Abstract
Purpose
To evaluate the effect of aprotinin withdrawal in 2008 on patient outcomes, to assess the likely risks and benefits of its re-introduction, and to consider the relevance of existing evidence from clinical trials to ‘real-world’ practice.
Methods
We performed a nested case–control study of two cohorts undergoing adult cardiac surgery in a single tertiary centre. The first group underwent surgery between 1 January 2005 and 30 July 2007 (n = 3,578), prior to aprotinin withdrawal; the second group underwent surgery between 1 January 2009 and 31 December 2010 (n = 3,030), after aprotinin withdrawal. Propensity matching was used to select patients matched for 24 covariates in both groups (n = 3,508). We also estimated the effect of aprotinin withdrawal on a subgroup of high-risk patients (n = 1,002). Results were expressed as adjusted odds ratios (OR) and 95 % confidence intervals (CI) for categorical data and hazard ratios (HR) for time-to-event data.
Results
In propensity-matched cohorts, the withdrawal of aprotinin from clinical use was associated with more bleeding, higher rates of emergency re-sternotomy, OR 2.10 (1.04–4.25), and acute kidney injury (AKI), OR 1.86 (1.53–2.25). In high-risk patients, the increases in bleeding and AKI following aprotinin withdrawal were of a greater magnitude. Aprotinin withdrawal was also associated with a significant increase in 30-day mortality, HR 2.51 (1.00–6.29), in the high-risk group. The results were not altered by sensitivity analyses that adjusted for potential selection bias, time series bias and unmeasured confounders.
Conclusions
Aprotinin withdrawal was associated with increased complication rates and patient deaths following cardiac surgery. These real-world findings are at odds with those of randomised trials and cohort studies that have considered the clinical role of aprotinin.
Similar content being viewed by others
Introduction
Coagulopathic haemorrhage and the subsequent requirement for large-volume blood transfusion are important risk factors for poor clinical outcomes in cardiac surgery [1–3]. This remains a significant problem; 25 % of all UK cardiac surgery patients audited over a 3-month period in 2010 received four or more red blood cell (RBC) units or non-RBC components for coagulopathic bleeding [4]. This reflects the increasingly elderly patients and complex case mix referred for surgery, as well as the increased emphasis on early revascularisation of patients with unstable ischaemic heart disease who are often receiving multiple anti-thrombotic drugs [5]. Safe and effective blood management is therefore a key component of high-quality care, particularly in high-risk patients [6, 7]. Aprotinin, a serine protease inhibitor with broad anti-inflammatory and pro-haemostatic effects, was initially licensed for use in high-risk coronary artery bypass grafting, where it was used widely and had demonstrable efficacy in terms of reducing bleeding, transfusion and re-sternotomy rates [8]. However, between 2005 and 2008 several large observational studies raised safety concerns relating to the use of aprotinin, reporting associations between aprotinin use and acute kidney injury (AKI), myocardial infarction and stroke [9–11]. This was most evident when aprotinin was compared to the lysine derivatives tranexamic acid or epsilon-aminocaproic acid (EACA), which can also reduce perioperative haemorrhage in cardiac patients. Following the publication of the blood conservation using antifibrinolytics in a randomised trial (BART) [12] in 2008, which reported an apparent increase in mortality in the aprotinin group when compared to groups receiving tranexamic acid and EACA, the drug was withdrawn from the market and licensure was suspended. However, subsequent revisiting of the BART study data raised questions as to the validity of this safety signal [13], and following a comprehensive review it was re-licensed in Europe and Canada in 2010. The aims of the current study were to evaluate the effect of aprotinin withdrawal in 2008 on patient outcomes in a large tertiary cardiac unit, to assess the likely risks and benefits to patients from its re-introduction and to cast light on the relevance of the existing evidence from clinical trials to ‘real-world’ practice.
Methods
This retrospective observational case–control study was approved by the South West research ethics committee under reference 11/SW/0075. The requirement for written informed consent was waived. Our research objectives and methods were specified prior to execution. A more detailed description of our methodology is available in an eSupplement.
Study population and data sources
The Bristol Royal Infirmary (Bristol, UK) established a database of adult cardiac surgical patients in April 1996 [Patient Analysis and Tracking System (PATS), Dendrite Clinical Systems, London, UK] for which standardised perioperative and postoperative data are routinely and prospectively collected. All patients ≥16 years old in the PATS database whose data could be linked to the hospital’s biochemistry databases (for the determination of AKI) and the blood bank database (of blood products issued and used) between 1 January 2005 and 31 December 2010 were initially included (n = 8,795).
Institutional blood management
Aprotinin was administered according to individual physician preference, in many cases without published international guidelines [6]. Alternatively, tranexamic acid (Cyclokapron®, Pfizer, UK) was administered as a 2-g intravenous bolus, plus an additional 2-g dose administered to the pump prime for procedures utilising cardiopulmonary bypass. The use of intraoperative cell salvage was recorded in the PATS database only for those patients where salvaged blood was processed and autotransfused (Fresenius C.A.T.S.®, Terumo, Surrey, UK). Clinical concern about bleeding was managed by a Thromboelastogram (TEG®, Haemoscope Corporation, USA) guided algorithm, with administration of up to two cycles of four units of fresh frozen plasma (FFP) and one pooled unit of platelets, followed by four units of cryocoprecipitate and then recombinant activated factor VIIa (Novoseven®, Novo Nordisk Inc., NJ, USA).
Definitions of exposures of interest
We defined two cohorts for comparison based on the declining use of aprotinin at the hospital (eFigure 1): the first group was operated on between 1 January 2005 and 30 June 2007 (n = 3,578); the second group was operated on between 1 January 2009 and 31 December 2010 (n = 3,030). To determine the effect of withdrawing aprotinin upon high-risk patients, we also extracted a subgroup defined by their receipt of aprotinin in the first group (n = 756) and compared them with a propensity-matched subgroup who underwent surgery in the second group.
Definitions of outcomes of interest
Three co-primary outcomes were prespecified: (1) the transfusion of allogenic blood components (RBC, FFP, platelets and cryoprecipitate); (2) re-sternotomy due to bleeding and blood lost postoperatively on the intensive care unit (ITU); (3) measures of morbidity including low cardiac output, new onset AKI (defined by the creatinine criteria of the KDIGO classification), pulmonary complications and infective complications. We also examined all-cause mortality and the durations of ventilation, ITU stay and overall hospital stay.
Statistical analysis
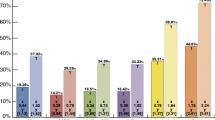
We selected 24 preoperative covariates (recorded on the y-axis of Fig. 1) for inclusion in a propensity model [14]. Propensity scores were then estimated for patients for whom all 24 preoperative characteristics were recorded via logistic regression using the methods of Rosenbaum and Rubin [15].
Our specification of a one-to-one ‘nearest neighbour’ propensity-matching algorithm with replacement permitted was chosen to minimise selection bias at the expense of variance. A total of 1,754 post-cessation patients were retrospectively matched to 1,754 pre-cessation counterparts. To maximise the number of unique patients in the pre-cessation arm of the high-risk subgroup analysis, 501 pre-cessation patients were prospectively matched to 501 post-cessation equivalents. After matching, t tests for the equality of covariate means in pre- and post-withdrawal groups were found to be non-significant (eTable 1), and standardised differences across all covariates (a metric of covariate balance defined by Rosenbaum and Rubin [16]) were found to be <5 % in the all-patient analysis and <10 % in the high-risk analysis (Fig. 1). We then proceeded to derive adjusted frequencies and odds ratios for our pre-specified outcomes. Length-of-stay and mortality outcomes were considered as time-to-event data. We employed Cox proportional hazards regression to compute hazard ratios across the entire duration of follow-up and also for epochs of follow-up time. Log-rank tests for differences between groups provided associated P values. To account for the correlation introduced by propensity matching, we present cluster confidence intervals for all results.
Given the high likelihood of outcomes changing over time independent of the effect of aprotinin withdrawal, we conducted a sensitivity analysis to investigate the potential bias introduced by the temporal definitions of pre- and post-withdrawal groups. The patient sample was serially decreased in size by excluding 3-month periods from the beginning and end of the time series (eFigure 1), and the outcomes re-quantified. We conducted further analyses to determine the sensitivity of our results to alternative propensity-matching strategies and to simulated unobserved confounders using the methods proposed by Ichino et al. [17]. Finally, we examined the interaction between aprotinin and alternate blood management strategies on outcomes using multivariate logistic regression. All analyses were performed in STATA 12 (Stata Corp., Texas, USA).
Results
Study population
Of the 8,795 cardiac surgical patients whose preoperative characteristics we present in Table 1 and eTable 2, transfusion data were available for 8,377. Blood loss on ITU was recorded for 7,218 subjects. Postoperative complications could be derived for all patients, with the exception of AKI for 8,500 patients. It was possible to calculate ventilator time, ITU stay and hospital stay for 8,178, 8,773 and 8,772 cases, respectively. All-cause mortality was recorded for all patients, 213 (2.4 %) of whom died in hospital. A total of 756 (8.6 %) patients were administered aprotinin. Comparisons of the pre- and post-cessation cohorts indicated that patients’ preoperative Euroscores, procedural complexity and CPB duration were greater in the latter cohort.
Transfusion requirements
Patients received more blood product transfusions after aprotinin had been withdrawn (Table 2). Adjusted odds ratios for the associations of aprotinin withdrawal with RBC, platelet, FFP and cryoprecipitate transfusion were 1.24 (CI 1.04–1.49), 2.16 (CI 1.69–2.76), 1.81 (CI 1.33–2.45) and 3.90 (CI 1.32–11.51), respectively. In the high-risk group, adjusted odds ratios for the associations of aprotinin withdrawal for RBC, platelet, FFP and cryoprecipitate transfusions were 1.22 (CI 0.89–1.66), 1.89 (CI 1.34–2.65), 1.63 (CI 1.11–2.39) and 2.64 (CI 0.81–8.65), respectively.
Blood loss and re-sternotomy
Table 2 demonstrates that the distribution of blood lost on ITU shifted from lower volumes (<1,000 ml) towards higher volumes (≥1,000 ml) after aprotinin had been withdrawn. This effect was more marked amongst high-risk patients where patients losing ≥1,000 ml of blood increased from 12.8 to 20.4 % [adjusted odds ratio 1.75 (CI 1.15–2.64)]. Overall, the adjusted rate of re-sternotomy due to bleeding increased from 1.8 % pre-cessation to 3.8 % post-cessation, with an associated odds ratio of 2.10 (CI 1.04–4.25). The cessation of use of aprotinin had a greater effect upon re-operation in the high-risk subgroup: re-sternotomy increased from 2.0 to 5.4 %, yielding an adjusted odds ratio of 2.80 (CI 1.25–6.25).
Postoperative morbidity
Postoperative cardiac, pulmonary and infectious morbidity decreased after aprotinin had been withdrawn. This effect was manifested both before and after propensity matching (eTable 3). Adjusted odds ratios for low cardiac output, pulmonary and infective complications were 0.62 (CI 0.52–0.74), 0.52 (CI 0.41–0.66) and 0.47 (CI 0.36–0.62), respectively. In contrast, aprotinin withdrawal conferred a significant increase in postoperative AKI from 23.4 to 36.2 % of all patients [adjusted odds ratio 1.86 (CI 1.53–2.25)]. High-risk patients were similarly affected by the cessation of use of aprotinin, with corresponding adjusted odds ratios of 0.58 (CI 0.42–0.79), 0.38 (CI 0.25–0.59) and 0.29 (CI 0.17–0.50) for low cardiac output, pulmonary and infective complications, respectively. Postoperative AKI increased from 32.5 to 41.3 % of high-risk subjects [adjusted odds ratio 1.46 (CI 1.06–2.01)].
Resource utilisation
Time-to-event analyses showed that aprotinin withdrawal was associated with shorter ITU [HR 0.87 (CI 0.80–0.95)] and hospital [HR 0.91 (CI 0.85–0.99)] stays (eTable 4). In high-risk patients, aprotinin withdrawal was not associated with significant reductions in resource use: ITU stay [HR 0.91 (CI 0.77–1.07)], hospital stay [HR 0.96 (CI 0.82–1.11)], and in fact ventilation time was increased in this group [HR 1.33 (CI 1.14–1.54)].
Mortality
We present Kaplan-Meier curves of cumulative deaths over the first postoperative year and adjusted hazard ratios for the effect of aprotinin withdrawal on mortality in Fig. 2, eFigure 2 and eTable 4. A trend toward increased all-cause mortality was evident in the first postoperative month following the withdrawal of the drug [HR 2.10 (CI 0.94–4.69)]. The relative increase in mortality was greater amongst high-risk patients [HR 2.51 (CI 1.00–6.29)]. Examination of the causes of in-hospital death amongst pre- versus post-aprotinin withdrawal did not demonstrate any clear change in the types of major morbidity preceding death, although this may reflect low statistical power for this analysis (eTable 5).
Kaplan-Meier failure functions showing the cumulative proportion of patients dying from any cause over the first postoperative year for patients pre-cessation (black) and post-cessation (red) in the high-risk subgroup. Dashed lines depict corresponding 95 % confidence intervals. Hazard ratios were calculated for epochs 0–30 days and 30 days to 1 year. See eFigure 2 for corresponding plots for the all-patient analysis
Sensitivity analyses
Sensitivity analyses performed on our binary outcomes demonstrated that both their magnitudes and directions of effect were highly robust to potentially unobserved confounders, alternate propensity-matching strategies and serially reducing durations of the pre- and post-withdrawal eras (eTables 6, 7 and eFigure 3). For example, our simulated confounders reduced the mean effect of aprotinin withdrawal on AKI in all patients by just 10–17 %. Re-calculating this outcome with alternate matching strategies conferred anything between an 11 % reduction and a 6 % increase. Alternative propensity-matching strategies including ‘greedy matching’ and stipulation of the ‘common support’ condition did not alter our results (eTables 8, 9). Serial restrictions in the duration of the pre- and post-withdrawal eras reduced the odds ratio for the effect of aprotinin withdrawal on AKI in all patients by no more than 21 % (eFigure 3).
Interaction among alternate blood management strategies, aprotinin and outcome
Associations between aprotinin and adverse outcomes may be confounded by alternate blood management strategies and by the administration of allogenic blood components. In this cohort, aprotinin withdrawal was associated with an increased use of tranexamic acid and recombinant factor VIIa (rFVIIa). In an exploratory analysis of the entire cohort, we estimated the associations among aprotinin, tranexamic acid or rFVIIa administration, increased bleeding and transfusion and death using multivariate logistic regression. This demonstrated significant associations between aprotinin, re-sternotomy for bleeding, the administration of allogenic RBC and non-RBC blood components, and 30-day mortality (Table 3). Examination of the contribution of each of these variables to the variance of the outcome measure indicated that RBC transfusion explained 13 % of the variance, whereas aprotinin explained 2 % and the administration of tranexamic acid 0.4 %.
Discussion
The main finding of this observational nested case-control study is that after adjustment for differences in baseline risk factors, the withdrawal of aprotinin from clinical use was associated with more bleeding and transfusions, higher rates of emergency re-sternotomy and AKI, and significant reductions in pulmonary, infectious and cardiac morbidity. In high-risk patients, the increases in bleeding, transfusion and AKI were of a greater magnitude. Aprotinin withdrawal was also associated with a significant increase in 30-day mortality in the high-risk group.
These results are at odds with the findings of systematic reviews of both randomised trials and observational cohort studies that have attempted to define the indications for aprotinin in cardiac surgery [7, 18–22]. Early randomised controlled trials (RCT) demonstrated clear clinical benefits attributable to aprotinin when compared to placebo [7]. However, the very high transfusion rates in the placebo arm for what were low-risk procedures by modern standards and the introduction of cheap alternative anti-fibrinolytics, such as the lysine analogues tranexamic acid and EACA, which themselves have proven efficacy, now mean that these findings have limited relevance to contemporary practice. When aprotinin was compared to these lysine analogues in a mixed risk contemporary cardiac surgery population in the BART study [12], no clinically important benefit for aprotinin relative to the other treatments beyond reduced blood loss was identified. More importantly, BART raised the possibility of increased mortality attributable to aprotinin use. This finding has been supported by a subsequent systematic review conducted by several of the BART investigators [18]. However, reviews of similar data by other groups have not supported these findings [18, 19]. Importantly, no contemporary review of the evidence from RCTs has shown a survival benefit from aprotinin, as suggested in our own study. Observational cohort studies have also failed to demonstrate clinically important benefits attributable to aprotinin use, although these types of studies are limited by systematic bias, unmeasured confounders, and a lack of standardisation of analyses and reporting between studies. For example, cohort studies that use propensity matching and/or logistic regression modelling to account for differences in the types of patients that do or do not receive aprotinin often demonstrate strong associations between aprotinin and adverse outcomes in mixed populations where aprotinin is used in selected high-risk patients [7–11]. Conversely, when this bias is reduced in cohort studies where aprotinin use is non-selective, or where high bleeding risk cohorts are considered in isolation, the safety signal attributable to aprotinin is absent [23–26].
The inclusion or omission of important confounders such as, for example, alternative haemostatic agents or other anti-fibrinolytics also produces conflicting results in observational cohort studies [27, 28]. We identified this as a potential source of confounding in the current study: alternative antifibrinolytics, rFVII, and the administration of blood components changed significantly following aprotinin withdrawal and may affect outcomes. In an exploratory analysis we established that the independent association between aprotinin and mortality was diminished following adjustment for volume of RBC transfusion, non-RBC blood components and alternative haemostatic strategies. This may be interpreted as showing that the adverse effects associated with aprotinin withdrawal are due to large-volume blood transfusions. However, this was not the primary aim of this study and we would caution against over-interpretation of this result.
Importantly, and unlike the results of RCTs and cohort studies, our real-world study suggested that the withdrawal of aprotinin was associated with increased complication rates and patient deaths following cardiac surgery. This apparent contradiction mandates a careful examination of the limitations of the current study. We attempted to minimise the bias attributable to the selected use of aprotinin by adopting a ‘before and after’ nested case-control design with propensity matching to select cohorts matched for key covariates and by performing two analyses; one that compared the entire cohort before and after aprotinin withdrawal, as well as a second comparing the high-risk cohort who received aprotinin before 2008 versus a matched cohort who we estimated would have received aprotinin after 2008. Bias will have been reduced by the fact that aprotinin was no longer available after 2008, by the use of a highly restrictive matching strategy that resulted in very similar patient groups in the before and after cohorts, and by the consistency of our observations in both mixed and high-risk populations. The chief limitation of this approach, as with all ‘before and after’ studies, is the high likelihood of time series bias. Mortality following elective coronary artery bypass grafting (CABG) in the UK has fallen from 2.2 to 0.8 % between 2005 and 2012 despite an increasingly high-risk case mix [6] and we have also reported improvements in clinical outcomes in our own unit over this period [29]. Time series bias in this case may have been expected to result in improvements in outcome in the later cohort, and indeed we did observe improvements in pulmonary and cardiac morbidity and resource use in this group consistent with historical trends. Importantly, however, the effect on bleeding and renal morbidity was in the opposite direction, and this result was not altered by our sensitivity analyses that utilised different historical cohorts, adjusted for simulated unmeasured confounders and used alternate matching strategies. The mixed positive and negative effects of aprotinin withdrawal argue against our results simply reflecting the increasingly high-risk and complex case mix in the latter cohort. Our findings mirror those of other ‘before and after’ studies from Europe [30], North America [31] and Asia [32, 33], where aprotinin withdrawal has increased bleeding complications, blood component exposure and perioperative morbidity. Importantly, our results also show an increase in mortality attributable to aprotinin withdrawal, an effect that was greater in the high-risk group. The possibility of time series bias notwithstanding, the mixed positive and negative effects of aprotinin withdrawal merit comment. Other ‘before and after’ studies have reported mixed changes in the frequencies of adverse events after aprotinin withdrawal; Von Heymann and colleagues documented reductions in stroke [30], whereas Martin et al. [34] reported lower rates of myocardial infarction in CABG patients, despite increased bleeding and transfusion post-aprotinin withdrawal. The trend towards increased mortality attributable to aprotinin in the BART trial has not been adequately explained and one possibility is that serine protease inhibition by aprotinin may have mixed adverse and beneficial clinical effects that are as yet poorly understood.
In conclusion, our study highlights the clinical importance of coagulopathic haemorrhage, and the need for improved, evidence-based blood management in cardiac surgery. Our study does not demonstrate a causal relationship between aprotinin withdrawal and adverse outcome. However, it demonstrates the disparity between frequently cited systematic reviews of randomised trials, the results of observational cohort studies that have considered the therapeutic role of aprotinin and our real-world experience of aprotinin withdrawal on patient outcomes. We suggest that this represents sufficient evidence of clinical equipoise to justify further evaluation of aprotinin in appropriately designed and powered multicentre RCTs.
References
Karkouti K, Wijeysundera DN, Yau TM, Beattie WS, Abdelnaem E, McCluskey SA, Ghannam M, Yeo E, Djaiani G, Karski J (2004) The independent association of massive blood loss with mortality in cardiac surgery. Transfusion 44:1453–1462
Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH (2006) Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med 34:1608–1616
Unsworth-White MJ, Herriot A, Valencia O, Poloniecki J, Smith EE, Murday AJ, Parker DJ, Treasure T (1995) Resternotomy for bleeding after cardiac operation: a marker for increased morbidity and mortality. Ann Thorac Surg 59:664–667
UK Transfusion audit. Bridgewater B, Keogh B Demonstrating Quality: The Society of Cardiothoracic Surgeons of Great Britain and Ireland National Sixth Adult Cardiac Surgical Database Report 2008. Dendrite Clinical Systems, Oxfordshire
Bridgewater B, Keogh B Demonstrating Quality: The Society of Cardiothoracic Surgeons of Great Britain and Ireland National Sixth Adult Cardiac Surgical Database Report 2008. Dendrite Clinical Systems, Oxforshire
Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, Baker RA, Dickinson TA, Fitzgerald DJ, Likosky DS, Shann KG (2011) 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 91:944–982
Ranucci M, Aronson S, Dietrich W, Dyke CM, Hofmann A, Karkouti K, Levi M, Murphy GJ, Sellke F, Shore-Lesserson L, von Heymann C (2011) Patient blood management during cardiac surgery: do we have enough evidence for clinical practice? J Thorac Cardiovasc Surg 142:249.e1–249.e32
Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Ker K (2011) Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev 16:CD001886
Mangano DT, Tudor IC, Dietzel C, Multicenter Study of Perioperative Ischemia Research Group, Ischemia Research and Education Foundation (2006) The risk associated with aprotinin in cardiac surgery. N Engl J Med 354:353–365
Shaw AD, Stafford-Smith M, White WD, Phillips-Bute B, Swaminathan M, Milano C, Welsby IJ, Aronson S, Mathew JP, Peterson ED, Newman MF (2008) The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med 358:784–793
Schneeweiss S, Seeger JD, Landon J, Walker AM (2008) Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med 358:771–783
Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM et al (2008) A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med 358:2319–2331
Beattie WS, Karkouti K (2011) The post-BART anti-fibrinolytic dilemma. J Cardiothorac Vasc Anesth 25:3–5
Gayat E, Pirracchio R, Resche-Rigon M, Mebazaa A, Mary JY, Porcher R (2010) Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. doi:10.1007/s00134-010-1991-5
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55
Rosenbaum PR, Rubin DB (1985) Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 39:33–38
Ichino A, Mealli F, Mannicini T (2008) From temporary help jobs to permanent employment: what can we learn from matching estimators and their sensitivity? J Appl Econom 23:203–227
Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A (2012) Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ 345:e5798
Howell N, Senanayake E, Freemantle N, Pagano D (1013) Putting the record straight on aprotinin as safe and effective: results from a mixed treatment meta-analysis of trials of aprotinin. J Thorac Cardiovasc Surg 145:234–240
Brown JR, Birkmeyer NJ, O’Connor GT (2007) Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation 115:2801–2813
Gagne JJ, Griesdale DE, Schneeweiss S (2009) Aprotinin and the risk of death and renal dysfunction in patients undergoing cardiac surgery: a meta-analysis of epidemiologic studies. Pharmacoepidemiol Drug Saf 18:259–268
Meybohm P, Herrmann E, Nierhoff J, Zacharowski K (2013) Aprotinin may increase mortality in low and intermediate risk but not in high risk cardiac surgical patients compared to tranexamic acid and epsilon-aminocaproic acid—a meta-analysis of randomised and observational trials over 30,000 patients. PLoS ONE. doi:10.1371/journal.pone.0058009
Pagano D, Howell NJ, Freemantle N, Cunningham D, Bonser RS, Graham TR, Mascaro J, Rooney SJ, Wilson IC, Cramb R, Keogh BE (2008) Bleeding in cardiac surgery: the use of aprotinin does not affect survival. J Thorac Cardiovasc Surg 135:495–502
Wang X, Zheng Z, Ao H, Zhang S, Wang Y, Zhang H, Hu S (2010) Effects of aprotinin on short-term and long-term outcomes after coronary artery bypass grafting surgery. Ann Thorac Surg 89:1489–1495
Dietrich W, Busley R, Boulesteix AL (2008) Effects of aprotinin dosage on renal function: an analysis of 8,548 cardiac surgical patients treated with different dosages of aprotinin. Anesthesiology 108:189–198
Karkouti K, Wijeysundera DN, Yau TM, McCluskey SA, Tait G, Beattie WS (2010) The risk-benefit profile of aprotinin versus tranexamic acid in cardiac surgery. Anesth Analg 110:21–29
Ngaage DL, Cale AR, Cowen ME, Griffin S, Guvendik L (2008) Aprotinin in primary cardiac surgery: operative outcome of propensity score-matched study. Ann Thorac Surg 86:1195–1202
Furnary AP, Wu Y, Hiratzka LF, Grunkemeier GL, Page US 3rd (2007) Aprotinin does not increase the risk of renal failure in cardiac surgery patients. Circulation 116:127–133
Bryan AJ, Cohen AM, Davies A. Bristol Heart Institute Cardiac Services Adult Cardiac Surgery Activity Report 2009-10. Available via http://www.uhbristol.nhs.uk/media/1467094/acsar09-10_ad_2_feb_2012.pdf. Accessed 1st Jul 2013
Sander M, Spies CD, Martiny V, Rosenthal C, Wernecke KD, von Heymann C (2010) Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: a retrospective analysis. Crit Care 14:R148
Strouch ZY, Drum ML, Chaney MA (2009) Aprotinin use during cardiac surgery: recent alterations and effects on blood product utilization. J Clin Anesth 21:502–507
Wang X, Zheng Z, Ao H, Zhang S, Wang Y, Zhang H, Li L, Hu S (2009) A comparison before and after aprotinin was suspended in cardiac surgery: different results in the real world from a single cardiac center in China. J Thorac Cardiovasc Surg 138:897–903
Sniecinski RM, Chen EP, Makadia SS, Kikura M, Bolliger D, Tanaka KA (2010) Changing from aprotinin to tranexamic acid results in increased use of blood products and recombinant factor VIIa for aortic surgery requiring hypothermic arrest. J Cardiothorac Vasc Anesth 24:959–963
Martin K, Wiesner G, Breuer T, Lange R, Tassani P (2008) The risks of aprotinin and tranexamic acid in cardiac surgery: a one-year follow-up of 1188 consecutive patients. Anesth Analg 107:1783–1790
Acknowledgments
This article presents independent research supported by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference no. RP-PG-0407-10384). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. We thank Dr Neil Davies for his assistance with the statistical analyses.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This retrospective observational case-control study was approved by the South West (UK) research ethics committee under reference 11/SW/0075.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Walkden, G.J., Verheyden, V., Goudie, R. et al. Increased perioperative mortality following aprotinin withdrawal: a real-world analysis of blood management strategies in adult cardiac surgery. Intensive Care Med 39, 1808–1817 (2013). https://doi.org/10.1007/s00134-013-3020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-3020-y