Abstract
Purpose
This study aimed at determining whether intravenous artesunate is safe and effective in reducing multiple organ dysfunction syndrome in trauma patients with major hemorrhage.
Methods
TOP-ART, a randomized, blinded, placebo-controlled, phase IIa trial, was conducted at a London major trauma center in adult trauma patients who activated the major hemorrhage protocol. Participants received artesunate or placebo (2:1 randomization ratio) as an intravenous bolus dose (2.4 mg/kg or 4.8 mg/kg) within 4 h of injury. The safety outcome was the 28-day serious adverse event (SAE) rate. The primary efficacy outcome was the 48 h sequential organ failure assessment (SOFA) score. The per-protocol recruitment target was 105 patients.
Results
The trial was terminated after enrolment of 90 patients because of safety concerns. Eighty-three participants received artesunate (n = 54) or placebo (n = 29) and formed the safety population and 75 met per-protocol criteria (48 artesunate, 27 placebo). Admission characteristics were similar between groups (overall 88% male, median age 29 years, median injury severity score 22), except participants who received artesunate were more shocked (median base deficit 9 vs. 4.7, p = 0.042). SAEs occurred in 17 artesunate participants (31%) vs. 5 who received placebo (17%). Venous thromboembolic events (VTE) occurred in 9 artesunate participants (17%) vs. 1 who received placebo (3%). Superiority of artesunate was not supported by the 48 h SOFA score (median 5.5 artesunate vs. 4 placebo, p = 0.303) or any of the trial’s secondary endpoints.
Conclusion
Among critically ill trauma patients, artesunate is unlikely to improve organ dysfunction and might be associated with a higher VTE rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This single-center randomized control trial showed that intravenous artesunate did not reduce multiple organ dysfunction in critically injured patients with trauma hemorrhage. In this patient cohort, artesunate may be associated with increased rates of venous thromboembolism. |
Introduction
Multiple organ dysfunction syndrome (MODS) is common after major trauma, affecting up to two thirds of patients with critical injuries [1, 2]. Post-trauma MODS is associated with a mortality over 20% and poor long-term outcomes in those who survive [1,2,3]. Prolonged critical care and hospital admissions place substantial economic burdens on healthcare systems [4]. Current management is supportive and there are no specific pharmacological agents that prevent organ dysfunction [5]. A therapeutic agent that reduces the incidence, severity or duration of MODS could have a major global impact on patient outcomes and healthcare resource utilization.
Artesunate, a semi-synthetic artemisinin derived from the plant Artemisia annua, is used globally as the first line treatment for severe malaria [6]. The pharmacological effects of artemisinins extend beyond eradication of Plasmodium to include cytotoxicity against cancer cells, viruses and fungi [7,8,9,10] and they demonstrate powerful anti-inflammatory effects in experimental asthma, pancreatitis, arthritis and sepsis [11,12,13,14]. We recently discovered that intravenous artesunate (2.4 or 4.8 mg/kg at 90min after hemorrhage) reduces organ dysfunction caused by severe hemorrhage and resuscitation in the rat. Subsequent investigation into the potential mechanism of action (MOA) of artesunate (using RNA-seq transcriptomics and pathway analysis) revealed pro-survival and anti-inflammatory properties. Artesunate activates the Akt-endothelial nitric oxide synthase (eNOS) cell survival pathway and inhibits a range of pro-inflammatory molecules, including interleukin-1 receptor-associated kinase 1 (IRAK1), forkhead box O4 (FOXO4), glycogen synthase kinase-3β (GSK-3β) and transcription factor nuclear factor kappa B (NF-κB) [15]. Artesunate also demonstrates cardioprotective effects in rat models of myocardial ischaemia and reperfusion, which are associated with activation of RISK (PI3K/Akt/ERK ½) and STAT3 (SAFE) pathways, activation of eNOS and inhibition of GSK-3β and NF-κB [16]. Similar molecular pathways are implicated in humans during the hyperacute period after critical injury [17].
Artesunate is safe, simple to administer, and the dose does not require adjustment in patients with renal or liver dysfunction [6, 18]. The recommended intravenous bolus dose in malaria is 2.4 mg/kg although safety studies in humans show tolerance in doses up to 18 mg/kg [19,20,21]. Artesunate has been used in millions of malaria patients without major adverse effects [22,23,24,25,26,27]. The potential benefit of artesunate in reducing MODS after trauma haemorrhage, coupled with an extensive pre-existing favourable safety profile in humans, provided a strong rationale for proceeding to early phase II studies examining the use of artesunate in patients with major trauma hemorrhage.
The primary objectives of this study were to evaluate the safety and efficacy of early artesunate administration in a cohort of severely injured and bleeding trauma patients. We designed and conducted a phase IIa single-center randomized control trial of artesunate in major trauma patients at risk of developing MODS at a single major trauma center in the United Kingdom (UK).
Methods
Design and setting
A prospective single-center randomized placebo-controlled phase IIa trial of an investigational medicinal product (IMP), intravenous artesunate, was conducted at the Royal London Hospital Major Trauma Centre (London, UK). Ethical approvals were granted by London City and East Research Ethics Committee (reference: 16/LO/003) and the report follows Consolidated Standards of Reporting Trials guidance [28]. Full methodological details are available in the trial protocol (Supplementary material 1) and statistical analysis plan (Supplementary material 2).
Participants
Adult trauma patients (16 years old and over) were eligible for inclusion if they activated the local massive hemorrhage protocol (MHP). The criteria for MHP activation were a systolic blood pressure < 90 mmHg, AND suspected haemorrhage, AND minimal response to small volume fluid resuscitation. This participant group was selected based on previous studies that identified high base rates of MODS (approximately 30%) in this population [29]. Participant agreement or agreement on behalf of incapacitated patients was required before enrolment. Exclusion criteria were as follows: admission > 2 h after injury; intervention not attainable within 4 h of injury; patient not expected to survive to reach the 48 h primary efficacy endpoint; severe traumatic brain injury (TBI); pregnancy or breastfeeding; suspected non-hemorrhagic shock; MHP activation > 1 h after arrival; concurrent participation in another clinical trial of an IMP, and known allergy to artesunate.
Informed consent procedures
Due to the emergency nature of the trial intervention, most participants were incapacitated at the time of eligibility (e.g., critical injury, mechanical ventilation, sedation). Consent on behalf of incapacitated participants was obtained from relatives (if present and appropriate), or two doctors (one independent of the trial), consistent with national and international guidance [30, 31]. Once a participant regained capacity, their informed consent was sought for continuation in the trial. For participants that did not regain capacity, consent for continuation was sought from a relative or other appropriate representative.
Study intervention
Intravenous artesunate in this trial was administered under a clinical trial authorization. A good manufacturing practice formulation of artesunate was provided free of charge by Sigma Tau (now Alfa-Wassermann Spa, Italy). Approval was granted by the Medicines and Healthcare products Regulation Authority (ref: 21313/0052/001-0001). Artesunate was supplied as crystalline powder that required re-constitution with diluent (10 mg/ml, phosphate buffer solution) immediately before delivery. The placebo consisted of diluent drawn to the equivalent volume necessary to reconstitute artesunate. Artesunate or placebo was administered as a single intravenous bolus dose within 4 h of injury.
Randomization, group allocation and concealment
The study used a parallel-group two-stage dosing regimen. In the first stage participants received low dose artesunate (LDA, 2.4 mg/kg) or placebo based on standard malaria dosing. Following an interim review of safety data participants in the second stage received high dose artesunate (HDA, 4.8 mg/kg) or placebo based on animal studies showing superior efficacy of HDA in reducing MODS after trauma haemorrhage [15]. Participants were assigned to artesunate or placebo (2:1 ratio) using a random block-size randomization method. The randomization algorithm was generated by the trial statistician and validated by an external statistician. Sealed randomization cards were manufactured by an independent provider and opened sequentially by a study investigator after the decision to randomize a participant. Allocations were concealed from the participant, clinical team and investigating team collecting outcomes data. Due to the requirement to inspect drug re-constitution visually, the investigator who administered the intervention was not blind to treatment allocation.
Assessments
Participants were screened for eligibility upon arrival to the resuscitation room. Their medical and trauma history was reviewed, and a pregnancy test obtained for women of childbearing age. After eligibility confirmation and consent, baseline vital signs and blood gases were measured, and the intervention was dispensed according to the randomization sequence. Pharmacokinetic blood samples were drawn before intervention delivery and at 5min, 30min, 60min, 4h and 12h after administration. Blood samples for routine investigations were drawn at admission and days 1–7, 14, 21 and 28. Patient demographics, injury characteristics and treatments were recorded. Injury severity was estimated using injury severity score (ISS) [32]. Sequential organ failure assessment (SOFA) scores [33] were calculated daily from admission to 7 days. MODS was defined as any daily SOFA > 5 [2]. Outcomes and safety data were reviewed daily until 28 days. Outcomes were also reviewed at discharge and 90 days.
Endpoints
The safety endpoint was the serious adverse event (SAE) rate in the first 28 days. The primary efficacy endpoint was the SOFA scale score at 48 h after admission. Secondary endpoints included: maximum SOFA; average SOFA (of days 3, 4 and 5) [34]; combined time to composite organ failure resolution (CTCOFR) at 14 days [35]; ventilator free days [36]; hospital and critical care length of stay (LOS); acute lung injury (ALI) [37]; acute kidney injury (AKI) [38]; infection [39]; prolonged (PR)MODS (SOFA > 5 on day 7) [2]; alive and free of MODS at 48 h; and mortality (28 days, discharge, 90 days).
Sample size calculation
Primary efficacy analysis was on the per-protocol (PP) cohort. We chose a clinically meaningful reduction in 48 h SOFA as a fall of two points. At 2:1 randomization, the study required 70 intervention and 35 control subjects to detect this difference with 80% power (α = 0.05; σ = 3.43, t-test).
Statistical analysis
Efficacy analyses were performed on PP and intention-to-treat (ITT) populations. The PP cohort included all patients randomized and treated according to the study protocol, whereas the ITT cohort included all patients randomized to their treatment allocation irrespective of whether they received the treatment. The safety analysis included all patients who received artesunate or placebo and was performed according to treatment received. Comparisons included all artesunate vs. placebo, LDA vs. placebo and HDA vs. placebo. Data are presented as median, mean and interquartile ranges (IQR) for continuous variables and numbers and percentages for categorical variables. Baseline characteristics and outcomes were compared using Mann–Whitney U-tests and Pearson Chi-squared tests. Confidence intervals (CI) on mean differences between group outcomes were estimated using non-parametric empirical bootstrap. Mortality was estimated using the Kaplan–Meier method. SAEs were analysed by number and type, and CIs were estimated for the difference in proportions of participants with one or more SAEs in each group [40]. Routine bloods were examined for safety. Plasma concentrations of artesunate and its active metabolite, dihydroartemisinin, were analysed at the Mahidol Oxford Tropical Medicine Research Unit (Bangkok, Thailand) using a validated LC–MS/MS [41] assay and presented graphically. The main analyses were done in SPSS (version 26) by JMS and validated by ARB using R (version 3.6.3).
Exploratory post-hoc safety analyses
Post-hoc analyses were conducted to examine a potential association between artesunate and venous thromboembolism (VTE). VTE was classified as deep vein thrombosis (DVT), pulmonary embolism (PE) and ‘other’, which referred to any other thrombotic or embolic vascular event deemed significant by the study team and the drug monitoring committee (DMC). We recorded DVTs and PEs that were clinically diagnosed, but we did not specifically screen patients for VTE. We also determined if patients were commenced on daily prophylactic low molecular weight heparin (LMWH; tinzaparin 4500 units) within 48 h of admission. The relative risk (RR) of VTE between placebo and artesunate groups was compared using a Chi-squared test. Logistic regression was used to determine whether differences in baseline characteristics (gender, shock) or receipt of early LMWH contributed to higher numbers of SAEs and VTE in the artesunate group. We also compared coagulation values (international normalised ratio—INR, platelet count) and the proportion of coagulopathic patients in each group defined by an INR ≥ 1.3 [42].
Exploratory post-hoc efficacy analyses
Post-hoc linear regression was performed to determine whether imbalances in baseline characteristics (age, gender, ISS, base deficit—BD) contributed to differences in the primary outcome between the groups. We also conducted post-hoc subgroup analyses of outcomes in blunt and penetrating trauma cohorts.
Drug monitoring committee and trial steering committee review
The DMC reviewed the SAE profile after recruitment of every 15 patients. There were no formal stopping rules due to difficulty in assigning causality to the intervention. Progress to the next stage of the trial depended on whether a meaningful difference in the safety profile was detected during the interim review. The DMC provided their report to the independent trial steering committee (TSC), who were responsible for recommending trial continuation.
Results
Participant recruitment occurred between 21st March 2017 and 15th May 2019. Follow-up was completed by 10th August 2019. Recruitment was suspended between April 30th to September 29th 2017 (pending delivery of a new batch of diluent), and between 30th June and 14th November 2018 (pending the interim review outcome). The trial was halted after 90 patients were enrolled, following a DMC review of safety data.
Study participants
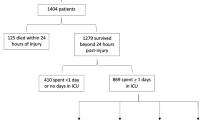
During the recruitment period 211 trauma calls activated the MHP, from which 105 patients met eligibility criteria, and 90 were randomized to intervention or comparator groups (Fig. 1). All patients, except those who subsequently declined consent (n = 5), were included in ITT analyses (n = 85) and all patients who received artesunate or placebo were included in safety analyses (n = 83). Seventy-five patients were evaluable for PP analysis after exclusions for protocol violations (n = 10).
CONSORT recruitment flow diagram. HDA high dose artesunate, ITT intention-to-treat, LDA low dose artesunate, MHP major hemorrhage protocol, PP per-protocol, TBI traumatic brain injury. aTrauma patient who activates the MHP. bExcluded before intervention given. cExcluded after intervention given. dLDA ITT analysis exclusions: declined consent = 4. eLDA safety analysis exclusions: did not receive intervention = 4. One participant was randomized to the placebo group but received LDA in error; they were analysed in the LDA safety analysis. fHDA ITT analysis exclusions: declined consent = 1. gHDA safety analysis exclusions: did not receive intervention = 2. hPlacebo ITT analysis exclusions: none. iPlacebo safety analysis exclusions: incorrect intervention = 1
The study cohort (Table 1, PP population) was predominantly male (88%, 66/75) with a median age of 29 years (IQR 19–49) and 51% (38/75) had sustained penetrating injury. Participants were severely injured (median ISS 22), and in shock on admission (median BD 6.2). The artesunate group had a lower proportion of males (81% vs. 100% p = 0.016), lower admission Glasgow Coma Scores—GCS (median 14 vs. 15, p = 0.044) and higher admission BD values (median 9 vs. 4.7, p = 0.042) vs. placebo. Findings were similar in ITT and safety populations (Supplementary material 3 Tables 1 and 2). Plasma concentrations of artesunate and dihydroartemisinin were within the therapeutic range previously reported in malaria patients (Supplementary material 3 Fig. 1).
Safety endpoints
The total number of SAEs (Table 2, Supplementary material 3 Table 3) was higher in the artesunate (vs. placebo) group (23 vs. 8), as was the proportion of patients who experienced an SAE; 31% vs.17% (17/54 vs. 5/29, relative risk—RR 1.8 [95% CI 0.8–4.4], p = 0.161). Further scrutiny showed a high proportion of SAEs in the artesunate group were due to VTE (48%, 11/23) vs. placebo (13%, 1/8). There were no differences in mortality (Supplementary material 3 Fig. 2c, Supplementary material 3 Table 4a and b). Routine blood tests did not identify any immediate safety concerns (Supplementary material 3 Appendix Tables 1–10).
Early trial termination
Safety data were reviewed by the DMC after recruitment of 15, 30, 51 (interim analysis), 66 and 79 patients. At the interim review, 14 SAEs were recorded, and of these, four were related to VTE and occurred in the same treatment group. The DMC requested a separate analysis of VTE in subsequent reports. After the final DMC review, the trial was suspended because of further data supporting an imbalance in VTE frequency between treatment groups. A second ad hoc interim analysis (total 90 patients) was requested by the TSC and compiled by the trial statistician (ARB). Based on this report, the TSC advised early termination of the trial due to a potentially increased VTE risk in the artesunate group, and insufficient evidence supporting superiority of artesunate in trauma hemorrhage, unlikely to be altered by full sample recruitment.
Primary efficacy endpoint
There was no statistically significant difference in 48 h SOFA in the PP population between study arms (median 5.5 vs. 4, p = 0.303). This included when LDA (median 6 vs. 4, p = 0.411) and HDA (median 5 vs. 4, p = 0.326) arms were analysed separately (Table 3). Findings were similar in ITT analyses (Supplementary material 3 Table 7a).
Secondary endpoints
There was little difference in the secondary endpoints between artesunate and placebo groups in both PP (Table 4, Fig. 2, and Supplementary material 3 Fig. 2a) and ITT analyses (Supplementary material 3 Table 7b and Supplementary material 3 Fig. 2b). Overall, 68% (51/75) of patients developed MODS and 21% (16/75) developed PRMODS, with similar proportions in artesunate vs. placebo groups (MODS: 69% vs. 67%, PRMODS: 21% vs. 22%). Maximum SOFA scores were similar (median 10 vs. 9), while analysis of trajectories showed average SOFA scores were initially higher in the artesunate group but became similar between days 5 and 7 (Fig. 2). Despite this, critical care usage was similar, and non-significantly lower in the artesunate group (median 8 vs. 11 days, p = 0.736). The overall 90-days mortality rates were low (4%, 3/75) with no differences between the groups (artesunate, 4%, 2/48 vs. placebo, 4%, 1/27; p = 0.992).
Mean SOFA scores (line) and standard error (bar) by day of admission for artesunate and placebo groups (per-protocol population). a All patients (placebo, n = 27; artsunate, n = 48); b patients with multiple organ dysfunction syndrome (MODS)(placebo, n = 18; artesunate, n = 33). MODS is defined as a SOFA score > 5 during days 0 to 7 after admission
Exploratory post-hoc safety results
Post-hoc analysis to examine the potential association between artesunate and VTE (Table 3) found the proportion of patients with VTE was non-significantly higher in the artesunate group; 17% vs. 3% (9/54 vs. 1/29, RR 4.8 [95% CI 0.6–36.3], p = 0.078). However, potentially fewer patients in the artesunate group received prophylactic LMWH within 48 h of admission; 35% vs. 53% (18/51 vs. 15/28, RR 0.7 [95% CI 0.4–1.1], p = 0.115). Binary logistic regression examining the effects of group imbalances on SAE and VTE rates (Supplementary material 3 Table 5) showed no effect of treatment group on the odds ratio (OR) of SAE or VTE; however, early prophylactic LMWH was associated with a reduced OR of SAE in this model (OR 0.248, p = 0.049). These analyses are limited by the small sample size. Examination of available coagulation parameters from admission to day 28 (Supplementary material 3, Table 6) showed patients in the artesunate group had a higher median INR values on day 1 (1.2 vs. 1.1; p = 0.012) and day 2 (1.2 vs. 1.1; p = 0.036), and lower platelet counts on day 2 (Day 2, 91 vs 122; p = 0.011) compared with the placebo group. There were non-significantly higher proportions of coagulopathic patients in the artesunate group on day 1 (33%, 16/48 vs. 13%, 3/23, p = 0.071) and day 2 (40%, 18/45 vs. 21%, 5/23, p = 0.123), despite similar proportions on admission (28%, 13/46 vs. 26%, 7/26, p = 0.786).
Exploratory post-hoc efficacy results
Post-hoc multiple regression examining the effects of imbalances in baseline characteristics on the primary outcome (Supplementary material 3 Table 8) found independent effects of age, shock, and ISS on the 48 h SOFA score, but the observed adjusted effect of treatment was unchanged (48 h SOFA, β 0.34, CI − 1.4 to 2.1, p = 0.698). A post-hoc subgroup analysis of blunt and penetrating injury did not reveal any differences in efficacy or safety outcomes (Supplementary material 3 Tables 9a & b).
Discussion
The TOP-ART blinded placebo-controlled randomized clinical trial evaluated the safety and efficacy of intravenous artesunate in patients with severe trauma hemorrhage. The trial was terminated early because of safety concerns, with a potential imbalance of VTE rates and lack of evidence of treatment efficacy.
The reasons for the potentially increased VTE frequency in the artesunate group are not explained by this study. Artesunate has been used extensively in critically ill malaria patients over the past two decades without adverse effects, and to our knowledge no previous studies show an association between VTE and artesunate. VTE has a known association with shock and coagulopathy in trauma populations [43, 44], and the risk increases in the absence of prophylactic LMWH [45, 46]. It is possible that the more profound shock in the artesunate group, coupled with reduced rates of early thromboprophylaxis may partially explain the difference in post-treatment VTE events. Higher rates of early coagulation abnormality in the artesunate group may also have contributed to the increase in VTE, although it is not clear whether these differences in coagulation are related to artesunate or to the degree of shock on admission. VTE prevalence in placebo recipients was also low (3%). Further studies are required to clarify whether artesunate increases VTE in trauma hemorrhage or whether the result is spurious.
Artesunate was well positioned as a potentially effective therapeutic agent for post-injury MODS. Our pre-clinical research suggested that artesunate attenuated the severity of organ dysfunction after trauma hemorrhage and appeared to ameliorate maladaptive immune responses to injury by promoting cell survival pathways while inhibiting pro-inflammatory pathways [15]. These findings were supported by human transcriptomic studies showing upregulation of pro-inflammatory signaling pathways in critically injured patients [17, 47], and enrichment of pathways associated with cell survival and death (e.g., apoptosis, necrosis) in those with MODS [17]. Our study design, with a defined cohort of patients at high risk of MODS, early therapeutic administration and clinically validated endpoints, aimed to address issues in previous human trauma trials of organ protective agents [5]. Our final patient cohort’s incidence and severity of MODS is in line with our original projections for study design and sample size estimate. Despite this we did not identify any evidence of efficacy for artesunate in this indication.
The small sample size of the PP population led to imbalances between intervention and placebo groups. In particular, patients in the artesunate arm were substantially more shocked on admission, and this is known to be a key driver of MODS [1, 34]. Post-hoc analyses adjusting for shock and other baseline factors did not indicate a treatment effect, although these were limited by the sample size. The artesunate arm also had a very high proportion of penetrating trauma (51%), a group thought to have potentially different injury responses [48] and which were excluded from our previous human transcriptomic analyses [17]. However, post-hoc subgroup analyses did not reveal any differences in outcomes between blunt and penetrating trauma cohorts. Our cohort also had comparably low late mortality (4%) considering the degree of shock and injury severity on admission [1, 2]. While the low mortality may be explained by the exclusion of patients expected to die within the first 48 h, the cohort may not fully represent the total trauma population at risk of MODS and its consequences.
The immune response to trauma is complex, and the types of response that pre-dispose patients to MODS remain incompletely understood. The immunological pathways targeted by artesunate may not alter the course of MODS in human trauma patients. Alternatively, the dose and/or timing of artesunate used in our study may not be sufficient to achieve a therapeutic effect in human trauma patients. Our PK/PD analyses showed participants given a 2.4 mg/kg or 4.8 mg/kg bolus of artesunate had plasma levels within the therapeutic range for malaria treatment. However, animal models showed a dose-dependent relationship for artesunate benefit so a higher target range, or repeat dosing, may be required to achieve efficacy and impact upon organ dysfunction in human trauma. Future studies that interrogate biobank data in this cohort may provide further insight as to whether artesunate impacts on the inflammatory response in humans, while not affecting organ dysfunction as measured by the SOFA score. Finally, the clinical characterization of MODS is evolving [1, 2, 34, 49] and it is possible that artesunate may only have efficacy against specific forms of MODS. Future research that matches underlying cell pathways to different clinical forms of MODS may enable more targeted patient and endpoint selection.
There are several limitations to this study. First, the trial was terminated early and did not reach full recruitment, limiting the power and precision of the primary end-point assessment. While the results did not convincingly demonstrate a benefit for the treatment, it is possible that one might still exist on the primary endpoint. However, the 95% CI did not support the presence of a clinically significant reduction in the 48 h SOFA with treatment, and the mean scores were higher overall in the treatment arm. Therefore, at the time of stopping the trial there was no signal to suggest a clinically meaningful benefit of treatment. Second, despite randomization, there were imbalances in the baseline characteristics between treatment groups, which affect group comparisons for both efficacy and VTE. The placebo group only included male participants and therefore results are most applicable for a male trauma population. Lastly, our study was not powered to detect differences in VTE rates between the groups. Future analyses that compare MODS and VTE outcomes between our dataset and matched cohorts from existing datasets (e.g., ACIT-2 [50]) might provide further insight.
In this study of critically injured trauma patients with major hemorrhage, artesunate did not improve organ dysfunction and was potentially associated with an increased VTE risk. Future studies that focus on immune responses to injury and associated clinical forms of MODS may elucidate the reasons for this and facilitate identification of alternative pharmaceutical agents that attenuate MODS in trauma hemorrhage.
Data availability
The data that support the findings of this trial are available from the corresponding author (JMS) upon reasonable request.
References
Cole E, Gillespie S, Vulliamy P et al (2020) Multiple organ dysfunction after trauma. Br J Surg 107:402–412. https://doi.org/10.1002/bjs.11361
Shepherd JM, Cole E, Brohi K (2017) Contemporary patterns of multiple organ dysfunction syndrome in trauma. Shock 47:429–435. https://doi.org/10.1097/SHK.0000000000000779
Ulvik A, Kvåle R, Wentzel-Larsen T, Flaatten H (2007) Multiple organ failure after trauma affects even long-term survival and functional status. Crit Care 11:R95. https://doi.org/10.1186/cc6111
Sauaia A, Moore EE, Johnson JL et al (2014) Temporal trends of postinjury multiple-organ failure. J Trauma Acute Care Surg 76:582–593. https://doi.org/10.1097/TA.0000000000000147
Lord JM, Midwinter MJ, Chen Y-F et al (2014) The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet (London, England) 384:1455–1465. https://doi.org/10.1016/S0140-6736(14)60687-5
WHO (2015) Guidelines for the treatment of malaria, 3rd edn. World Health Organisation, Geneva
Ho WE, Peh HY, Chan TK, Wong WSF (2014) Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 142:126–139. https://doi.org/10.1016/j.pharmthera.2013.12.001
Singh NP, Lai H (2001) Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci 70:49–56. https://doi.org/10.1016/s0024-3205(01)01372-8
Efferth T, Romero MR, Wolf DG et al (2008) The antiviral activities of artemisinin and artesunate. Clin Infect Dis 47:804–811. https://doi.org/10.1086/591195
Moore CM, Hoey EM, Trudgett A, Timson DJ (2011) Artemisinins act through at least two targets in a yeast model. FEMS Yeast Res 11:233–237. https://doi.org/10.1111/j.1567-1364.2010.00706.x
Ho WE, Cheng C, Peh HY et al (2012) Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma. Free Radic Biol Med 53:498–507. https://doi.org/10.1016/j.freeradbiomed.2012.05.021
Zhao M, Xue D-B, Zheng B et al (2007) Induction of apoptosis by artemisinin relieving the severity of inflammation in caerulein-induced acute pancreatitis. World J Gastroenterol 13:5612–5617. https://doi.org/10.3748/wjg.v13.i42.5612
Li Y, Wang S, Wang Y et al (2013) Inhibitory effect of the antimalarial agent artesunate on collagen-induced arthritis in rats through nuclear factor kappa B and mitogen-activated protein kinase signaling pathway. Transl Res 161:89–98. https://doi.org/10.1016/j.trsl.2012.06.001
Li B, Yu M, Pan X et al (2014) Artesunate reduces serum lipopolysaccharide in cecal ligation/puncture mice via enhanced LPS internalization by macrophages through increased mRNA expression of scavenger receptors. Int J Mol Sci 15:1143–1161. https://doi.org/10.3390/ijms15011143
Sordi R, Nandra KK, Chiazza F et al (2017) Artesunate protects against the organ injury and dysfunction induced by severe hemorrhage and resuscitation. Ann Surg 265:408–417. https://doi.org/10.1097/SLA.0000000000001664
Khan AI, Kapoor A, Chen J et al (2018) The antimalarial drug artesunate attenuates cardiac injury in a rodent model of myocardial infarction. Shock 49:675–681. https://doi.org/10.1097/SHK.0000000000000963
Cabrera CP, Manson J, Shepherd JM et al (2017) Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: a prospective cohort study. PLoS Med 14:e1002352. https://doi.org/10.1371/journal.pmed.1002352
Rosenthal PJ (2008) Artesunate for the treatment of severe falciparum malaria. N Engl J Med 358:1829–1836. https://doi.org/10.1056/NEJMct0709050
Li Q, Melendez V, Cantilena LR et al (2009) Pharmacokinetic profiles of artesunate after single intravenous doses at 0.5, 1, 2, 4, and 8 mg/kg in healthy volunteers: a phase i study. Am J Trop Med Hyg 81:615–621. https://doi.org/10.4269/ajtmh.2009.09-0150
Miller RS, Li Q, Cantilena LR et al (2012) Pharmacokinetic profiles of artesunate following multiple intravenous doses of 2, 4, and 8 mg/kg in healthy volunteers: phase 1b study. Malar J 11:255. https://doi.org/10.1186/1475-2875-11-255
Deeken JF, Wang H, Hartley M et al (2018) A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol 81:587–596. https://doi.org/10.1007/s00280-018-3533-8
Dondorp A, Nosten F, Stepniewska K et al (2005) Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366:717–725. https://doi.org/10.1016/S0140-6736(05)67176-0
Dondorp AM, Fanello CI, Hendriksen IC et al (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376:1647–1657. https://doi.org/10.1016/S0140-6736(10)61924-1
Saito M, Mansoor R, Kennon K et al (2020) Efficacy and tolerability of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: a systematic review and individual patient data meta-analysis. Lancet Infect Dis 20:943–952. https://doi.org/10.1016/S1473-3099(20)30064-5
Ribeiro IR, Olliaro P (1998) Safety of artemisinin and its derivatives. A review of published and unpublished clinical trials. Med Trop (Mars) 58:50–53
McIntosh HM, Olliaro P (2000) Artemisinin derivatives for treating severe malaria. Cochrane Database Syst Rev 1998:CD000527. https://doi.org/10.1002/14651858.CD000527
Sinclair D, Donegan S, Isba R, Lalloo DG (2012) Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev 2012:CD005967. https://doi.org/10.1002/14651858.CD005967.pub4
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 340:c332–c332. https://doi.org/10.1136/bmj.c332
Minei JP, Cuschieri J, Sperry J et al (2012) The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock*. Crit Care Med 40:1129–1135. https://doi.org/10.1097/CCM.0b013e3182376e9f
Department of Constitutional Affairs (2007) Mental capacity act 2005 code of practice. London Stationary Office, London
World Medical Association (2013) World Medical Association Declaration of Helsinki. JAMA 310:2191. https://doi.org/10.1001/jama.2013.281053
Baker SP, O’Neill B, Haddon W Jr, Long WB (1974) The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14:187–269
Vincent J-L, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710. https://doi.org/10.1007/BF01709751
Liu D, Namas RA, Vodovotz Y et al (2020) Unsupervised clustering analysis based on MODS severity identifies four distinct organ dysfunction patterns in severely injured blunt trauma patients. Front Med 7:46. https://doi.org/10.3389/fmed.2020.00046
Nadel S, Goldstein B, Williams MD et al (2007) Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 369:836–843. https://doi.org/10.1016/S0140-6736(07)60411-5
Yehya N, Harhay MO, Curley MAQ et al (2019) Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med 200:828–836. https://doi.org/10.1164/rccm.201810-2050CP
Definition Task Force ARDS, Ranieri VM, Rubenfeld GD et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533. https://doi.org/10.1001/jama.2012.5669
Bellomo R, Ronco C, Kellum JA et al (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. https://doi.org/10.1186/cc2872
Cole E, Davenport R, Willett K, Brohi K (2014) The burden of infection in severely injured trauma patients and the relationship with admission shock severity. J Trauma Acute Care Surg 76:730–735. https://doi.org/10.1097/TA.0b013e31829fdbd7
Newcombe RG (1998) Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 17:873–890. https://doi.org/10.1002/(sici)1097-0258(19980430)17:8%3c873::aid-sim779%3e3.0.co;2-i
Hanpithakpong W, Kamanikom B, Dondorp AM et al (2008) A liquid chromatographic-tandem mass spectrometric method for determination of artesunate and its metabolite dihydroartemisinin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 876:61–68. https://doi.org/10.1016/j.jchromb.2008.10.018
Frith D, Goslings JC, Gaarder C et al (2010) Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost 8:1919–1925. https://doi.org/10.1111/j.1538-7836.2010.03945.x
Hamada SR, Espina C, Guedj T et al (2017) High level of venous thromboembolism in critically ill trauma patients despite early and well-driven thromboprophylaxis protocol. Ann Intensive Care 7:97. https://doi.org/10.1186/s13613-017-0315-0
Moore EE, Moore HB, Kornblith LZ et al (2021) Trauma-induced coagulopathy. Nat Rev Dis Prim 7:30. https://doi.org/10.1038/s41572-021-00264-3
Geerts WH, Code KI, Jay RM et al (1994) A prospective study of venous thromboembolism after major trauma. N Engl J Med 331:1601–1606. https://doi.org/10.1056/NEJM199412153312401
Jacobs BN, Cain-Nielsen AH, Jakubus JL et al (2017) Unfractionated heparin versus low-molecular-weight heparin for venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg 83:151–158. https://doi.org/10.1097/TA.0000000000001494
Xiao W, Mindrinos MN, Seok J et al (2011) A genomic storm in critically injured humans. J Exp Med 208:2581–2590. https://doi.org/10.1084/jem.20111354
Pfeifer R, Teuben M, Andruszkow H et al (2016) Mortality patterns in patients with multiple trauma: a systematic review of autopsy Studies. PLoS One 11:1–9. https://doi.org/10.1371/journal.pone.0148844
Eriksson J, Nelson D, Holst A et al (2021) Temporal patterns of organ dysfunction after severe trauma. Crit Care 25:165. https://doi.org/10.1186/s13054-021-03586-6
Centre for Trauma Sciences Activation of Inflammation and Coagulation in Trauma (ACIT). https://www.c4ts.qmul.ac.uk/research-programmes/acit. Accessed 1 Oct 2021
Acknowledgements
The authors would like to thank Col Brian Smith (U.S. Army Medical Materiel Development Activity) for providing phase I data for the Investigator Brochure; Sanofi for providing the Investigational Medicinal Product Dossier and confidential data to the Medicines and Healthcare products Regulation Authority; and Sigma Tau (now Alfa Wassermann Spa) for provision of Good Manufacturing Practice artesunate free of charge. We would like to acknowledge the contributions of trial research assistants Ayoyemi Macauly and Haya Akkad, and the trauma research fellows at the Centre for Trauma Sciences who recruited study participants and collected follow-up data.
Funding
This research was funded by the Wellcome Trust (Grant Reference Number: 101012/Z/13/Z) and Department of Health (Grant Reference Number: HICFR7405).
Author information
Authors and Affiliations
Contributions
The study was conceived by CT and KB. Methods were developed by JMS, CR, ARB, JT, NW, CT, and KB. Data acquisition was performed by JMS, JR, LA, and CR. The formal analysis was by JMS and ARB. The first draft of the manuscript was written by JMS, and it was reviewed and edited by ARB, JT, NW, KB and CT. All authors commented on previous versions of the manuscript and read and approved the final manuscript. Funding acquisition was by CT.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflict of interest, including financial interests, activities, relationships, and affiliations in relation with the TOP-ART Trial.
Ethical approval
The trial was authorized by the Medicines and Healthcare products Regulation Authority (ref: 21313/0052/001-0001) on the 22nd January 2016 and approved by London City and East Research Ethics Committee (reference: 16/LO/003) on 11th March 2016. The trial was registered prospectively with the EU Clinical Trials Register (Identifier 2015-000301-40) and the ISRCTN (Identifier 15731357) on the 28th August 2015.
Consent to participate
The trial was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants or, in cases of impaired capacity, their closest relative or appropriate representative.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shepherd, J.M., Ross, J., Anton, L. et al. Safety and efficacy of artesunate treatment in severely injured patients with traumatic hemorrhage. The TOP-ART randomized clinical trial. Intensive Care Med 49, 922–933 (2023). https://doi.org/10.1007/s00134-023-07135-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-023-07135-3