, Patterson T, Weiss SJ, Bavaria JE (2001) Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg 71:14–21
Ruijter BJ, Tjepkema-Cloostermans MC, Tromp SC, van den Bergh WM, Foudraine NA, Kornips FHM, Drost G, Scholten E, Bosch FH, Beishuizen A, van Putten M, Hofmeijer J (2019) Early electroencephalography for outcome prediction of postanoxic coma: a prospective cohort study. Ann Neurol 86:203–214
Sandroni C, Geocadin R (2015) Neurological prognostication after cardiac arrest. Curr Opin Crit Care 21:209–214
May TL, Ruthazer R, Riker RR, Friberg H, Patel N, Soreide E, Hand R, Stammet P, Dupont A, Hirsch KG, Agarwal S, Wanscher MJ, Dankiewicz J, Nielsen N, Seder DB, Kent DM (2019) Early withdrawal of life support after resuscitation from cardiac arrest is common and may result in additional deaths. Resuscitation 139:308–313
Scarpino M, Carrai R, Lolli F, Lanzo G, Spalletti M, Valzania F, Lombardi M, Audenino D, Contardi S, Celani MG, Marrelli A, Mecarelli O, Minardi C, Minicucci F, Politini L, Vitelli E, Peris A, Amantini A, Sandroni C, Grippo A, ProNe CAsg, (2020) Neurophysiology for predicting good and poor neurological outcome at 12 and 72 h after cardiac arrest: The ProNeCA multicentre prospective study. Resuscitation 147:95–103
Youn CS, Park KN, Kim SH, Lee BK, Cronberg T, Oh SH, Jeung KW, Cho IS, Choi SP, Korean Hypothermia Network I (2022) External validation of the 2020 ERC/ESICM prognostication strategy algorithm after cardiac arrest. Crit Care 26:95
Paul M, Bougouin W, Geri G, Dumas F, Champigneulle B, Legriel S, Charpentier J, Mira JP, Sandroni C, Cariou A (2016) Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med 42:1128–1136
Giacino JT, Fins JJ, Laureys S, Schiff ND (2014) Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 10:99–114
Magliacano A, De Bellis F, Panico F, Sagliano L, Trojano L, Sandroni C, Estraneo A, (2023) Long-term clinical evolution of patients with prolonged disorders of consciousness due to severe anoxic brain injury: A meta-analytic study. Eur J Neurol
Sandroni C, D’Arrigo S, Cacciola S, Hoedemaekers CWE, Westhall E, Kamps MJA, Taccone FS, Poole D, Meijer FJA, Antonelli M, Hirsch KG, Soar J, Nolan JP, Cronberg T (2022) Prediction of good neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med 48:389–413
Acknowledgements
The members of the TTM2 Trial Collaborators are listed here: Steering Group: Niklas Nielsen, Lund University, Helsingborg Hospital, Department of Clinical Sciences Lund, Anesthesiology and Intensive care, Lund, Sweden (Chair and Chief Investigator); Jan Bělohlávek, 2nd Department of Medicine, First Faculty of Medicine, Charles University in Prague and General University Hospital, Prague, Czech Republic (NI); Clifton Callaway, Department of Emergency Medicine, University of Pittsburgh, Pittsburgh, PA, USA (NI); Alain Cariou, Descaretes University of Paris and Cochin University Hospital, Paris, France (NI); Tobias Cronberg, Lund University, Skåne University Hospital Lund, Department of Clinical Sciences, Neurology, Lund, Sweden (Senior Investigator); Josef Dankiewicz, Lund University, Skåne University Hospital Lund, Department of Clinical Sciences, Cardiology, Lund, Sweden (Coordinating Investigator); Glenn Eastwood, The Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne, Australia; David Erlinge, Lund University, Skåne University Hospital Lund, Department of Clinical Sciences, Cardiology, Lund, Sweden; Hans Friberg, Lund University, Skåne University Hospital Malmö, Department of Clinical Sciences, Anesthesia & Intensive care, Lund, Sweden (Senior Investigator); Jan Hovdenes, Department of Anesthesiology and Intensive Care, Oslo University Hospital, Rikshospitalet, Oslo, Norway (NI); Janus Christian Jakobsen, Copenhagen Trial Unit, Capital Region, Copenhagen, Denmark; Department of Regional Health Research, The Faculty of Health sciences, University of Southern Denmark, Denmark (Trialist); Michael Joannidis, Division of Intensive and Emergency Medicine, Department of Internal Medicine, Medical University Innsbruck, Innsbruck, Austria (NI); Hans Kirkegaard, Research Center for Emergency Medicine, Department of Clinical Medicine, Aarhus University Hospital and Aarhus University, Aarhus N, Denmark (NI); Helena Levin, Lund University, Skåne University Hospital Lund, Department of Clinical Sciences, Anesthesiology and Intensive care, Lund, Sweden (Clinical Trial Manager); Gisela Lilja, Lund University, Skåne University Hospital Lund, Department of Clinical Sciences, Neurology, Lund, Sweden (Follow-up Coordinator); Matt P. G. Morgan, Adult Critical Care, University Hospital of Wales, Cardif, United Kingdom; Alistair D. Nichol, University College Dublin—Clinical Research Centre at St Vincent’s University Hospital, Dublin, Ireland. Per Nordberg, Department of Medicine, Center for Resuscitation Science, Karolinska Institute, Solna, Sweden; Mauro Oddo, Neuroscience Critical Care Group, Adult Intensive Care Medicine Service, CHUV-Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland (NI); Paolo Pelosi, Anesthesiology and Critical Care, San Martino Policlinico Hospital, IRCCS for Oncology and Neurosciences, Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy (NI); Christian Rylander, Department of Anesthesiology and Intensive Care Medicine, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (NI); Manoj Saxena, Division of Critical Care and Trauma, George Institute for Global Health. Bankstown-Lidcombe Hospital, South Western Sydney Local Health District, Sydney, Australia (NI); Christian Storm, Department of Nephrology and Medical Intensive Care, Charité—Universitäts‑medizin Berlin, Germany (NI); Fabio S. Taccone, Department of Intensive Care, Erasme University Hospital, Université Libre de Bruxelles (ULB), Brussels, Belgium (NI); Susann Ullén, Clinical Studies Sweden—Forum South, Skåne University Hospital, Lund, Sweden (Chief Statistician); Matt P. Wise, Adult Critical Care, University Hospital of Wales, Cardif, United Kingdom (NI); Paul J. Young, Medical Research Institute of New Zealand, Intensive Care Unit, Wellington Hospital, Wellington, New Zealand (NI). NI-National Coordinating Investigator. Independent Data Monitoring and Safety Committee: Kathy Rowan, Intensive Care National Audit & Research Centre, UK (Chair); David Harrison, Intensive Care National Audit & Research Centre, UK; Paul Mouncey, Intensive Care National Audit & Research Centre, UK; Manu Shankar-Hari, Guy’s and St Thomas’s NHS Foundation Trust, London, UK; Duncan Young, Nufeld Department of Clinical Neurosciences, University of Oxford, UK. Statisticians: Susann Ullén, Clinical Studies Sweden—Forum South, Skåne University Hospital, Lund, Sweden (Chief Statistician); Theis Lange, Department of Biostatistics, University of Copenhagen, Copenhagen, Denmark (Independent Statistician); Karolina Palmér, Department of Medical Statistics and Epidemiology, Malmö, Sweden Independent statistician). Coordinating Organizations and Trial Management: , Helsingborg Hospital, Helsingborg, Sweden (Sponsor). Lund University, Lund, Sweden. Core management group: Niklas Nielsen (Chair and Chief Investigator), Josef Dankiewicz (Coordinating Investigator), Tobias Cronberg (Senior Investigator, Neurology), Hans Friberg (Senior Investigator, Intensive Care), Gisela Lilja (Follow-up Coordinator), Helena Levin. (Clinical Trial Manager), Janus Christian Jakobsen (Trialist), Susann Ullén (Chief Statistician). Trial financial management: Helsingborg Hospital: Ulla-Britt Karlsson; Lund University: Simon Heissler. Australia: The George Institute for Global Health, Sydney (Local Sponsor): Manoj Saxena, Frances Bass, Naomi Hammond, John Myburgh, Colman Taylor. France: Clinical Research Unit, Paris Descartes Necker Cochin, Paris (Local Representative): Alain Cariou, Adele Bellino. Trial Coordinators and Monitors: Australia: The George Institute for Global Health, Sydney: Marwa Abel-all, Ben Finfer, Carolyn Koch, Yang Li, Anne O’Connor, Julia Pilowsky, Tina Schneider, Anna Tippett; Monash University, Melbourne: Bridget Ady, Tessa Broadley, Amanda Brown, Liz Melgaard, Mimi Morgan, Vanessa Singh, Rebecca Symons. Austria: Medical University Innsbruck, Innsbruck: Kathrin Becker. Belgium: NVS Consulting, Brussels: Nathalie Van Sante. Czech Republic: Aixial, Brno: Vendula Saleova, Silvie Zerzanova. Denmark: Lund University, Lund, Sweden: Helena Levin. France: Clinical Research Unit, Paris Descartes Necker Cochin, Paris: Samia Sefr-Kribel. Germany: Charité Universitätsmedizin, Berlin: Ute Lübeck. Italy: Mario Negri Institute for Pharmacological Research, Milan: Martina Carrara. New Zealand: Medical Research Institute of New Zealand (MRINZ), Wellington: Kathryn Fernando, Diane Mackle, Leanlove Navarra, Judith Riley. Norway: Oslo University Hospital, Oslo: Elin Westerheim; Haukeland University Hospital, Bergen: Marianne Flatebø. Sweden: Helsingborg Hospital, Helsingborg: Ameldina Ceric, Zana Haxhija, Lovisa Terling; Skåne University Hospital, Lund: Lena Bossmar, Liz Jergle, Helén Holm Månsson. Switzerland: Lausanne University Hospital (CHUV), Lausanne: Samia Abed Maillard, Andreja Vujicic Zagar; Cantonal Hospital St. Gallen, St. Gallen: Christina Jodlauk. United Kingdom: University Hospital of Wales, Cardif: Helen Hill; Niche Science & Technology, Richmond: Jennifer Scrivens; The HRB Irish Critical Care—Clinical Trials Network (ICC-CTN), Dublin, Ireland: Kate Ainscough, Ciara Fahey. Sites, Principal Investigators, and Site Personnel: Australia: Austin Hospital, Melbourne: Rinaldo Bellomo (PI), Glenn Eastwood, Leah Peck, Helen Young; Concord Repatriation General Hospital, Sydney: Winston Cheung (PI), Rosalba Cross, Michael Hayes, Nitin Jain, Mark Kol, Asim Shah, Atul Wagh, Helen Wong; John Hunter Hospital, Newcastle: F. Eduardo Martinez (PI), Gail Brinkerhof, Dustin Bush; Liverpool Hospital, Sydney: Antony Stewart (PI), Anders Aneman, Lien Lombardo, Peter McCanny, James Penketh; Nepean Hospital, Sydney: Ian Seppelt (PI), Rebecca Gresham, Julie Lowrey, Kristy Masters, Christina Whitehead; Princess Alexandra Hospital, Brisbane: James Walsham (PI), Meg Harward, Josephine Mackay, Jason Meyer, Emma Saylor, Ellen Venz, Krista Wetzig; Royal North Shore Hospital, Sydney: Wade Stedman (PI), Angela Ashelford, Frances Bass, Naomi Hammond, Sharon Mar, Julia Pilowsky, Miyuki Tokumitsu, Elizabeth Yarad; St Vincent’s Hospital, Sydney: Hergen Buscher (PI), Claire Reynolds; The Alfred Hospital, Melbourne: Andrew Udy (PI), Aidan Burrell, Jasmin Collins, Dashiell Gantner, Victoria Emma-Leah Martin, Phoebe Mccracken, Vinodh Nanjayya, AlistairNichol, Alexander Sacha Richardson, Meredith Young; The Northern Hospital, Melbourne: Angaj Ghosh (PI), Simone Said. Austria: Medical University Innsbruck, Innsbruck: Michael Joannidis (PI), Ronny Beer, Frank Hartig, Raimund Helbok, Sebastian Klein, Andreas Peer. Belgium: Erasme University Hospital, Brussels: Fabio S. Taccone (PI), Jacques Creteur, Dominique Durand; Ziekenhuis Oost-Limburg, Genk: Matthias Dupont (PI), Sigrid Christiaens, Carola Claes, Sebastiaan Deckx, Bert Ferdinande, Sanne Lenaerts, Wilifred Mullens, Sarah Stroobants, Evi Theunissen, David Verhaert. Czech Republic: General University Hospital, Prague: Ondřej Šmíd (PI), Marek Flaksa, David Kemlink, Jan Malík, Michal Otáhal, Jan Rulíšek, Michal Šíranec, Zdeněk Stach, Anna Valeriánová, Petra Zavadilova; University Hospital Hradec Králové, Hradec Králové: Miroslav Solař (PI), Róber Bánszky, Jana Červená, Renata Černá Pařízková, Libor Šimůnek, Filip Varhaník; Regional Hospital Liberec, Liberec: Jiří Karásek (PI), Matěj Strýček. Denmark: Aarhus University Hospital, Aarhus: Anders Grejs (PI), Stefen Christensen, Peter Juhl-Olsen, Ida Katrine Thomsen, Lisa Gregersen Østergaard. France: Cochin University Hospital (APHP), Paris: Alain Cariou (PI), Albert Cao, Pierre Dupland, Ariane Gavaud, Paul Jaubert, Mathieu Jozwiak, Nathalie Marin, Guillaume Savary; Lariboisiere University Hospital (APHP), Paris: Nicolas Deye (PI), Bruno Megarbane, Pierre Mora, Laetitia Sutterlin; Centre Hospitalier de Versailles, Le Chesnay: Stephane Legriel (PI), Hugo Bellut, Alexis Ferre, Guillaume Lacave, Marine Paul; CHU de Nantes,Nantes: Jean-Baptiste Lascarrou (PI), Emmanuel Canet, Charlotte Garret, Arnaud Felix Miaihle, Jean Reignier; Dupuytren Teaching Hospital, Limoges: Philippe Vignon (PI), Thomas Daix, Arnaud Desachy, Bruno Evrard, Bruno Francois, Anne-Laure Fedou, Marine Goudelin. Germany: Charité Universitätsmedizin, Berlin: Christian Storm (PI), Gabriele Kress, Christoph Leithner, Jens Nee, Kaspar Josche Streitberger. Italy: San Martino Policlinico Hospital, Genoa: Iole Brunetti (PI), Lorenzo Ball, Denise Battaglini, Giulia Bonatti, Iacopo Firpo, Paolo Frisoni, Arianna Iachi, Simona Maiani, Maura Mandelli, Chiara Robba, Fabio Tarantino; Civil Hospital, Baggiovara, Modena: Alberto Barbieri (PI), Elisabetta Bertellini, Enrico Giuliani, Gabriele Melegari; University of Trieste, Trieste: Erik Roman-Pognuz (PI), Giorgio Berlot, Umberto Lucangelo, Elisabetta Macchini. Norway: Oslo University Hospital, Rikshospitalet, Oslo: Jan Hovdenes (PI), Vibeke Aune, Tomas Drægni, Simon Jacobsen, Søren Pieschke, Åse Rasmussen, Gro Ringstad Akselsen; St. Olav’s University Hospital, Trondheim: Halvor Langeland (PI), Daniel Bergum, Therese M. Erbe, Pål Klepstad, Helle M. Næss; Sorlandet Hospital, Arendal: Roy Bjørkholt Olsen (PI), Lena Eriksen Skjelnes, Marius Holen, Joakim Iver Post; Haukeland University Hospital, Bergen: Rune Fanebust (PI), Linda Hårteig Sørensen, Ken Åge Kårstad, Carsten Fredrik Wickman. New Zealand: Wellington Regional Hospital, Wellington: Paul Young (PI), Colin Barnes, Ben Barry, Nina Beehre, Dick Dinsdale, Sam Edney, Anna Hunt, Harriet Judd, Charlotte Latimer-Bell, Cassie Lawrence, James Moore, Shaanti Olatunji, Alex Psirides, Chelsea Robinson, Kate Tietjens, Jason Wright; Christchurch Hospital, Christchurch: David Knight (PI), Brandon. Birker, David Bowie, Tara Burke, David Closey, Rosalind Crombie, Neil Davidson, Seton Henderson, Louise Hitchings, James McKay, Jan Mehrtens, Emmeline Minto, Stacey Morgan, Anna Morris, Jay Ritzemar-Carter, Jessica Roberts, Geofrey Shaw, Katherine Townend, Kymbalee Vander Heyden. Sweden: Sahlgrenska University Hospital, Gothenburg: Christian Rylander (PI), Marita Ahlqvist, Roman Desta Lindgren, Ingrid Eiving, Andreas Lundin, Patrik Martner, Elisabeth Myhrman, Birgitta Ryding; Skåne University Hospital, Malmö: Joachim Düring (PI), Mattias Bergström, Mattias Bohm, Ingrid Didriksson, Petrea Frid, Katarina Heimburg, Marina Larsson, Oscar Lundberg, Stefan Olsson Hau, Simon Schmidbauer; Skåne University Hospital, Lund: Ola Borgquist (PI), Anne Adolfsson, Anna Bjärnroos, Erik Blennow-Nordström, Irina Dragancea, Thomas Kander, Anna Lybeck, Gustav Mattiasson, Olof Persson, Malin Rundgren, Susann Schrey, Erik Westhall; Helsingborg Hospital, Helsingborg: Martin Annborn (PI), Sara Andertun, Florian Ebner, Nerida Gustavsson, Lisa Hassel, Jesper Johnsson, Marie Nelderup, Heléne Petersson, Jörgen Petersson, Frideriki Staflidou; Hallands Hospital, Halmstad: Johan Undén (PI), Frida Antonsson, Git Bergman, Jörgen Gamroth, Maria Meirik, Katarina Rudolfsson, Helena Sandberg, Martin Thorsson; Karlstad Central Hospital, Karlstad: Kristin Savolainen (PI), Maria Hansbo, Malin Helliksson, Björne Nödtveidt, Johan Sanner, Victoria Sem, Camilla Sund Lindquist; Södersjukhuset, Karolinska Institute, Stockholm: Per Nordberg (PI), Akil Awad, Anna-Sofa Börjesson, Malin Hedberg, Mia Henning, Jacob Hollenberg; Northern Älvsborg County Hospital, Trollhättan: Per Petersen (PI), Emelia Dahlberg, Johan Forshammar, Veronica Svensson; Capio S:t Görans Hospital, Stockholm: Michael Wanecek (PI), Håkan Eskilsson; Skaraborg Hospital, Skövde: Daniel Rodriguez-Santos (PI), Åsa Appelqvist, Henrietta Jidbratt, Elisabeth Johansson, Lars Kiszakiewicz, Åsa Nilsson, Sinnika Olsson, Anders Paulsson, Urszula Stempel, Andreas Thoren; Örebro University Hospital, Örebro: Stefan Persson (PI), Ida Berglund, Eric Bergström, Cathrine Törnqvist, Ingela Östman; Uppsala University Hospital, Uppsala: Sten Rubertsson (PI), Ing-Marie Larsson, Elin Söderman, Ewa Wallin, Joanna Wessbergh; Linköping University Hospital, Linköping: Thomas Halliday (PI), Filippa Engvall. Switzerland: Lausanne University Hospital (CHUV), Lausanne: Mauro Oddo (PI), Nawfel Ben-Hamouda, Adriano Bernini, Pierre-Nicolas Carron, Philippe Eckert, Eva Favre, John-Paul Miroz, Paola Morelli, Olivier Muller, Jan Novi, Andrea Rosseti, Madeleine Schnorf; Bern University Hospital, Bern: Matthias Haenggi (PI), Anja Levis, Sandra Nansoz, Marianne Roth, Nicole Söll; Cantonal Hospital St. Gallen, St.Gallen: Claudia Schrag (PI), Mensur Alicajic, Philipp Baier, Joel Dütschler, Dominique Flügel, Edith Fässler, Ruth Gamio-Veis, Marc Güpfert, Yvonne Hilpertshauser, Stefan Hägele-Link, Gian-Reto Kleger, Peter Krähenmann, Maria Elisabeth Mair, Nadja Schai, Christoph Strohmaier, Peter Tangl, Dominik Zieglgänsberger; University Hospital Zurich, Zurich: Marco Maggiorini (PI), Gabriele Claus, Gabi Consani Vogel, Lukas Imbach, Samira Kaiser, Eva-Maria Kleinert, Pedro David Wendel Garcia; Cardiocentro Ticino, Lugano: Tiziano Cassina (PI), Pamela Agazzi, Bruno Capelli, Gabriele Casso, Martino Regazzi, Hervé Schlotterbeck, Gabriele Via, Michele Villa. United Kingdom: University Hospital of Wales, Cardif: Matt P. Wise (PI), Jenny Brooks, Eve Cocks, Jade Cole, Jacqueline Curtin, Michelle Davies, Rhys Davies, Stephen Fernandez, Julie Highfeld, Helen Hill, Matt P. G. Morgan, Lydia Pennant, Sofa Rose, Emma Thomas, Angharad Williams; Royal Victoria Hospital, Belfast: Peter McGuigan (PI), Stephen Hafey, Aisling O’Neill, Kathryn Ward; Bristol Royal Infrmary, Bristol: Matthew Thomas (PI), Jeremy Bewley, Anna Chillingworth, Julie Cloake, Libby Cole, Hilary Galvin, Zoe Garland, Lisa Grimmer, Bethany Gumbrill, Lucy Howie, Rebekah Johnson, Chloe Searles, Agnieszka Skorko, Katie Sweet, Victoria Taylor, Denise Webster; Essex Cardiothoracic Centre, Basildon: Thomas Keeble (PI), Gill Adams, Rajesh K Aggarwal, Jo-Anne Cartwright, Steven Church, Gerald J Clesham, John R Davies, Kelly Farrell, Reto Gamma, Jane Harding, Rohan Jagathesan, Alamgir Kabir, Paul A Kelly, Lauren Kittridge, Maria Maccaroni, Gracie Maloney, Marco Mion, Naveen Nain, Raghunath Nalgirkar, Gyanesh Namjoshi, Stacey Pepper, Emily Redman, Nicholas M Robinson, Jeremy Sayer, Amanda Solesbury, Kare H Tang, Sali Urovi, Kunal Waghmare, Noel Watson, Teresa Webber; University Hospitals Birmingham NHS Foundation Trust, Birmingham: Peter Isherwood (PI), Conor Bentley, Colin Bergin, Ronald Carrera, Amy Clark, Lauren Cooper, Liesl Despy, Natalie Dooley, Karen Ellis, Emma Fellows, Stephanie Goundry, Samantha Harkett, Christopher McGhee, Aoife Neal, Hazel Smith, Catherine Snelson, Elaine Spruce, Tony Whitehouse, Kamal Yakoub; Royal Berkshire Hospital, Reading: Andrew Walden (PI), Shauna Bartley, Parminder Bhuie, Matthew Frise, Nicola Jacques, Liza Keating; Queen Alexandra Hospital, Portsmouth: David Pogson (PI), Zoe Daly, Steve Rose; Manchester Royal Infirmary, Manchester: Jonathan Bannard-Smith (PI), Rachael Quayle; Royal Bournemouth Hospital, Bournemouth: Nigel Chee (PI), Nina Barratt, Katie Bowman, Debbie Branney, Elizabeth Howe, Maria Letts, Sally Pitts, Luke Vamplew. USA: University of Pittsburgh, Pittsburgh PA: Clifton W. Callaway (PI), Sara Difore Sprouse, Ankur A. Doshi; Mayo Clinic, Rochester MN: Jennifer Fugate (PI), Amy M. Headlee, Eelco F.M.Wijdicks. PI—Principal Investigator. Related Organizations: Copenhagen Trial Unit, Copenhagen, Denmark; Clinical Studies Sweden—Forum South, Skåne University Hospital, Lund, Sweden; The George Institute for Global Health, Sydney, Australia; Australian and New Zealand Intensive Care Research Centre, Monash University, Melbourne, Australia; Medical Research Institute of New Zealand—(MRINZ), Wellington, New Zealand; Clinical Research Unit, Paris Descartes Necker Cochin, Paris, France; Scandinavian Critical Care Trials Group (SCCTG); The HRB Irish Critical Care-Clinical Trials Network (ICC-CTN); The ANZICS Clinical Trials Group (CTG); Spiral Software, Wellington, New Zealand; Allianz Global Corporate & Specialty SE, Copenhagen, Denmark; IBBL (Integrated BioBank of Luxembourg), Dudelange, Luxembourg.
Statistical analysis: Susann Ullén, PhD and statistician within our academic institution (Clinical Studies Sweden—Forum South, Skane University Hospital, Lund, Sweden) supervised the statistical analysis and performed the regression models.
Funding
Open access funding provided by Lund University. The study was supported by independent research grants from nonprofit or governmental agencies (the Swedish Research Council, Swedish Heart–Lung Foundation, Stig and Ragna Gorthon Foundation, Knutsson Foundation, Laerdal Foundation, Hans-Gabriel and Alice Trolle-Wachtmeister Foundation for Medical Research, and Regional Research Support in Region Skane) and by governmental funding of clinical research within the Swedish National Health Service.
Author information
Authors and Affiliations
Consortia
Contributions
EW and TC designed the EEG protocol. ST and EW performed the statistical analyses. ST, CS and EW drafted the manuscript. The manuscript was critically revised for intellectual content and finally approved by all the co-authors. The following co-authors are members of the TTM2-trial core management group: NN is the chair and chief investigator; JD is the coordinating investigator; TC and HF are the senior investigators; GL is the follow-up coordinator; JCJ is the TTM2 Trialist; HL is clinical trial manager. CL, AOR, and FZ are the members of the EEG group of the TTM2-trial and represent sites which included more than 30 EEGs in the present study.
Corresponding author
Ethics declarations
Conflicts of interest
CS is Associate Editor of Intensive Care Medicine. All the other authors report no disclosures relevant to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the TTM2-trial investigators are mentioned in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Turella, S., Dankiewicz, J., Friberg, H. et al. The predictive value of highly malignant EEG patterns after cardiac arrest: evaluation of the ERC-ESICM recommendations. Intensive Care Med 50, 90–102 (2024). https://doi.org/10.1007/s00134-023-07280-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07280-9