Abstract
Purpose
The life-saving role of oxygen therapy in African children with severe pneumonia is not yet established.
Methods
The open-label fractional-factorial COAST trial randomised eligible Ugandan and Kenyan children aged > 28 days with severe pneumonia and severe hypoxaemia stratum (SpO2 < 80%) to high-flow nasal therapy (HFNT) or low-flow oxygen (LFO: standard care) and hypoxaemia stratum (SpO2 80–91%) to HFNT or LFO (liberal strategies) or permissive hypoxaemia (ratio 1:1:2). Children with cyanotic heart disease, chronic lung disease or > 3 h receipt of oxygen were excluded. The primary endpoint was 48 h mortality; secondary endpoints included mortality or neurocognitive sequelae at 28 days.
Results
The trial was stopped early after enrolling 1852/4200 children, including 388 in the severe hypoxaemia stratum (median 7 months; median SpO2 75%) randomised to HFNT (n = 194) or LFO (n = 194) and 1454 in the hypoxaemia stratum (median 9 months; median SpO2 88%) randomised to HFNT (n = 363) vs LFO (n = 364) vs permissive hypoxaemia (n = 727). Per-protocol 15% of patients in the permissive hypoxaemia group received oxygen (when SpO2 < 80%). In the severe hypoxaemia stratum, 48-h mortality was 9.3% for HFNT vs. 13.4% for LFO groups. In the hypoxaemia stratum, 48-h mortality was 1.1% for HFNT vs. 2.5% LFO and 1.4% for permissive hypoxaemia. In the hypoxaemia stratum, adjusted odds ratio for 48-h mortality in liberal vs permissive comparison was 1.16 (0.49–2.74; p = 0.73); HFNT vs LFO comparison was 0.60 (0.33–1.06; p = 0.08). Strata-specific 28 day mortality rates were, respectively: 18.6, 23.4 and 3.3, 4.1, 3.9%. Neurocognitive sequelae were rare.
Conclusions
Respiratory support with HFNT showing potential benefit should prompt further trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In Africa, in children hospitalised with severe pneumonia with oxygen saturations between 80 and 91% who did not receive oxygen, mortality assessed at 48 h (1.4%) was comparable to the usual method of oxygen delivery (low-flow oxygen; LFO (2.5%)) and in those receiving high-flow nasal therapy (HFNT, 1.1%). The potential impact of HFNT on patient-centred outcomes and on resources, particularly oxygen supplies, should stimulate further exploration particularly in children with severe pneumonia managed in low resource settings. |
Introduction
In Africa, severe pneumonia remains the leading cause of mortality in children under 5 years old [1], posing a major disease burden on health systems. The World Health Organization (WHO) recommends presumptive antibiotic treatment and oxygen for those with clinicallydefined severe pneumonia and/or hypoxaemia (peripheral oxygen saturation (SpO2) < 90%) [2]. However, the evidence under-pinning the use of oxygen therapy is weak [3] and it is generally poorly targeted using non-specific clinical signs [4] since many paediatric services lack pulse oximeters [5, 6]. In-hospital mortality among pneumonia cases is high (9–16%) with hypoxemic children at fivefold greater risk of death [6, 7]. WHO recommends research on the targeted use of oxygen therapy together with simple, non-invasive methods of respiratory support as cost-effective strategies for improving outcome [3], but these have not yet been tested in adequately powered randomised controlled trials [8, 9].
A key challenge for the trial design is the significant gap between supply and demand for oxygen in resource-limited African hospitals. Expense and logistic challenges mean that many lack sustainable provision of bottled oxygen [10], as highlighted in a survey of 231 health facilities (12 African countries) showing only 43% had an uninterrupted source of oxygen, 24% had a functioning oxygen concentrator (the WHO-preferred source of oxygen [2]) and an only 81 (35%) had uninterrupted electricity supply (necessary for oxygen concentrators) [11]. Thus, in many hospitals children with severe pneumonia receive little or no oxygen, whether this contributes to the poor outcomes is unknown.
The Children’s Oxygen Administration Strategies Trial (COAST) simultaneously addressed two hypotheses. First, whether liberal oxygenation strategies in children with SpO2 ≥ 80–91% will decrease mortality (at 48 h and up to 28 days) compared with a permissive hypoxia strategy. Second, whether respiratory support with high-flow nasal therapy (OptiFlow™) decreases mortality (at 48 h and up to 28 days) compared with low-flow oxygen delivery (standard care) [12].
Methods
COAST was a two-stratum multicentre, open, fractional-factorial RCT (see statistical methods, Supplemental Appendix) conducted in four Ugandan and two Kenyan hospitals. Children aged 28 days to 12 years, hospitalised with a history of respiratory illness and any one of the 2013 WHO clinical definitions of severe pneumonia [13] plus hypoxaemia (SpO2 < 92%) were enrolled into either the severe hypoxaemia stratum (SpO2 < 80%) or the hypoxaemia stratum (SpO2 80–91%). Children with previous diagnosed but uncorrected cyanotic heart disease, chronic lung disease (excluding asthma), children given oxygen given at another health facility (or > 3 h at the current hospital) or previous COAST enrolment were excluded. In the severe hypoxaemia stratum, eligible children were randomised (ratio 1:1) to high-flow nasal therapy (HFNT) via OptiFlow™ or low-flow oxygen delivery (LFO: standard practice). In the hypoxaemia stratum, eligible children were randomised (ratio 1:1:2) to HFNT via OptiFlow™, LFO delivery (standard practice) or permissive hypoxaemia since pre-existing data indicated no differences in mortality across the SpO2 range 80–89% [12, 14] (see Supplemental Appendix and Trial Protocol).
Screening and randomisation
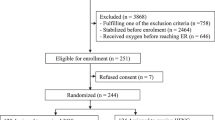
Children hospitalised with suspected severe pneumonia were clinically assessed for eligibility including oxygen saturation measurement (BITMOS sat 801 +), which are capable of measuring oxygen saturations accurately during motion and low peripheral perfusion. Children eligible for the hypoxaemia stratum required two SpO2 readings of 80–91% 5 min apart. Where prior written consent from parents/legal guardians could not be obtained, ethics committees approved verbal assent with delayed written informed consent as soon as practicable [15]. Otherwise informed written consent was obtained from parents or guardians before randomization. The trial statistician in London generated and kept the sequential randomization list, computer-generated using variably sized permuted blocks stratified by trial centre. Randomisation occurred using consecutively numbered packs containing randomised links to opaque sealed envelopes ensuring allocation concealment.
Study procedures
Children were managed on general paediatric wards; mechanical ventilation facilities were largely unavailable. Training in triage and emergency paediatric life support was given throughout the trial to optimize case recognition, supportive management and protocol adherence. Basic infrastructural support for emergency care, patient monitors, haemoglobin, glucose and lactate point-of-care tests, blood cultures and chest X-rays were provided by the study. A structured clinical case report form was completed at admission and on reviews at 1 2, 4, 8, 16, 24, 36 and 48 h.
Oxygen therapy and respiratory support
HFNT was delivered by AIRVO™2 device (https://www.fphcare.com/), which contains a humidifier with integrated flow generator that delivers, to spontaneous breathing patients, high flow warmed and humidified air/oxygen blend. HFNT was initiated on FiO2 of 21% (room air) with flow rates increase and oxygen titrated in using a structured protocol. Reliable sources of oxygen including electricity power-back up for the AIRVO™2 and oxygen concentrators were provided to ensure oxygen delivery was uninterrupted [11]. LFO was delivered by nasal canulae/prongs and escalated to higher flow rates delivered by standard masks. Saturations were checked at 15-, 30-, and 60-min post-enrolment and during the structured reviews. Per-protocol the permissive hypoxaemia arm received LFO if SpO2 fell below 80%. Children unable to tolerate HFNT were switched to LFO. From 2-h post-enrolment oxygen could be weaned/stopped if SpO2 remained ≥ 92% in room air and restarted if SpO2 dropped to < 92%. At 48 h children on HFNT were switched to LFO (extra details in Supplemental Methods).
All children received standard treatments including intravenous maintenance fluids (2.5–4 mls/kg/hour) [16], antibiotics, antimalarials, antipyretics, anticonvulsants, and transfusion for haemoglobin < 5 g/dl according to national guidelines. At the scheduled follow-up visit, children were clinically assessed (including neurodevelopmental assessment) at 28 day post-randomization. Clinicians were trained on the structured ‘Kilifi Developmental Milestones Assessment which covers three broad domains of child functioning: motor, language and personal–social development [17]. From previous experience [18], neurocognitive changes can be transient, children who exhibiting neurocognitive sequalae at 28 days were re-assessed at 90 days. Nurses/doctors were unblinded; laboratory tests were assayed blinded.
Endpoints
The primary outcome was mortality at 48-h post-randomization (a timepoint capturing the majority of in-patient deaths [16]) and deaths to Day 28 [19]. Secondary outcomes included day-28 mortality, treatment failure at 48 h (persistent hypoxaemia: SpO2 < 92% plus respiratory distress), time to hypoxaemia resolution (SpO2 ≥ 92%), duration of respiratory (oxygen/HFNT) support, length of initial hospital stay, Day-28 neurocognitive/developmental sequelae, re-admissions and anthropometric status (See Statistical Analysis Plan). Adverse events were graded using the Common Toxicity Criteria for Adverse Events v4.0 [20]. An Endpoint Review Committee (ERC) reviewed all deaths blinded to treatment arm.
Statistical analysis
The sample size was determined using simulations, assuming a 1:2 ratio between the respective strata and 48 h mortality in the LFO arms of 26 and 9% in severely hypoxaemia and hypoxaemia strata respectively [12]. Overall, 4200 children provided at least 90% power to detect a 33% relative risk reduction (RR) for liberal (HTNT or LFO) vs permissive hypoxaemia and a 25% RR for HFNT vs. LFO. Two (of three) planned interim analyses were reviewed by an independent Data Monitoring Committee using Haybittle–Peto criterion (p < 0.001) for early stopping for either efficacy or harm.
Patients were analysed according to their randomised groups, following a prespecified statistical analysis plan. The primary outcome was analysed as a binary outcome using multilevel logistic regression including both treatment allocation variables simultaneously (HFNT vs. LFO, and liberal (any respiratory support) vs. permissive hypoxaemia and adjusted for the stratifying factors of baseline SpO2 (grouped as < 80, 80–84, 85–89, 90–91%) and trial site (as a random factor). Adjusted odds ratios (aOR) with 95% confidence intervals (CI) were calculated to compare any respiratory support/oxygen vs permissive hypoxaemia (hypoxaemia stratum only), and HFNT vs. LFO (primary effect estimates). Each treatment allocation variable was tested for significance using a likelihood ratio test of the reduced compared to full regression model fit. (More details in Supplemental Methods).
Results
The trial was stopped prematurely in by the Trial Steering Committee on the grounds of feasibility as a result of a campaign to terminate the trial in Uganda, which deemed permissive hypoxaemia (permissive hypoxaemia) unethical (detailed in the Supplemental Methods).
Between 14th Feb 2017 and when enrolment ceased (28th Feb 2020) 1842 eligible children were enrolled into the COAST trial and included in all analyses (Fig. 1), Of 388 in the severe hypoxaemia stratum, 194 children were randomised to HFNT and 194 to LFO. Of 1454 children in the hypoxaemia stratum, 363 to were randomised to HFNT, 364 to LFO and 727 to permissive hypoxaemia. Baseline characteristics are presented in Table 1 demonstrating multiple features of severe pneumonia were present in all children. In the respective strata median SpO2 was 75 (68, 78) and 88 (85, 90); 75 and 65% had radiographically confirmed pneumonia. Additional baseline features and working diagnoses reported at 48 h are summarised in Supplemental Tables S2a, b.
Adherence to randomisation
Most oxygen therapy was started within 30 min of screening except for three children on HFNT who died before this timepoint. Adherence to the randomisation strategy was excellent (Table 2). Per protocol LFO was started in the permissive hypoxaemia arm in 107/726 (14.7%) for drops of SpO2 < 80% and in 2 whose SpO2 was > 80%. Three children unable to tolerate HFNT were switched to LFO.
Use of oxygen and respiratory support
In the severe hypoxaemia stratum, median duration (interquartile range, IQR) on respiratory support by HFNT was longer than for LFO 36.6 h (9, 48) versus 32.1 h (7.4, 47.7). In the hypoxaemia stratum duration of support by HFNT and LFO were similar (8.4 (2.8, 26.8) and 6.8 (2.5, 25.3) hours respectively (Fig. 2; Tables 2 and Supplemental T2). In the hypoxaemia stratum only 198/362 (54.7%) on HFNT had oxygen titrated in. In the severe hypoxaemia stratum although the number of hours of supplemental air/oxygen blend was similar over the 48 h period, the mean (standard deviation) volume of oxygen (litres) used was lower in HFNT: 2731L/child (2733) than LFO: 3591L/child (4128). In the hypoxaemia stratum both the time receiving supplemental air/oxygen [9.8 (14.6) versus 15.9 (16.6)] hours and oxygen volume received [969L/child (1890) versus 1481L/child (2480)] were considerably lower in HFNT than LFO. The permissive hypoxaemia strategy had lower hours/volume of oxygen therapy than the liberal strategies. Baseline SpO2 levels in children requiring oxygen in the permissive hypoxaemia arm were similar to those never receiving oxygen (Supplemental Figure S1).
Mortality and neurocognitive sequelae
Vital status at 48 h (primary endpoint) was known in all children 388 (100%) in the severe hypoxaemia stratum and in 363 (100%), 363 (99.7%) and 724 (99.6%) in the hypoxaemia stratum children, respectively. By Day 28 vital status was known in 194 (100%) and 192 (99%) in the severe hypoxaemia stratum and in 362 (99.7%), 362 (99.7%) and 718 (99.2%) in the hypoxaemia stratum children respectively (Supplemental Figure S2). By 48 h in the severe hypoxaemia stratum, 18 (9.3%) in HFNT versus 26 (13.4%) in LFO groups had died. In the hypoxaemia stratum, 4 (1.1%), 9 (2.5%) and 10 (1.4%) children in HFNT vs LFO vs permissive hypoxaemia had died. The aOR for liberal versus permissive hypoxaemia was 1.16 (95% CI 0.49, 2.74) p = 0.728 and for HTNT versus LFO was 0.60 (0.33, 1.06) p = 0.076 (Table 3). By 28 days, 36 (18.6%) and 45 (23.4%) children in the severe hypoxaemia stratum and 12(3.3%), 15(4.1%) and 28(3.9%) in the hypoxaemia stratum had died [aOR for LFO versus permissive hypoxaemia 0.92 (0.53, 1.59); aOR for HFNT versus LFO was 0.75 (0.49, 1.16)]. Most neurocognitive sequelae primarily reported at Day 28 in survivors were transient, and all had resolved by Day 90. Details on blind ERC review of deaths and relationship are given in Table 3. Overall, there was one event identified by this process that was possibly related to treatment arm; most deaths appeared to be attributable to the severity of the underlying condition or comorbidity.
Other clinical outcomes
Treatment failure to 48 h (defined as SpO2 < 92% plus respiratory distress) was somewhat lower in HFNT versus LFO aOR 0.75 (0.40, 1.41) and lower for liberal versus permissive hypoxaemia strategy aOR 0.37 (0.19, 0.71) (Table 3; Supplemental Figure S3). Further exploration of these treatment failures by severity of hypoxaemia (< 80, 80–89 and 90–91%) and comorbidities are reported in Supplemental Table S3. Overall, in the severe hypoxaemia stratum, 14/33 (42.4%) and 12/33 (36%) treatment failures were for saturations of < 80 and 80–89%, respectively. Day-28 hospital readmissions were low (≤ 3%) across all groups/strata (Supplemental Table S4). There was no evidence of a difference in mean hospital stay in the HFNT versus LFO [difference in means 0.26 (95% CI − 0.43, 0.94)] or liberal versus permissive hypoxaemia [0.62 (0.02, 1.22)] groups nor differences in anthropometric status at Day 28 (Table 3).
Discussion
The premature termination of the COAST trial meant that it was unable answer the two specific hypotheses it was designed to address. First, whether liberal oxygenation therapy for children with hypoxaemia (SpO2 80–91%) is superior to permissive hypoxaemia. In this stratum, however, the overall 48 h and Day 28 mortality was low across all arms including the permissive hypoxaemia arm. Second, as the trial was stopped prematurely there were insufficient data to demonstrate that HFNT was superior to LFO, but caution that the interpretation may be subject to a Type II error i.e. false negative (no benefit of HFNT versus LFO) since it was underpowered to uncover true effects, should they exist. However, the size of reduction (40%) in 48-h mortality warrants further investigation particularly for children with severe hypoxaemia (SpO2 < 80%). Notably, across both strata the method of HFNT administration was also relatively oxygen-sparing, using room air/oxygen blends without any evidence of harm. In the hypoxaemia stratum in many cases HFNT was provided on room air, leading to correction of hypoxaemia, without any evidence of harm.
Although the trial does not provide definitive data to inform treatment guidelines, the knowledge acquired from the trial adds a new level of uncertainty to the liberal use of oxygen as a supportive therapy [21]. Equipoise, centred on the degree of uncertainty about the relative benefits (or risks) of a clinical intervention, requires a clear distinction between pre-existing knowledge (evidence) and opinion or personal preference [22]. In the case of severe pneumonia, pre-existing evidence demonstrating clear benefits of oxygen therapy was poor [3]. On that basis the trial was both ethically and scientifically sound [23]. Substantial uncertainty behoves clinicians to conduct RCTs so that in future societies will know which treatments are better and ensuring patients will not be exposed to inferior or harmful treatments.
Whilst oxygen, as a supportive therapy, has been considered the standard treatment for pneumonia for a large part of the last century, the recognition of potential oxygen toxicity is relatively recent [24]. In several areas of emergency care and resuscitation, the use of oxygen (and other therapies included in guidelines [25]) are now being challenged by emerging evidence from clinical trials, including in paediatric populations [26, 27], and in systematic reviews [21, 28]. In neonates, medical oxygen used during resuscitation increases mortality, myocardial injury and renal injury [29].
COAST was designed with cognisance of the significant gaps between supply and demand for oxygen in hospitals in developing countries [11]. Sustainable provision of bottled oxygen is expensive and logistically challenging [30]; therefore, the WHO-preferred source of oxygen is oxygen concentrators [2]. Nevertheless, technical reports on the operational quality, availability and reliability of cylinders and oxygen concentrators indicate that, even when available, these are often faulty and unsustainable due to high cost [10], or depend upon erratic electricity supply [11]. With regard to demand on health services, the routine use of pulse oximetry to target oxygen therapy is poorly implemented despite having been recommended by WHO for triage of sick children for over two decades [5]. Hence, oxygen therapy is generally targeted by non-specific clinical signs that predict hypoxaemia poorly [4], thus exposing large numbers of children without hypoxaemia to oxygen therapy, and raising questions over safety [28, 31], cost and demand for a constrained health resource [32]. Our findings indicating that hypoxaemia in children with severe pneumonia can be corrected without additional oxygen (including those on HFNT using room air alone) is timely given the major demand on health services for oxygen therapy as a result of the COVID-19 epidemic.
Recognising the resource limitations and costs of oxygen therapy, the protocol incorporated relatively oxygen-sparing strategies in the investigational groups (HFNT and permissive hypoxaemia) and vital sign monitoring to ensure early weaning in both liberal (HFNT and LFO) strategies (Supplemental Figure S4). Thus, over 48 h even in the most liberal of the strategies (LFO) the median volume of oxygen in the respective strata (3279 and 1337 L/child) were substantially lower than in a LFO equivalent strategy (5990L over a median of 2.5 days) reported in the multicentre study in Nigeria investigating oxygen use in children with pneumonia [6].
One key limitation of the COAST trial was its premature termination. Nevertheless, the COAST trial represents the largest trial of oxygen therapy ever conducted in children and the only multicentre controlled trial. Another limitation was that we were unable to confirm the pulse oximetry readings with arterial blood gases. We chose BITMOS sat 801 + , since it incorporates Masimo Signal Extraction Technology® and was the instrument of choice in the large multicentre pneumonia aetiology study [19]. The substantially lower mortality in both strata of the trial, contrast to the mortality rates of 9–10 and 26–30%, respectively, reported in previous studies in African children, on which our power calculation was based [4, 6, 7, 33]. Most were conducted prior to the introduction of vaccines against the lead bacterial causes of paediatric pneumonia (Haemophilus influenzae type b and Streptococcus pneumoniae) into national immunization programs (including Uganda and Kenya) and/or the scaling-up of prevention and anti-retroviral medications for HIV. The resultant change in the aetiologic profile of childhood pneumonia was recently demonstrated by a multi-country case-control hypoxaemia studies of HIV-negative children with radiologically-confirmed pneumonia. Only 56/1749 (3·2%) cases had a positive blood culture and viruses accounted for 61% of cases. Respiratory syncytial virus, which is associated with low mortality [34], was the commonest pathogen [19]. The WHO severe pneumonia definition maximizes sensitivity over specificity, resulting in substantial overlap with other medical conditions [4, 35]. Notable in the COAST trial was the large proportion of previously undiagnosed cardiac conditions particularly in the sub-group with treatment failure. Nevertheless, radiologically confirmed pneumonia was present in a very large proportion of our study population, thus generalisable to children hospitalised with acute pneumonia in resource-limited hospitals.
HFNT has been shown in other populations to reduce the need for mechanical ventilation [36]. In that vast majority of hospitals in Africa access to mechanical ventilation or specialist intensive care is not standard. We, therefore, proposed that HFNT was a feasible alternative source of respiratory support owing to its relative simplicity in implementation, humidification, low risk of nosocomial infection [37]. In addition, the ability to blend both oxygen and room air to deliver positive end-expiratory pressure, thus, limiting exposure to high concentrations of oxygen (and potential toxicity) and with the prospect of reducing costs to health services. Bubble continuous positive airway pressure (bCPAP) is an alternative means of providing respiratory support. However, a recent trial in Malawian children showing worse outcomes in children receiving bCPAP than usual care advocates caution regarding implementation of bCPAP in a real-world setting without physician oversight [9].
In conclusion, our findings support the need for future trials with similar designs, particularly in settings where access to oxygen and/or mechanical respiratory support are restricted. The scale of the mortality reduction of HFNT over LFO, particularly in severely hypoxaemic children (40%) warrants further investigation. Oxygen-sparing strategies potentially offer cost-effective approaches to reducing overall oxygen requirements in overburdened health services and adds to the general findings in critical care than ‘less is more’ [38].
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
28 May 2021
The Open Access Licence has been corrected.
References
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE (2016) Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388(10063):3027–3035
Hospital Care for Children. Guidelines for the management of common childhood illnesses. Second Edition. In. Geneva: World Health Organization; 2013.
Recommendations for management of common childhood conditions: evidence for technical update of pocket book recommendations. In. Geneva: World Health Organization; 2012.
Mwaniki MK, Nokes DJ, Ignas J, Munywoki P, Ngama M, Newton CR, Maitland K, Berkley JA (2009) Emergency triage assessment for hypoxaemia in neonates and young children in a Kenyan hospital: an observational study. Bull World Health Organ 87(4):263–270
English M, Gathara D, Mwinga S, Ayieko P, Opondo C, Aluvaala J, Kihuba E, Mwaniki P, Were F, Irimu G, Wasunna A, Mogoa W, Nyamai R (2014) Adoption of recommended practices and basic technologies in a low-income setting. Arch Dis Child 99(5):452–456
Graham H, Bakare AA, Ayede AI, Oyewole OB, Gray A, Peel D, McPake B, Neal E, Qazi SA, Izadnegahdar R, Duke T, Falade AG (2019) Hypoxaemia in hospitalised children and neonates: a prospective cohort study in Nigerian secondary-level hospitals. EClinicalMedicine 16:51–63
Lazzerini M, Sonego M, Pellegrin MC (2015) Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle-income countries: systematic review and meta-analysis. PLoS One 10(9):e0136166
Chisti MJ, Salam MA, Smith JH, Ahmed T, Pietroni MA, Shahunja KM, Shahid AS, Faruque AS, Ashraf H, Bardhan PK, Sharifuzzaman S, Graham SM, Duke T (2015) Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: an open, randomised controlled trial. Lancet 386(9998):1057–1065
McCollum ED, Mvalo T, Eckerle M, Smith AG, Kondowe D, Makonokaya D, Vaidya D, Billioux V, Chalira A, Lufesi N, Mofolo I, Hosseinipour M (2019) Bubble continuous positive airway pressure for children with high-risk conditions and severe pneumonia in Malawi: an open label, randomised, controlled trial. Lancet Respir Med 7(11):964–974
Dobson MB (2001) Oxygen concentrators and cylinders. Int J Tuberc Lung Dis 5(6):520–523
Belle J, Cohen H, Shindo N, Lim M, Velazquez-Berumen A, Ndihokubwayo JB, Cherian M (2010) Influenza preparedness in low-resource settings: a look at oxygen delivery in 12 African countries. J Infect Dev Ctries 4(7):419–424
Maitland K, Kiguli S, Opoka RO, Olupot-Olupot P, Engoru C, Njuguna P, Bandika V, Mpoya A, Bush A, Williams TN, Grieve R, Sadique Z, Harrison D, Rowan K (2017) Children’s Oxygen Administration Strategies Trial (COAST): a randomised controlled trial of high flow versus oxygen versus control in African children with severe pneumonia. Wellcome Open Res 2:100
Pocket Book of Hospital Care for Children: Guidelines for the management of common childhood illnesses. In., Second edn. Geneva: World Health Organization; 2013.
Maitland K (2017) Different targets in children. In: 37th International Symposium on Intensive Care and Emergency Medicine Brussels.
Maitland K, Molyneux S, Boga M, Kiguli S, Lang T (2011) Use of deferred consent for severely ill children in a multi-centre phase III trial. Trials 12:90
Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC et al (2011) Mortality after fluid bolus in African children with severe infection. N Engl J Med 364(26):2483–2495
Abubakar A, Holding P, Van de Vijver F, Bomu G, Van Baar A (2010) Developmental monitoring using caregiver reports in a resource-limited setting: the case of Kilifi Kenya. Acta Paediatr 99(2):291–297
Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S et al (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376(9753):1647–1657
Pneumonia Etiology Research for Child Health Study G (2019) Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 394(10200):757–779.
Common Terminology Criteria for Adverse Events (CTCAE) (2009) In. Edited by SERVICES USDOHAH, vol. Version 4. National Institutes of Health National Cancer Institute.
Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schunemann HJ, Neary JD, Alhazzani W (2018) Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 391(10131):1693–1705
Djulbegovic B (2007) Articulating and responding to uncertainties in clinical research. J Med Philos 32(2):79–98
Peto R, Baigent C (1998) Trials: the next 50 years Large scale randomised evidence of moderate benefits. BMJ 317(7167):1170–1171
Jackson RM (1985) Pulmonary oxygen toxicity. Chest 88(6):900–905
Perner A, Finfer S (2018) Do trials that report a neutral or negative treatment effect improve the care of critically ill patients? Yes. Intensive Care Med 44(11):1985–1988
Cunningham S, Rodriguez A, Adams T, Boyd KA, Butcher I, Enderby B, MacLean M, McCormick J, Paton JY, Wee F, Thomas H, Riding K, Turner SW, Williams C, McIntosh E et al (2015) Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet 386(9998):1041–1048
Peters MJ, Jones GAL, Wiley D, Wulff J, Ramnarayan P, Ray S, Inwald D, Grocott M, Griksaitis M, Pappachan J, O’Neill L, Eaton S, Mouncey PR, Harrison DA, Rowan KM et al (2018) Conservative versus liberal oxygenation targets in critically ill children: the randomised multiple-centre pilot Oxy-PICU trial. Intensive Care Med 44(8):1240–1248
Martin DS, Grocott MP (2013) Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med 41(2):423–432
Davis PG, Tan A, O’Donnell CP, Schulze A (2004) Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet 364(9442):1329–1333
Dauncey JW, Olupot-Olupot P, Maitland K (2019) Healthcare-provider perceptions of barriers to oxygen therapy for paediatric patients in three government-funded eastern Ugandan hospitals; a qualitative study. BMC Health Serv Res 19(1):335
Gilbert-Kawai ET, Mitchell K, Martin D, Carlisle J, Grocott MP (2014) Permissive hypoxaemia versus normoxaemia for mechanically ventilated critically ill patients. Cochrane Database Syst Rev 5:CD009931
Bradley BD, Howie SR, Chan TC, Cheng YL (2014) Estimating oxygen needs for childhood pneumonia in developing country health systems: a new model for expecting the unexpected. PLoS One 9(2):e89872
Djelantik IG, Gessner BD, Sutanto A, Steinhoff M, Linehan M, Moulton LH, Arjoso S (2003) Case fatality proportions and predictive factors for mortality among children hospitalized with severe pneumonia in a rural developing country setting. J Trop Pediatr 49(6):327–332
Nokes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, Scott JA, Cane PA, Medley GF (2009) Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis 49(9):1341–1349
Agweyu A, Lilford RJ, English M, Clinical Information Network Author G (2018) Appropriateness of clinical severity classification of new WHO childhood pneumonia guidance: a multi-hospital, retrospective, cohort study. Lancet Glob Health 6(1):e74–e83
Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, Mekontso-Dessap A, Schreiber A, Azoulay E, Mercat A, Demoule A, Lemiale V, Pesenti A, Riviello ED, Mauri T et al (2019) High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med 45(5):563–572
Hui DS, Chow BK, Lo T, Ng SS, Ko FW, Gin T, Chan MTV (2015) Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest 147(5):1336–1343
Perner A, Hjortrup PB, Pettila V (2018) Focus on randomised clinical trials. Intensive Care Med 44(12):2257–2259
Acknowledgements
We thank all the participants and staff from all the centres participating in the COAST trial. A special thank to: A. Turnbull, A. Odit (Endpoint Review Committe); E. Molyneux (chair), I. Lubega, W. Macharia, J. Crawley, M. Peters (Trial Steering Committee); T. Peto (chair), P. Musoke, F. Were, C. Semple, J. Todd (Data Monitoring Committee). This paper is published with permission from the Director of KEMRI. We thank the independent members of the trial steering committee and data monitoring committee for their support.
Funding
COAST was funded by the UK Joint Global Health Trials scheme: Medical Research Council, Foreign Commonwealth and Department Office, Department of Health and Social Care through the National Institute for Health Research) and Wellcome Trust (Grant Number MR/L004364/1). Fisher and Paykel Healthcare donated the AIRVO™2 machines and oxygen interfaces for the trial.
The funders and the sponsor (Imperial College, London) had no role in study design, data collection, data analysis, data interpretation, or report writing.
Author information
Authors and Affiliations
Consortia
Contributions
KM, KR, DAH, POO, SK, and JF designed the study; SK POO, MH, FA, ROO, AT, VB, AM, HM, WO, MN, CE, EO, and TNW gathered the data; KT/DAH analyzed the data; all authors vouch for the data and analysis; KM wrote the original draft paper which all authors commented on; all authors decided to publish.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
The School of Medicine Research Ethics Committee, Makerere University, Kampala, Uganda, Kenya Medical Research Institute Scientific Ethics and Review Unit, Nairobi, Kenya and Imperial College Research Ethics Committee approve the trial.
Consent for publication
We did not obtain consent to publish individual data and were unable to obtain or retrospectively seek consent from individual patients or their legal guardians for the publication of their data. All data were anonymised prior to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maitland, K., Kiguli, S., Olupot-Olupot, P. et al. Randomised controlled trial of oxygen therapy and high-flow nasal therapy in African children with pneumonia. Intensive Care Med 47, 566–576 (2021). https://doi.org/10.1007/s00134-021-06385-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06385-3