Abstract
The continuing shortage of deceased donor organs for transplantation, and the limited number of potential donors after brain death, has led to a resurgence of interest in donation after circulatory death (DCD). The processes of warm and cold ischemia threaten the viability of DCD organs, but these can be minimized by well-organized DCD pathways and new techniques of in situ organ preservation and ex situ resuscitation and repair post-explantation. Transplantation survival after DCD is comparable to donation after brain death despite higher rates of primary non-function and delayed graft function. Countries with successfully implemented DCD programs have achieved this primarily through the establishment of national ethical, professional and legal frameworks to address both public and professional concerns with all aspects of the DCD pathway. It is unlikely that expanding standard DCD programs will, in isolation, be sufficient to address the worldwide shortage of donor organs for transplantation. It is therefore likely that reliance on extended criteria donors will increase, with the attendant imperative to minimize ischemic injury to candidate organs. Normothermic regional perfusion and ex situ perfusion techniques allow enhanced preservation, assessment, resuscitation and/or repair of damaged organs as a way of improving overall organ quality and preventing the unnecessary discarding of DCD organs. This review will outline exemplar controlled and uncontrolled DCD pathways, highlighting practical and logistical considerations that minimize warm and cold ischemia times while addressing potential ethical concerns. Future perspectives will also be discussed.



Similar content being viewed by others
References
Manyalich M, Nelson H, Delmonico FL (2018) The need and opportunity for donation after circulatory death worldwide. Curr Opin Organ Transplant 23:136–141
Thuong M, Ruiz A, Evrard P, Kuiper M, Boffa C, Akhtar MZ, Neuberger J, Ploeg R (2016) New classification of donation after circulatory death donors definitions and terminology. Transpl Int 29:749–759
Manara AR, Murphy PG, O’Callaghan G (2012) Donation after circulatory death. Br J Anaesth 108(Suppl 1):i108–i121
Foss S, Nordheim E, Sorensen DW, Syversen TB, Midtvedt K, Asberg A, Dahl T, Bakkan PA, Foss AE, Geiran OR, Fiane AE, Line PD (2018) First Scandinavian protocol for controlled donation after circulatory death using normothermic regional perfusion. Transplant Direct 4:e366
Inci I (2017) Donors after cardiocirculatory death and lung transplantation. J Thorac Dis 9:2660–2669
Summers DM, Watson CJ, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM, Bradley JA (2015) Kidney donation after circulatory death (DCD): state of the art. Kidney Int 88:241–249
Tang JX, Na N, Li JJ, Fan L, Weng RH, Jiang N (2018) Outcomes of controlled donation after cardiac death compared with donation after brain death in liver transplantation: a systematic review and meta-analysis. Transplant Proc 50:33–41
Hulme W, Allen J, Manara AR, Murphy PG, Gardiner D, Poppitt E (2016) Factors influencing the family consent rate for organ donation in the UK. Anaesthesia 71:1053–1063
Siminoff LA, Gordon N, Hewlett J, Arnold RM (2001) Factors influencing families’ consent for donation of solid organs for transplantation. JAMA 286:71–77
Mitro G, Warnock R, Wiederhold P, Jiles K, Ortiz J (2018) Consistency of DCD procurement procedures across organ procurement organizations—preliminary findings [abstract]. American Transplant Congress Archives—ATC Abstracts 2018. https://atcmeetingabstracts.com/abstract/consistency-of-dcd-procurement-procedures-across-organ-procurement-organizations-preliminary-findings/. Accessed Nov 2018
Moers C, Leuvenink HG, Ploeg RJ (2010) Donation after cardiac death: evaluation of revisiting an important donor source. Nephrol Dial Transplant 25:666–673
Reich DJ, Mulligan DC, Abt PL, Pruett TL, Abecassis MM, D’Alessandro A, Pomfret EA, Freeman RB, Markmann JF, Hanto DW, Matas AJ, Roberts JP, Merion RM, Klintmalm GB (2009) ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant 9:2004–2011
Citerio G, Cypel M, Dobb GJ, Dominguez-Gil B, Frontera JA, Greer DM, Manara AR, Shemie SD, Smith M, Valenza F, Wijdicks EF (2016) Organ donation in adults: a critical care perspective. Intensive Care Med 42:305–315
Algahim MF, Love RB (2015) Donation after circulatory death: the current state and technical approaches to organ procurement. Curr Opin Organ Transplant 20:127–132
Kotsopoulos AMM, Boing-Messing F, Jansen NE, Vos P, Abdo WF (2018) External validation of prediction models for time to death in potential donors after circulatory death. Am J Transplant 18:890–896
Neyrinck A, Van RD, Monbaliu D (2013) Donation after circulatory death: current status. Curr Opin Anaesthesiol 26:382–390
de Groot YJ, Lingsma HF, Bakker J, Gommers DA, Steyerberg E, Kompanje EJ (2012) External validation of a prognostic model predicting time of death after withdrawal of life support in neurocritical patients. Crit Care Med 40:233–238
Rabinstein AA, Yee AH, Mandrekar J, Fugate JE, de Groot YJ, Kompanje EJ, Shutter LA, Freeman WD, Rubin MA, Wijdicks EF (2012) Prediction of potential for organ donation after cardiac death in patients in neurocritical state: a prospective observational study. Lancet Neurol 11:414–419
Rusinova K, Cerny V, Kukal J, Pokorna E (2015) Expanding the DCD donor pool: prediction of time to death after removal of life-sustaining treatments. Intensive Care Med 41:1719–1720
Wind J, Snoeijs MG, Brugman CA, Vervelde J, Zwaveling J, van Mook WN, van Heurn EL (2012) Prediction of time of death after withdrawal of life-sustaining treatment in potential donors after cardiac death. Crit Care Med 40:766–769
Schlegel A, Kalisvaart M, Scalera I, Laing RW, Mergental H, Mirza DF, Perera T, Isaac J, Dutkowski P, Muiesan P (2018) The UK DCD Risk Score: a new proposal to define futility in donation-after-circulatory-death liver transplantation. J Hepatol 68:456–464
Dikdan GS, Mora-Esteves C, Koneru B (2012) Review of randomized clinical trials of donor management and organ preservation in deceased donors: opportunities and issues. Transplantation 94:425–441
Dhanani S, Hornby L, Ward R, Shemie S (2012) Variability in the determination of death after cardiac arrest: a review of guidelines and statements. J Intensive Care Med 27:238–252
Morrissey PE, Monaco AP (2014) Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation 97:258–264
Institue of Medicine Committee on Increasing Organ Donation Rates (2006) Organ donation: opportunities for action. Washington, D.C., National Academies Press. https://www.nap.edu/read/11643/chapter/1. Accessed Nov 2018
Wall SP, Kaufman BJ, Williams N, Norman EM, Gilbert AJ, Munjal KG, Maikhor S, Goldstein MJ, Rivera JE, Lerner H, Meyers C, Machado M, Montella S, Pressman M, Teperman LW, Dubler NN, Goldfrank LR (2016) Lesson from the New York City out-of-hospital uncontrolled donation after circulatory determination of death program. Ann Emerg Med 67:531–537
Grasner JT, Lefering R, Koster RW, Masterson S, Bottiger BW, Herlitz J, Wnent J, Tjelmeland IB, Ortiz FR, Maurer H, Baubin M, Mols P, Hadzibegovic I, Ioannides M, Skulec R, Wissenberg M, Salo A, Hubert H, Nikolaou NI, Loczi G, Svavarsdottir H, Semeraro F, Wright PJ, Clarens C, Pijls R, Cebula G, Correia VG, Cimpoesu D, Raffay V, Trenkler S, Markota A, Stromsoe A, Burkart R, Perkins GD, Bossaert LL (2016) EuReCa ONE-27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation 105:188–195
Jabre P, Bougouin W, Dumas F, Carli P, Antoine C, Jacob L, Dahan B, Beganton F, Empana JP, Marijon E, Karam N, Loupy A, Lefaucheur C, Jost D, Cariou A, Adnet F, Rea TD, Jouven X (2016) Early identification of patients with out-of-hospital cardiac arrest with no chance of survival and consideration for organ donation. Ann Intern Med 165:770–778
Navalpotro-Pascual JM, Echarri-Sucunza A, Mateos-Rodriguez A, Peinado-Vallejo F, Del Valle PF, Alonso-Moreno D, Del Pozo-Perez C, Mier-Ruiz MV, Ruiz-Azpiazu JI, Bravo-Castello J, Martinez-Cuellar N, Saez-Jimenez A, Lopez-Unanua C, Anton-Ramas R, Escriche-Lopez MDC, Giraldo-Sebastia JM, Garcia-Ochoa MJ, Martin-Sanchez E, Borraz-Clares D, Del Valle MM, Carriedo-Scher C, Rosell-Ortiz F (2018) Uncontrolled donation programs after out-of-hospital cardiac arrest. An estimation of potential donors. Resuscitation 122:87–91
Bossaert LL, Perkins GD, Askitopoulou H, Raffay VI, Greif R, Haywood KL, Mentzelopoulos SD, Nolan JP (2015) European Resuscitation Council guidelines for resuscitation 2015: section 11. The ethics of resuscitation and end-of-life decisions. Resuscitation 95:302–311
Dominguez-Gil B, Haase-Kromwijk B, Van LH, Neuberger J, Coene L, Morel P, Corinne A, Muehlbacher F, Brezovsky P, Costa AN, Rozental R, Matesanz R (2011) Current situation of donation after circulatory death in European countries. Transpl Int 24:676–686
Dominguez-Gil B, Duranteau J, Mateos A, Nunez JR, Cheisson G, Corral E, De JW, Del RF, Valero R, Coll E, Thuong M, Akhtar MZ, Matesanz R (2016) Uncontrolled donation after circulatory death: European practices and recommendations for the development and optimization of an effective programme. Transpl Int 29:842–859
Zamperetti N, Bellomo R, Latronico N (2016) Heart donation and transplantation after circulatory death: ethical issues after Europe’s first case. Intensive Care Med 42:93–95
Minambres E, Rubio JJ, Coll E, Dominguez-Gil B (2018) Donation after circulatory death and its expansion in Spain. Curr Opin Organ Transplant 23:120–129
Donación en Asistolia en España: Situación actual y Recomendaciones. Documento de Consenso 2012. Donación en Asistolia en España. 2012. http://www.ont.es/infesp/DocumentosDeConsenso/DONACIÓN%20EN%20ASISTOLIA%20EN%20ESPAÑA.%20SITUACIÓN%20ACTUAL%20Y%20RECOMENDACIONES.pdf. Accessed Nov 2018
Fondevila C, Hessheimer AJ, Flores E, Ruiz A, Mestres N, Calatayud D, Paredes D, Rodriguez C, Fuster J, Navasa M, Rimola A, Taura P, Garcia-Valdecasas JC (2012) Applicability and results of Maastricht type 2 donation after cardiac death liver transplantation. Am J Transplant 12:162–170
Del Río F, Andrés A, Padilla M et al (2019) Kidney transplantation from uncontrolled donors after circulatory death: the Spanish experience. Kidney Int 95:420–428
Delsuc C, Faure A, Berthiller J, Dorez D, Matillon X, Meas-Yedid V, Floccard B, Marcotte G, Labeye V, Rabeyrin M, Codas R, Chauvet C, Robinson P, Morelon E, Badet L, Hanf W, Rimmele T (2018) Uncontrolled donation after circulatory death: comparison of two kidney preservation protocols on graft outcomes. BMC Nephrol 19:3
Demiselle J, Augusto JF, Videcoq M, Legeard E, Dube L, Templier F, Renaudin K, Sayegh J, Karam G, Blancho G, Dantal J (2016) Transplantation of kidneys from uncontrolled donation after circulatory determination of death: comparison with brain death donors with or without extended criteria and impact of normothermic regional perfusion. Transpl Int 29:432–442
Molina M, Guerrero-Ramos F, Fernandez-Ruiz M, Gonzalez E, Cabrera J, Morales E, Gutierrez E, Hernandez E, Polanco N, Hernandez A, Praga M, Rodriguez-Antolin A, Pamplona M, de la Rosa F, Cavero T, Chico M, Villar A, Justo I, Andres A (2018) Kidney transplant from uncontrolled donation after circulatory death donors maintained by nECMO has long-term outcomes comparable to standard criteria donation after brain death. Am J Transplant 27:5. https://doi.org/10.1111/ajt.14991
Pieter Hoogland ER, van Smaalen TC, Christiaans MH, van Heurn LW (2013) Kidneys from uncontrolled donors after cardiac death: which kidneys do worse? Transpl Int 26:477–484
De Carlis R, Di Sandro S, Lauterio A, Ferla F, Dell’Acqua A, Zanierato M, De Carlis L (2017) Successful donation after cardiac death liver transplants with prolonged warm ischemia time using normothermic regional perfusion. Liver Transpl 23:166–173
Gomez-de-Antonio D, Campo-Canaveral JL, Crowley S, Valdivia D, Cordoba M, Moradiellos J, Naranjo JM, Ussetti P, Varela A (2012) Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 31:349–353
Dominguez-Gil B, Murphy P, Procaccio F (2016) Ten changes that could improve organ donation in the intensive care unit. Intensive Care Med 42:264–267
Manara A (2015) Bespoke end-of-life decision making in ICU: has the tailor got the right measurements? Crit Care Med 43:909–910
Lee YY, Ranse K, Silvester W, Mehta A, Van Haren F (2018) Attitudes and self-reported end-of-life care of Australian and New Zealand intensive care doctors in the context of organ donation after circulatory death. Anaesth Intensive Care 46:488–497
Academy of Medical Royal Colleges UK Donation Ethics Committee (2011) An ethical framework for controlled donation after circulatory death. 2011. http://www.aomrc.org.uk/wp-content/uploads/2016/04/Ethical_framework_donation_circulatory_death_1211-3.pdf. Accessed Nov 2018
Kotloff RM, Blosser S, Fulda GJ, Malinoski D, Ahya VN, Angel L, Byrnes MC, DeVita MA, Grissom TE, Halpern SD, Nakagawa TA, Stock PG, Sudan DL, Wood KE, Anillo SJ, Bleck TP, Eidbo EE, Fowler RA, Glazier AK, Gries C, Hasz R, Herr D, Khan A, Landsberg D, Lebovitz DJ, Levine DJ, Mathur M, Naik P, Niemann CU, Nunley DR, O’Connor KJ, Pelletier SJ, Rahman O, Ranjan D, Salim A, Sawyer RG, Shafer T, Sonneti D, Spiro P, Valapour M, Vikraman-Sushama D, Whelan TP (2015) Management of the potential organ donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med 43:1291–1325
Zavalkoff S, Shemie SD, Grimshaw JM, Chasse M, Squires JE, Linklater S, Appleby A, Hartell D, Lalani J, Lotherington K, Knoll G (2018) Potential organ donor identification and system accountability: expert guidance from a Canadian consensus conference. Can J Anaesth 18:5
van Dijk G, van Bruchem-Visser R, de Beaufort I (2018) Organ donation after euthanasia, morally acceptable under strict procedural safeguards. ClinTransplant 32:e13294
Dominguez-Gil B, Coll E, Elizalde J, Herrero JE, Pont T, Quindos B, Marcelo B, Bodi MA, Martinez A, Nebra A, Guerrero F, Mancino JM, Galan J, Lebron M, Minambres E, Matesanz R (2017) Expanding the donor pool through intensive care to facilitate organ donation: results of a Spanish multicenter study. Transplantation 101:e265–e272
Manara AR, Thomas I, Harding R (2016) A case for stopping the early withdrawal of life sustaining therapies in patients with devastating brain injuries. J Intensive Care Soc 17:295–301
Harvey D, Butler J, Groves J, Manara A, Menon D, Thomas E, Wilson M (2018) Management of perceived devastating brain injury after hospital admission: a consensus statement from stakeholder professional organizations. Br J Anaesth 120:138–145
Souter MJ, Blissitt PA, Blosser S, Bonomo J, Greer D, Jichici D, Mahanes D, Marcolini EG, Miller C, Sangha K, Yeager S (2015) Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management: a position statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care 23:4–13
Manara AR, Menon DK (2017) Withdrawal of treatment after devastating brain injury: post-cardiac arrest pathways lead in best practice. Anaesthesia 72:1179–1184
Broderick AR, Manara A, Bramhall S, Cartmill M, Gardiner D, Neuberger J (2016) A donation after circulatory death program has the potential to increase the number of donors after brain death. Crit Care Med 44:352–359
Manara AR, Dominguez-Gil B, Perez-Villares JM, Soar J (2016) What follows refractory cardiac arrest: death, extra-corporeal cardiopulmonary resuscitation (E-CPR), or uncontrolled donation after circulatory death? Resuscitation 108:A3–A5
Prabhu A, Parker LS (2017) New therapies raise new dilemmas in the emergency department. Am J Bioeth 17:6–16
Roncon-Albuquerque R Jr, Gaiao S, Figueiredo P, Principe N, Basilio C, Mergulhao P, Silva S, Honrado T, Cruz F, Pestana M, Oliveira G, Meira L, Franca A, Almeida-Sousa JP, Araujo F, Paiva JA (2018) An integrated program of extracorporeal membrane oxygenation (ECMO) assisted cardiopulmonary resuscitation and uncontrolled donation after circulatory determination of death in refractory cardiac arrest. Resuscitation 133:88–94
Shemie SD, Hornby L, Baker A, Teitelbaum J, Torrance S, Young K, Capron AM, Bernat JL, Noel L (2014) International guideline development for the determination of death. Intensive Care Med 40:788–797
Pana R, Hornby L, Shemie SD, Dhanani S, Teitelbaum J (2016) Time to loss of brain function and activity during circulatory arrest. J Crit Care 34:77–83
Nelson HM, Glazier AK, Delmonico FL (2016) Changing patterns of organ donation: brain dead donors are not being lost by donation after circulatory death. Transplantation 100:446–447
Ausania F, White SA, Pocock P, Manas DM (2012) Kidney damage during organ recovery in donation after circulatory death donors: data from UK National Transplant Database. Am J Transplant 12:932–936
Tsui SSL, Oniscu GC (2017) Extending normothermic regional perfusion to the thorax in donors after circulatory death. Curr Opin Organ Transplant 22:245–250
Minambres E, Suberviola B, Dominguez-Gil B, Rodrigo E, Ruiz-San Millan JC, Rodriguez-San Juan JC, Ballesteros MA (2017) Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant 17:2165–2172
Rojas-Pena A, Sall LE, Gravel MT, Cooley EG, Pelletier SJ, Bartlett RH, Punch JD (2014) Donation after circulatory determination of death: the University of Michigan experience with extracorporeal support. Transplantation 98:328–334
Ruiz P, Gastaca M, Bustamante FJ, Ventoso A, Palomares I, Prieto M, Fernandez JR, Salvador P, Pijuan JI, Valdivieso A (2018) Favorable outcomes after liver transplantation with normothermic regional perfusion from donors after circulatory death: a single-center experience. Transplantation. https://doi.org/10.1097/tp.0000000000002391
Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA (2013) Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 381:727–734
Dalle Ave AL, Shaw DM, Bernat JL (2016) Ethical Issues in the use of extracorporeal membrane oxygenation in controlled donation after circulatory determination of death. Am J Transplant 16:2293–2299
Perez-Villares JM, Rubio JJ, Del RF, Minambres E (2017) Validation of a new proposal to avoid donor resuscitation in controlled donation after circulatory death with normothermic regional perfusion. Resuscitation 117:46–49
Hosgood SA, Nicholson ML (2018) The evolution of donation after circulatory death donor kidney repair in the United Kingdom. Curr Opin Organ Transplant 23:130–135
Nicholson ML, Hosgood SA (2013) Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant 13:1246–1252
Hosgood SA, Saeb-Parsy K, Wilson C, Callaghan C, Collett D, Nicholson ML (2017) Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 7:e012237
Kollmann D, Selzner M (2017) Recent advances in the field of warm ex vivo liver perfusion. Curr Opin Organ Transplant 22:555–562
Mergental H, Perera MT, Laing RW, Muiesan P, Isaac JR, Smith A, Stephenson BT, Cilliers H, Neil DA, Hubscher SG, Afford SC, Mirza DF (2016) Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant 16:3235–3245
Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M, Chow CW, Chaparro C, Hutcheon M, Singer LG, Slutsky AS, Yasufuku K (2011) Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 364:1431–1440
Greer DM, Valenza F, Citerio G (2015) Improving donor management and transplantation success: more research is needed. Intensive Care Med 41:537–540
Dhital KK, Chew HC, Macdonald PS (2017) Donation after circulatory death heart transplantation. Curr Opin Organ Transplant 22:189–197
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MS is Editor in Chief of the Journal of Neurosurgical Anesthesiology. BDG is Director General at the Organización Nacional de Trasplantes, Spain. DG is Editor-in-Chief of Seminars in Neurology, and receives research support from Bard Medical, Inc, as PI for the INTREPID Clinical Trial. ARM is the Regional Clinical Lead in Organ Donation for the South West of England. MJS is the Medical Director for Lifecenter Northwest Organ Procurement agency, serving the Pacific Northwest of the USA. The authors have no other conflicts of interest to declare.
Ethical approval
An approval by an ethics committee was not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2019_5533_MOESM1_ESM.pptx
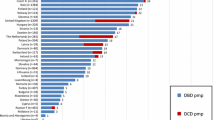
Supplementary material 1 (PPTX 92 kb). Fig. 1 Evolution of donation after circulatory death (rates per million population) in the most active countriesfrom 2003 to 2017. Source: Global Observatory on Organ Donation and Transplantation(http://www.transplant-observatory.org/)
134_2019_5533_MOESM2_ESM.docx
Supplementary material 2 (DOCX 13 kb). Table 1 Assessing the balance of whether an individual ante-mortem intervention is acceptable in an individualpatient
Rights and permissions
About this article
Cite this article
Smith, M., Dominguez-Gil, B., Greer, D.M. et al. Organ donation after circulatory death: current status and future potential. Intensive Care Med 45, 310–321 (2019). https://doi.org/10.1007/s00134-019-05533-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05533-0