Abstract
Purpose
Functional status and chronic health status are important baseline characteristics of critically ill patients. The assessment of frailty on admission to the intensive care unit (ICU) may provide objective, prognostic information on baseline health. To determine the impact of frailty on the outcome of critically ill patients, we performed a systematic review and meta-analysis comparing clinical outcomes in frail and non-frail patients admitted to ICU.
Methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, PubMed, CINAHL, and Clinicaltrials.gov. All study designs with the exception of narrative reviews, case reports, and editorials were included. Included studies assessed frailty in patients greater than 18 years of age admitted to an ICU and compared outcomes between fit and frail patients. Two reviewers independently applied eligibility criteria, assessed quality, and extracted data. The primary outcomes were hospital and long-term mortality. We also determined the prevalence of frailty, the impact on other patient-centered outcomes such as discharge disposition, and health service utilization such as length of stay.
Results
Ten observational studies enrolling a total of 3030 patients (927 frail and 2103 fit patients) were included. The overall quality of studies was moderate. Frailty was associated with higher hospital mortality [relative risk (RR) 1.71; 95% CI 1.43, 2.05; p < 0.00001; I 2 = 32%] and long-term mortality (RR 1.53; 95% CI 1.40, 1.68; p < 0.00001; I 2 = 0%). The pooled prevalence of frailty was 30% (95% CI 29–32%). Frail patients were less likely to be discharged home than fit patients (RR 0.59; 95% CI 0.49, 0.71; p < 0.00001; I 2 = 12%).
Conclusions
Frailty is common in patients admitted to ICU and is associated with worsened outcomes. Identification of this previously unrecognized and vulnerable ICU population should act as the impetus for investigating and implementing appropriate care plans for critically ill frail patients. Registration: PROSPERO (ID: CRD42016053910).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of clinical frailty describes a state or syndrome of reduced physical, physiologic, and cognitive reserve [1]. Frail patients are characterized by a heterogeneous combination of decreased mobility, weakness, reduced muscle mass, poor nutritional status, and diminished cognitive function; all of these render frail individuals more susceptible to extrinsic stressors. Although frailty is more common in older individuals [2], frailty and aging are not synonymous [3], and the former has been estimated to occur in approximately 25% of those over the age of 65 and over 50% of those over the age of 85 [4]. Frail individuals are more likely to require assisted living, be more susceptible to adverse events, and are more likely to die when compared to age-matched non-frail individuals [5, 6]. Frailty has characteristic molecular and physiologic features including increases in inflammatory markers [7] and epigenetic changes characterized by increased DNA methylation [8].
A number of validated tools to screen for, identify, and quantify frailty have been described [3, 9,10,11,12,13,14]. Frailty is increasingly recognized as a risk factor for poor outcomes across many disease states and healthcare interventions [15,16,17]. Similarly, there is emerging evidence that frailty status has important implications for individuals developing critical illness [18].
The increased prevalence of frailty with ageing and growing utilization of critical care services by older individuals [19] imply there is likely to be an increased number of frail patients being admitted to intensive care units (ICUs). Considering the diminished resilience and greater vulnerability of frail patients, they may be more likely to require and have longer durations of the life-sustaining ICU therapies but their effectiveness in this population is unclear. Studies to date of critically ill frail patients have utilized a variety of designs, include variable populations and report on a range of outcomes. There is a need to synthesize the evidence in its entirety to understand if it can inform prognostication or decision-making and to identify knowledge gaps to inform future research including the potential for targeted interventions. Therefore, we conducted a systematic review and meta-analysis of the impact of frailty on outcomes for critically ill frail patients admitted to the ICU. We hypothesized that frailty would be associated with higher hospital and long-term mortality, increased utilization of healthcare resources, and prolonged institutionalization. An abstract of this study has been accepted for presentation at the 2017 European Society of Intensive Care Medicine Conference [20].
Methods
This systemic review was conducted and reported according to Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Guidelines (see Appendix for Moose checklist) [21, 22]. The protocol was registered on PROSPERO (ID: CRD42016053910) in December 2016 after the initial literature search but before the literature search was subsequently updated in January and April of 2017. Eligible studies included observational studies or randomized controlled trials (RCT) that reported on frailty in ICU settings. Studies were included if they included adults (age ≥18 years) admitted to the ICU, reported on patient or health services outcomes, and used a validated tool to identify frailty. In order to best evaluate the impact of frailty, only studies comparing frail and non-frail populations were included. Narrative reviews, editorials, case reports, case-series, animal studies, and duplicate publications were excluded. Published abstracts were eligible for inclusion and there were no language restrictions.
Search strategy
We electronically searched MEDLINE, EMBASE, CINAHL, and PubMed databases initially in June 2016 which was then updated in December 2016 and April 2017. Our search strategy cross-referenced frailty and ICUs using appropriate medical subject headings (MeSH) and keywords (Appendix—Search strategies). The references from selected articles and reviews were manually searched for additional studies. We also searched trial registries and conference abstracts for completed but unpublished studies. The searches were developed and conducted in consultation with a research librarian. A protocol for this review has not been published separately.
Study selection
Two authors (AV and BW) independently evaluated the retrieved titles and abstracts of all articles to identify potentially relevant studies. Full-text review was conducted when either reviewer deemed that the abstract warranted further investigation on the basis of our a priori eligibility criteria. Any disagreement was resolved by discussion and consensus.
Data extraction
Data were independently extracted by AV and BW and subsequently verified by JM. Data extracted included the following: author, study design, frailty identification method, number of frail and non-frail patients, and outcomes of interest. Outcomes were chosen a priori and based on two domains; patient-centered outcomes and health services utilization. We collected both unadjusted data and adjusted data. The primary outcomes were in-hospital and long-term mortality (≥6 months following ICU admission). Although hospital mortality was initially chosen as the primary outcome, long-term mortality was later added to the primary outcome with increased availability of data for this outcome. Secondary patient-centered outcomes were ICU mortality and health-related quality-of-life (HRQL). Secondary health service utilization outcomes were ICU and hospital length of stay, receipt of vasoactive agents, receipt and duration of mechanical ventilation (MV), and discharge disposition.
Assessment of quality
The Newcastle-Ottawa Scale (NOS) was used to assess for study quality [23]. The NOS has three domains based on selection of the cohort, comparability of the groups, and quality of the outcomes. The NOS is a nine-point scale with a maximum of four points allocated to selection, two points for comparability, and three points for outcome. The reference for cohort selection was a general medical-surgical adult ICU population and the outcome reference was in-hospital mortality. Studies scoring 7 or more were considered high quality; 4–6, moderate quality; and 4 or less, low quality.
Data analysis
A meta-analysis was performed, where possible, using Review Manager 5.3 software (Cochrane Collaboration). We primarily pooled unadjusted data, although where possible we pooled adjusted data. For the purposes of data aggregation where more than one frailty scale was reported, we used the scale most commonly reported across all the included studies. We calculated pooled risk ratio (RR) and 95% confidence intervals (95% CI) using a random effects model for dichotomous outcomes and weighted mean difference with 95% CIs for continuous data. Where data were reported as medians it was converted to means and standard deviation [24]. Additional unpublished data were sought from authors. A priori planned subgroup analyses were conducted on the basis of the method of frailty identification, the severity of frailty, age of included subjects, and study quality. We hypothesized that the method of frailty identification would significantly change the effect estimate on outcomes, that increasing severity of frailty would be associated with higher mortality, that older frail patients would have higher mortality, and that there would be a decrease in the strength of association between frailty and outcomes in high quality studies.
Statistical heterogeneity was determined using the Mantel–Haenszel (M–H) Chi-squared test and the interclass correlation (I 2) statistic [25]. Significant heterogeneity was defined as I 2 > 50% or as p < 0.10 with the Mantel–Haenszel Chi-squared test. Funnel plots were used to visually inspect for publication bias. We considered an unadjusted, two-sided p < 0.05 to be statistically significant. To assess the probability that the results obtained were robust, we conducted trial sequential analysis (TSA) on long-term mortality with a two-sided α = 5%, a power of 90%, and the assumption that the absence of frailty would be associated with at least a 20% relative risk reduction in long-term all-cause mortality. The TSA was conducted with version 0.9.5.5 Beta (www.ctu.dk/tsa).
Results
Study selection
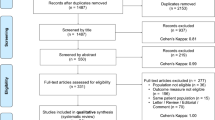
The initial search identified 1413 articles and abstracts (Appendix Fig. 1). After screening the titles and abstracts, 406 duplicates and 204 unrelated papers were excluded. A further 776 titles were excluded on the basis of publication type. Twenty-nine full-text articles were assessed; 17 studies did not meet inclusion criteria, leaving a total of 12 publications from 10 separate studies fulfilling eligibility since two studies reported new data in two separate publications each [26–37].
Summary of studies
The characteristics of the included studies are summarized in Tables 1 and 2. All were prospective observational cohort studies where frailty was measured on ICU admission; the majority were conducted in medical-surgical ICUs. Frailty was assessed using the clinical frailty scale (CFS) [3] in seven studies, a frailty index (FI) [38] in four, and the frailty physical phenotype (FP) [39] in two (Table 3). Of 3030 patients enrolled in the ten studies, 927 patients were classified as frail and 2103 as non-frail patients. The pooled prevalence of frailty in the ICU populations studied was 30% (95% CI 29–32%) (Fig. 1).
Study quality
There were no randomized controlled studies and the overall quality of the studies was moderate with mean (SD) NOS score of 6.5 (1.3) and a range of 5–8 (Table 4). There were five high quality studies with a score of 7 or above [26, 27, 32, 33, 35].
Mortality
All ten studies reported on mortality. Data could be abstracted for hospital mortality in nine studies, ICU mortality in six studies, and long-term mortality in six studies. Pooled unadjusted data using any frailty measure revealed an increased risk for frail patients compared to non-frail patients for hospital mortality (RR 1.71; 95% CI 1.43, 2.05; p < 0.00001; I 2 = 32%) and long-term mortality (RR 1.53; 95% CI 1.40, 1.68; p < 0.00001; I 2 = 0%) (Fig. 2). Pooled ICU mortality data revealed significantly increased risk of mortality for those identified as frail (RR 1.51; 95% CI 1.31, 1.75; p < 0.00001; I 2 = 8%) (Appendix Fig. 2). TSA for long-term mortality found that the required information size was 1514 and the Z line crossed both conventional boundaries and information size indicating that the association of frailty and long-term mortality was robust (Appendix Fig. 3).
ICU and hospital length of stay (LOS)
Six studies reported hospital LOS [26, 28, 29, 32, 33, 35] and five studies ICU LOS [26, 28, 29, 32, 35]. Pooled hospital and ICU LOS demonstrated non-statistically significant longer stays for frail patients with the mean differences being 3.39 days (95% CI −0.33, 7.10; p = 0.07; I 2 = 77%) and 0.33 days (95% CI −0.78, 1.44; p = 0.56; I 2 = 73%) (Appendix Fig. 4) respectively.
Mechanical ventilation and vasopressors
Five of the 10 studies, which included 703 frail and 1591 non-frail patients, reported on receipt of MV [26, 27, 29, 32, 35]. There was no difference between groups in the use of MV (80% vs 82% for frail vs. non-frail patients respectively: RR 1.01; 95% CI 0.93, 1.10; p = 0.81; I 2 = 67%). Only one study compared MV duration between groups and found no difference [28]. In addition, five of the 10 studies, which included 442 frail and 1008 non-frail patients, compared the use of vasoactive therapy between these groups [26,27,28,29, 35]. There was no difference in the use of vasoactive therapy (58% vs 56% for frail vs. non-frail patients respectively: RR 1.05; 95% CI 0.88, 1.26; p = 0.57; I 2 = 61%).
Discharge to home versus hospital or assisted living
Five of the 10 studies reported on discharge disposition [26, 28, 29, 31, 35]. The discharge destinations included home, rehabilitation facility, nursing home, or another acute care institution. As a result of the variety of post-discharge settings, we were only able to aggregate data for home which was reported in four studies [26, 28, 29, 35]. In these studies, reporting on 416 frail and 912 non-frail patients, frail patients were less likely to be discharged home (RR 0.59; 95% CI 0.49, 0.71; p < 0.00001; I 2 = 12%) (Fig. 3).
Quality of life
Only two studies reported on HRQL [26, 32, 40]. Both studies reported reduced quality of life at 1 year related to poor physical function in those who were identified as being frail on ICU admission (Table 2). Bagshaw et al. also found worsened quality life related to mental health.
Subgroup analyses
Frailty measure
We conducted subgroup analysis for the association of frailty, as measured with the CFS, FI, and FP, with hospital and long-term mortality (Fig. 4, Appendix Fig. 5). In the seven studies using the CFS data could be pooled including 775 frail and 1875 non-frail patients [26, 28, 31–33, 35, 37] and the RR for hospital mortality was 1.54; 95% CI 1.33, 1.77; p < 0.00001; I 2 = 0%. For the two studies pooled on the basis of an FI, the RR for hospital mortality was 3.71; 95% CI 0.22, 63.42; p = 0.36; I 2 = 76% [27, 29] and for two studies reporting on hospital mortality using the FP [28, 33] RR was 1.24; 95% CI 0.85, 1.81; p = 0.32; I 2 = 0%. On testing for interaction, there was not a statistically significant difference between the measures of frailty for the risk of hospital and long-term mortality (p = 0.49 and p = 0.26, respectively).
Severity of frailty
Of the ten studies, eight reported on the incremental risk of adverse outcomes, mainly mortality, with increasing frailty score; seven demonstrated increased risk with increased frailty severity while only one did not demonstrate an association. Differences in methods of reporting precluded pooling of data. Bagshaw et al. reported that increases in frailty severity as measured by the CFS incrementally increased risk of death adjusted for age, co-morbidities, and severity of illness at 1 year relative to those not frail [26]. Similarly, Brummel et al. reported a stepwise increase in 12-month mortality with each CFS point increase; a CFS of 1 was associated with approximately 90% 1-year survival rate, a CFS of 5 had 50% survival, and those with a CFS of 6/7 had a 35% survival rate [32]. Heyland et al. found that increasing FI was associated with decreased chance of being discharged home and that at 12 months, in multivariate models for every 0.2 increase in FI, the odds ratio of recovery to baseline physical function was 0.32 (0.19, 0.56; p < 0.0001) and survival was 0.56 (0.37, 0.85; p = 0.007) [35]. Kizilarslanoglu et al. categorized patients as robust (FI < 0.25), pre-frail (FI 0.25–0.40), and frail (FI > 0.40); 6-month mortality increased as the FI increased, 55.9%, 70.3%, and 84.6% respectively [27]. Le Maguet el al. demonstrated that increasing CFS scores and increasing FP frailty characteristics were associated with increased risk of mortality at 6 months [28]. Mueller et al. found that increasing FI correlated with reduced muscle mass as measured by ultrasound [29]. Similarly Zeng et al. found that the degree of frailty as measured by FI correlated with increased risk of mortality at both 30 days and 300 days [34]. Only one single-center study did not find a significant correlation between increasing CFS and mortality [31].
Impact of age
Six studies adjusted for age in the association between frailty and outcome [26, 27, 32–35] and in all of these studies, frailty was independently associated with adverse outcomes. Five of the studies included older adults of a minimum age as part of their inclusion criteria; one used the age of 50 [26], one used 60 [27], two used the age of 65 [28, 34], and one used the age of 80 [35]. The incidence of frailty in studies enrolling only older adults was 33.1% (95% CI 23.4, 43.5%) compared to 30% in all the included studies.
Study quality
There were five high quality studies [26, 27, 32, 33, 35] all reporting on hospital and long-term mortality. In these studies, frailty continued to be associated with increased risk of hospital and long-term mortality (RR 1.63; 95% CI 1.38, 1.91; p < 0.0001; I 2 = 15% and RR 1.51; 95% CI 1.37, 1.66; p < 0.0001, I 2 = 0%, respectively) (Appendix Figs. 6 and 7). On testing for interaction, we found that the increased risk for both hospital and long-term mortality was similar in both the high and low quality studies, (p = 0.54 and p = 0.15, respectively).
Adjusted outcomes
Nine studies reported outcomes adjusted for co-variates including age, illness severity, and co-morbidities, although there was a large degree of variability in the adjusted outcomes reported and the co-variates included in the adjustment models. All of the adjusted data reported in the studies is summarized in Table 5. We were only able to aggregate adjusted data for three studies reporting on long-term mortality [26, 28, 32]. In this pooled adjusted data (Appendix Fig. 8), frailty was associated with increased risk of long-term mortality with a hazard ratio of 1.75 (95% CI 1.36, 2.24; p < 0.0001; I 2 = 43%).
Publication bias
Publication bias was assessed visually using a funnel plot for hospital mortality; there was no significant evidence of publication bias (Appendix Fig. 9).
Discussion
Key findings
In this systematic review of 10 observational studies we found that frailty was common, occurring in approximately 30% of adult ICU admissions. We also found that frailty was associated with increased risk of hospital and long-term mortality and that frail patients were less likely to have home as a discharge destination. We found no significant difference among frail and non-frail patients in the receipt of mechanical ventilation, receipt of vasoactive therapy, or duration of ICU stay. Increasing severity of frailty was associated with worsened outcomes including hospital and long-term mortality and our findings were robust when we analyzed high quality studies, adjusted data, and in trial sequential analysis.
Context
Although frailty has been long recognized by geriatric medicine, it has only been recently identified as an important determinant of prognosis for critically ill patients and our systematic review supports this. Our findings are consistent with the observation that frail patients are at increased risk of poor outcomes in other settings and after healthcare interventions [41, 42]. Potential causes for poor outcomes experienced by critically ill frail patients include its underlying pathophysiology of neuromuscular weakness, sarcopenia, decreased oxygen utilization, inflammation, and immunosenescence [9, 18, 43] reflecting a wide range of age-related molecular and cellular deficits [44, 45]. These may increase susceptibility to inflammatory insults and nosocomial infections common in critical illness. Diminished reserve arising from the multisystem nature of frailty may increase adverse effects of critical illness treatments such as bed rest, sedation, polypharmacy, instrumentation, and MV. Additionally, the reduced resilience of frail patients and increased likelihood of comorbid conditions [46] may make their recovery more difficult [47] and prolonged with reduced probability of returning to baseline increasing the chances of institutionalization [5, 6, 18]. In our study, we found that frail ICU patients were at an increased risk of not being discharged home, although this was reported in only four studies.
We did not find significant differences in ICU LOS, although there was a non-statistically significant increase in hospital stay. The only study reporting duration of MV found no difference between frail and non-frail patients [28]. This is unexpected since there are many factors, including diminished resilience, that may increase recovery time in frail patients prolonging their ICU and hospital stays as compared to non-frail patients. For example, frail patients may be more difficult to wean from mechanical ventilation because of weakness, sarcopenia, and decreased oxygen uptake [9, 17, 18, 43]. Further, as a result of immunosenescence, frail patients may need more time to recover from infections including those nosocomially acquired [45]. Our results are not in keeping with data in surgical populations, which have demonstrated that frail patients have longer stays in hospital and recovery time [6]. Possible reasons for these results include incomplete reporting of data, impact of end-of-life care or limitations of care influenced by frailty status, and discharge practices. A further factor that may have influenced the LOS data and duration of organ support is survival bias. Frail patients may have died earlier than the non-frail and this may have been associated with reduced LOS, as well as the duration of organ support. Data which would have allowed examination of this, such as “days alive and free of organ support”, was rarely reported with only Bagshaw et al. finding that hospital LOS was prolonged in frail survivors. These data should be described in futures studies focused on frailty in ICU settings.
Implications for clinicians, policy, and research
An important aspect of this work is to determine if ICU processes of care can be modified to improve outcomes for those identified as frail. Examples of processes which may have differential impact in those who are frail include nutritional support, sedation practices, intensity of mobilization/rehabilitation etc. While research is being conducted on how to improve outcomes, ongoing awareness of frailty as a marker of risk is important and may lead to better advanced care planning. Implicit in this is the recognition that frailty is not only associated with the elderly but may even occur in younger ages [26, 32]. Moreover, frailty may provide a better method to evaluate the trajectory of chronic health and its determinants such as cognition, mobility, function, and social engagement leading to ICU admission. Currents methods such as co-morbidity indices and chronic health evaluations integrated into illness severity scores and mortality prediction models are likely insufficient given the incremental impact of frailty on outcomes after adjusting for illness. Our work supports the value for implementation of frailty screening at the time of ICU admission. Since all the scales used in the included studies correlated with worsened outcomes; after further validation, the CFS which is the most studied, least time intensive, and easy to apply would be the most promising candidate.
ICU researchers and clinicians, who routinely measure co-morbidities, may question why frailty should be additionally measured or measured instead. The value of frailty is that it is a reflection of overall function which is not the case for co-morbidity, although frailty and co-morbidities are inherently intertwined in relation to the degree of frailty [48]. Fried and colleagues attempted to “untangle” these constructs but there is considerable overlap which increases with age [11]. Work on defining health deficit accumulation through network modeling shows that what matters the most is the density of a deficits connections to other deficits which is not captured by simple counting of deficits [49–51]. As an individual ages and accumulates deficits, as would be the case in many older people who are critically ill, the more that frailty and co-morbidity are inextricably intertwined.
Limitations
Although the association between frailty and poor outcomes from critical illness is supported by its underlying pathophysiology, it should be emphasized that the studies in our review were observational, may have been prone to bias, and causation cannot be determined. Two key potential biases are selection and confirmation biases. None of the studies applied the gold standard for frailty determination which is a comprehensive geriatric assessment performed by a specialist in geriatric medicine [52]. All these studies identified patients after ICU admission and we have no data on frail and non-frail patients declined ICU admission. In addition, the perception and identification of frailty may have influenced care received and limitations of care. Similarly we are unable to ascertain the role of survival bias in our results. Furthermore, we were limited in our ability to pool adjusted data because of heterogeneity in its reporting. However, supporting the importance of frailty as a determinant of outcome was that high quality studies which controlled for age and other co-founders including illness severity found that frailty was independently associated with adverse outcomes. In addition, we found that frail and non-frail patients had similar rates of mechanical ventilation and use of vasopressors reducing the likelihood of care limitations. Moreover, in most of the studies there was a frailty dose response where increasing frailty correlated with increasingly worse outcomes.
An additional limitation is that the included studies used three different frailty measures: the CFS, FP, and FI. We included all of these studies since frailty measures generally correlate well with each other [13]. When we performed subgroup analysis the results remained similar across all measures of frailty. However, unanswered questions remain including which is the most appropriate measure in the ICU setting? Should there be an ICU-specific frailty measure? Does it matter which measure if they all show similar trends for outcome? If this is the case, the one that is least time consuming and most feasible may be a reasonable starting point. Limitations also include variable reporting of outcomes, data originating from different healthcare settings, and need to transform data for aggregation. Further, the late registry in PROSPERO, the addition of long-term mortality as an outcome, and lack of a published protocol with a statistical plan could all increase the risk of bias.
Conclusions
Clinically frail patients are at increased risk of adverse outcomes because of physiological vulnerability when stressors are experienced. In this study, we demonstrate significantly increased risk of mortality and adverse outcomes in critically ill frail patients. Routine assessment of frailty at ICU admission may provide clinicians prognostic information for survival and recovery for their frail ICU patients. Importantly, this may help patients and their families make informed decisions about goals-of-care when they are critically ill. Importantly, further research is required to determine if there are modifiable factors that can improve outcomes for critically ill frail patients.
References
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381:752–762
Mitnitski A, Rockwood K (2016) The rate of aging: the rate of deficit accumulation does not change over the adult life span. Biogerontology 17:199–204
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173:489–495
Hoover M, Rotermann M, Sanmartin C, Bernier J (2013) Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep 24:10–17
Joseph B, Pandit V, Zangbar B, Kulvatunyou N, Hashmi A, Green DJ, O’Keeffe T, Tang A, Vercruysse G, Fain MJ, Friese RS, Rhee P (2014) Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg 149:766–772
Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M (2009) Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 250:449–455
Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N (2016) Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 31:1–8
Breitling LP, Saum KU, Perna L, Schottker B, Holleczek B, Brenner H (2016) Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin Epigenet 8:21
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Freiheit EA, Hogan DB, Eliasziw M, Meekes MF, Ghali WA, Partlo LA, Maxwell CJ (2010) Development of a frailty index for patients with coronary artery disease. J Am Geriatr Soc 58:1526–1531
Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G (2004) Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 59:255–263
Rockwood K, Abeysundera MJ, Mitnitski A (2007) How should we grade frailty in nursing home patients? J Am Med Dir Assoc 8:595–603
de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW (2011) Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 10:104–114
Hogan DB, Maxwell CJ, Afilalo J, Arora RC, Bagshaw SM, Basran J, Bergman H, Bronskill SE, Carter CA, Dixon E, Hemmelgarn B, Madden K, Mitnitski A, Rolfson D, Stelfox HT, Tam-Tham H, Wunsch H (2017) A scoping review of frailty and acute care in middle-aged and older individuals with recommendations for future research. Can Geriatr J 20:22–37
Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M (2009) Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr 48:78–83
Kristjansson SR, Nesbakken A, Jordhøy MS, Skovlund E, Audisio RA, Johannessen HO, Bakka A, Wyller TB (2010) Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol 76:208–217
Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM (2010) Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 121:973–978
McDermid RC, Stelfox HT, Bagshaw SM (2011) Frailty in the critically ill: a novel concept. Crit Care 15:301
Nguyen YL, Angus DC, Boumendil A, Guidet B (2011) The challenge of admitting the very elderly to intensive care. Ann Intensive Care 1:29
Waters BVA, Bagshaw S, Boyd J, Maslove D, Sibley S, Rockwood E, Muscedere J (2017) The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med Exp, September abstract supplementary issue (in press)
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283:2008–2012
Waters B, Varambally A, Muscedere J (2017) The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016053910. Accessed 1 June 2017
Wells GABS, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohrica/programs/clinical_epidemiology/oxfordasp. Accessed 1 Jan 2017
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, Majumdar SR (2014) Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ 186:E95–102
Kizilarslanoglu MC, Civelek R, Kilic MK, Sumer F, Varan HD, Kara O, Arik G, Turkoglu M, Aygencel G, Ulger Z (2017) Is frailty a prognostic factor for critically ill elderly patients? Aging Clin Exp Res 29:247–255
Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, Mimoz O, Le Gac G, Somme D, Cattenoz C, Feuillet F, Malledant Y, Seguin P (2014) Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med 40:674–682
Mueller N, Murthy S, Tainter CR, Lee J, Riddell K, Fintelmann FJ, Grabitz SD, Timm FP, Levi B, Kurth T, Eikermann M (2016) Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? a prospective, observational cohort study. Ann Surg 264:1116–1124
Hope AAHS, Petti A, Hurtado-Sbordoni M, Gong MN (2015) Bedside frailty assessment and hospital outcomes in critically ill patients. J Am Geriatr Soc 63:S180
Fisher C, Karalapillai DK, Bailey M, Glassford NG, Bellomo R, Jones D (2015) Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care 43:361–368
Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, Thompson JL, Chandrasekhar R, Bernard GR, Dittus RS, Gill TM, Ely EW (2016) Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. doi:10.1164/rccm.201605-0939OC
Hope AA, Hsieh SJ, Petti A, Hurtado-Sbordoni M, Verghese J, Gong MN (2017) Assessing the usefulness and validity of frailty markers in critically ill adults. Ann Am Thorac Soc 14:952–959. doi:10.1513/AnnalsATS.201607.538OC
Zeng A, Song X, Dong J, Mitnitski A, Liu J, Guo Z, Rockwood K (2015) Mortality in relation to frailty in patients admitted to a specialized geriatric intensive care unit. J Gerontol A Biol Sci Med Sci 70:1586–1594
Heyland DK, Garland A, Bagshaw SM, Cook D, Rockwood K, Stelfox HT, Dodek P, Fowler RA, Turgeon AF, Burns K, Muscedere J, Kutsogiannis J, Albert M, Mehta S, Jiang X, Day AG (2015) Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med 41:1911–1920
Heyland D, Cook D, Bagshaw SM, Garland A, Stelfox HT, Mehta S, Dodek P, Kutsogiannis J, Burns K, Muscedere J, Turgeon AF, Fowler R, Jiang X, Day AG, Canadian Critical Care Trials Group, Canadian Researchers at the End of Life Network (2015) The very elderly admitted to ICU: a quality finish? Crit Care Med 43:1352–1360
Hope AA, Hsieh SJ, Hurtado-Sbordoni M, Petti AM, Gong NM (2015) Frailty assessment and hospital outcomes in critically ill patients. Am J Respir Crit Care Med 191:A2285
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 1:323–336
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:146–156
Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, Ibrahim Q, Majumdar SR (2015) Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 43:973–982
Tu KY, Wu MK, Chen YW, Lin PY, Wang HY, Wu CK, Tseng PT (2016) Significantly higher peripheral insulin-like growth factor-1 levels in patients with major depressive disorder or bipolar disorder than in healthy controls: a meta-analysis and review under guideline of PRISMA. Medicine (Baltimore) 95:e2411
Muscedere J, Andrew MK, Bagshaw SM, Estabrooks C, Hogan D, Holroyd-Leduc J, Howlett S, Lahey W, Maxwell C, McNally M, Moorhouse P, Rockwood K, Rolfson D, Sinha S, Tholl B, Canadian Frailty Network (CFN) (2016) Screening for frailty in Canada’s health care system: a time for action. Can J Aging 35:281–297
Xue QL (2011) The frailty syndrome: definition and natural history. Clin Geriatr Med 27:1–15
Rockwood K, Mitnitski A, Howlett SE (2015) Frailty: scaling from cellular deficit accumulation? Interdiscip Topics Gerontol Geriatr 41:1–14
Vina J, Tarazona-Santabalbina FJ, Perez-Ros P, Martinez-Arnau FM, Borras C, Olaso-Gonzalez G, Salvador-Pascual A, Gomez-Cabrera MC (2016) Biology of frailty: modulation of ageing genes and its importance to prevent age-associated loss of function. Mol Aspects Med 50:88–108
Hope AA, Gong MN, Guerra C, Wunsch H (2015) Frailty before critical illness and mortality for elderly medicare beneficiaries. J Am Geriatr Soc 63:1121–1128
Rajabali N, Rolfson D, Bagshaw SM (2016) Assessment and utility of frailty measures in critical illness, cardiology, and cardiac surgery. Can j cardiol 32:1157–1165
Theou O, Rockwood MR, Mitnitski A, Rockwood K (2012) Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 55:e1–8
Mitnitski AB, Rutenberg AD, Farrell S, Rockwood K (2017) Aging, frailty and complex networks. Biogerontology. doi:10.1007/s10522-017-9684-x
Farrell SG, Mitnitski AB, Rockwood K, Rutenberg AD (2016) Network model of human aging: frailty limits and information measures. Phys Rev E 94:052409
Taneja S, Mitnitski AB, Rockwood K, Rutenberg AD (2016) Dynamical network model for age-related health deficits and mortality. Phys Rev E 93:022309
Turner G, Clegg A, British Geriatrics Society, Age UK, Royal College of General Practitioners (2014) Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 43:744–747
Acknowledgements
We would like to acknowledge and are grateful to Dr. Nathan Brummel and Dr. Aluko Hope for providing additional data from their studies. We would also like to acknowledge the Canadian Frailty Network for facilitating this work.
Author information
Authors and Affiliations
Contributions
JM conceived and supervised the study, extracted data, performed data analysis, and developed the manuscript. BW extracted data, performed data analysis, and developed the manuscript. AV extracted data, performed data analysis, and provided input on manuscript development. SMB, JGB, DM, SS, and KR provided input on data interpretation, helped write the manuscript, and provided critical input on its revisions.
Corresponding author
Ethics declarations
Conflicts of interest
Braden Waters, Aditya Varambally, Sean M Bagshaw, Stephanie Sibley, and David Maslove declare that no conflicts of interest exist. J. Gordon Boyd: Dr. Boyd receives a stipend from the Trillium Gift of Life Network to support his role as the Hospital Donation Physician. Kenneth Rockwood: President and Chief Scientific Officer of DGI Clinical, which has contracts with pharma on individualized outcome measurement. In July 2015 he gave a lecture at the Alzheimer Association International Conference in a symposium sponsored by Otsuka and Lundbeck. At that time he presented at an Advisory Board meeting for Nutricia. He plans to attend a 2017 advisory board meeting for Lundbeck. He is a member of the Research Executive Committee of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, with additional funding from the Alzheimer Society of Canada and several other charities, as well as from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Nova Scotia Health Research Foundation, the Capital Health Research Fund, and the Fountain Family Innovation Fund of the Nova Scotia Health Authority Foundation. John Muscedere. Dr. Muscedere is the Scientific Director for the Canadian Frailty Network which is funded by the government of Canada.
Additional information
Braden Waters is the co-lead author.
Take-home message: Frailty is an important baseline characteristic of patients who are critically ill. In this meta-analysis, we show that critically ill frail patients, compared to non-frail patients, are at increased risk of mortality, adverse outcomes, and are less likely to be discharged home.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Muscedere, J., Waters, B., Varambally, A. et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med 43, 1105–1122 (2017). https://doi.org/10.1007/s00134-017-4867-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4867-0