Abstract
Purpose
Carbapenem-resistant (CR) Gram-negative pathogens have increased substantially. This study was performed to identify the risk factors for development of CR Gram-negative bacteremia (GNB) in intensive care unit (ICU) patients.
Methods
Prospective study; risk factors for development of CR-GNB were investigated using two groups of case patients: the first group consisted of patients who acquired carbapenem susceptible (CS) GNB and the second group included patients with CR-GNB. Both case groups were compared to a shared control group defined as patients without bacteremia, hospitalized in the ICU during the same period.
Results
Eighty-five patients with CR- and 84 patients with CS-GNB were compared to 630 control patients, without bacteremia. Presence of VAP (OR 7.59, 95 % CI 4.54–12.69, p < 0.001) and additional intravascular devices (OR 3.69, 95 % CI 2.20–6.20, p < 0.001) were independently associated with CR-GNB. Presence of VAP (OR 2.93, 95 % CI 1.74–4.93, p < 0.001), presence of additional intravascular devices (OR 2.10, 95 % CI 1.23–3.60, p = 0.007) and SOFA score on ICU admission (OR 1.11, 95 % CI 1.03–1.20, p = 0.006) were independently associated with CS-GNB. The duration of exposure to carbapenems (OR 1.079, 95 % CI 1.022–1.139, p = 0.006) and colistin (OR 1.113, 95 % CI 1.046–1.184, p = 0.001) were independent risk factors for acquisition of CR-GNB. When the source of bacteremia was other than VAP, previous administration of carbapenems was the only factor related with the development of CR-GNB (OR 1.086, 95 % CI 1.003–1.177, p = 0.042).
Conclusions
Among ICU patients, VAP development and the presence of additional intravascular devices were the major risk factors for CR-GNB. In the absence of VAP, prior use of carbapenems was the only factor independently related to carbapenem resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of Gram-negative bacterial pathogens resistant to multiple antimicrobial agents is increasing in hospitals, and particularly in intensive care unit (ICU) settings [1–7]. Carbapenems, such as meropenem and imipenem, are currently considered to be the preferred agents for the treatment of serious bacterial infections caused by multidrug-resistant Gram-negative pathogens, mainly Enterobacteriaceae, Pseudomonas aeruginosa, and nonfermenters, i.e., Acinetobacter baumannii [2, 3]. However, the emergence of carbapenem resistance among Gram-negative pathogens has been increasingly reported worldwide and is a matter of great concern, since it complicates both empirical and guided treatment. Moreover, carbapenem resistance is also associated with additional mechanisms of resistance to other antibiotic classes [8]. Local knowledge of the microbial etiologies and susceptibility patterns of isolates appears to be important due to geographical variation of occurrence of bacterial pathogens and antimicrobial drug resistance.
A predominance of multidrug-resistant Gram-negative pathogens has previously been observed in our ICU [9]. Subsequently, an increased rate of carbapenem resistant (CR) A. baumannii isolates was observed [10]. We conducted the present study to identify risk factors for acquisition of CR Gram-negative bacteremia (GNB) in the ICU. Such knowledge should be useful to identify patients at risk, so that they receive in time a targeted antimicrobial therapy. Unlike previous studies that have focused on risk factors for antibiotic-resistant organisms comparing case patients with resistant pathogens only to those with susceptible, as controls, we used two groups of case patients as proposed by Kaye et al. [11] with two separate case–control analyses to overcome limitations of the usual case–control studies.
Patients and methods
Setting
This prospective, observational study was conducted from January 2006 through August 2007 in the ICU of Evangelismos Hospital in Athens, Greece. This is a 25-bed university ICU in a 1,000-bed tertiary-care hospital for adults. It admits critically ill medical, surgical and trauma patients. Patients with acute coronary syndromes, cardiac surgery and transplantation are managed in special units and are admitted to the general ICU if they have a complicated course and multiple organ failure.
Study design and data collection
All patients consecutively admitted to the ICU for more than 48 h during the study period, were eligible for inclusion in the study. The data were prospectively collected and included demographics, diagnostic category, comorbidities, illness severity, use of mechanical ventilation, development of acute lung injury (ALI), acute respiratory distress syndrome (ARDS), length of ICU stay, laboratory examinations and antibiotic therapy regimen. The illness severity was evaluated by APACHE II and SOFA scoring systems [12, 13], calculated during the first 24 h of ICU admission. All patients had at least one central venous catheter, a peripheral arterial catheter, and a urinary catheter. Additional exposure to intravascular devices (i.e., pulmonary artery catheter, continuous veno-venous hemofiltration catheter, intraaortic balloon catheter or temporary pacemakers), was defined as the “presence of additional intravascular devices”. For patients who had more than one episode of GNB, only the first episode was considered. Twelve patients included in this study have also been included in a previous study comparing characteristics and outcome between patients with CR and CS A. baumannii bacteremia [10].
Selection of case and control patients
A nested case–case–control design was followed according to that previously proposed [11, 14–16] as an effective method for identifying risk factors for antimicrobial resistant pathogens: the first group consisted of patients who acquired carbapenem-susceptible (CS) GNB and the second group included patients who acquired CR-GNB. Both case groups were compared to a shared control group defined as patients without bacteremia, hospitalized in the ICU during the same period as the case patients.
Definitions
ICU-acquired GNB was defined as the isolation of Gram-negative bacilli in a blood culture specimen obtained more than 48 h after admission to the ICU. The onset of bacteremia was defined as the date of the blood sampling. Blood culture specimens were ordered by the attending physicians in the presence of clinical features compatible with systemic inflammatory response syndrome (SIRS) [17] or when infection was suspected. Blood cultures were obtained via peripheral venous puncture using a standard sterile technique or from a new central venous catheter immediately after placement and prior to breaking the sterile field that was used for the catheterization. Sources of bacteremia were defined according to the criteria proposed by the Centers for Disease Control and Prevention [18]. Documentation of more than one source was defined as multiple-source bacteremia.
For the risk factor analysis, antibiotic exposure was analyzed by classes of antibiotics given prior to bacteremia development in patients with GNB, and for the whole length of ICU stay in patients without bacteremia.
Microbiological methods
Blood cultures were performed using the BACTEC 9240 system (Becton–Dickinson Sparks, MD, USA). Identification and susceptibility of the blood isolates by determining the minimum inhibitory concentrations (MICs) to different antimicrobial agents was performed by the VITEK2 system (bioMERIEUX, Marcy l’Etoile, France). Carbapenem resistance was verified by determination of minimum inhibitory concentrations (MICs) using E-test (AB Biodisk, Solna, Sweden) strips. Interpretation breakpoints were used as follows: A. baumannii ≤ 4 mg/L as susceptible, ≥16 mg/L as resistant; P. aeruginosa ≤ 2 mg/L as susceptible, ≥8 mg/L as resistant; Enterobacteriaceae ≤ 1 mg/L as susceptible, ≥4 mg/L as resistant, according to the Clinical and Laboratory Standards Institute (CLSI) recommendations [19]. Intermediate susceptibility was considered as resistance.
Statistical analysis
Continuous variables were expressed as mean value ± standard deviation or as median and inter-quartile range when were not normally distributed. Groups’ comparisons were made by the analysis of variance (ANOVA) method followed by the Tykey-multiple comparisons test. For not-normally distributed data, the Kruskal–Wallis test was used and the Mann–Whitney test for two groups’ comparison. Associations between categorical variables were examined by the χ2 test or the Fisher exact test, when appropriate. In identifying the independent risk factors for development of carbapenem resistance, a (backward stepwise logistic regression) multiple analysis was performed to control for the effects of confounding factors. The variables initially entered into the analysis were those that were statistically significant in the univariate analysis. A p value of <0.05 was considered statistically significant. All independent variables in this study were tested for multicollinearity. The interaction between risk factor variables was investigated. All statistical analyses were performed using the SPSS version 11.5 for Windows (SPSS Inc. Chicago, IL, USA).
Results
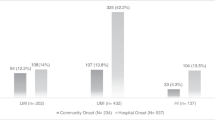
During the study period, 1,096 patients were admitted to the ICU. Of these patients, 241 stayed for <48 h and 13 patients were admitted with bacteremia, so they were excluded from further analysis. Of the remaining 842, 43 patients developed only Gram-positive bacteremia and/or candidemia, so they were also excluded. Finally, among the remaining 799 patients, 169 developed GNB giving an incidence of 16.3 per 1,000 patient-ICU days; 84 patients had bacteremia due to CS and 85 patients had bacteremia due to CR isolates (Fig. 1).
CS-GNBs were most common due to A. baumannii (48 patients, 57.2 %) and Klebsiella pneumoniae (21 patients, 25 %). CR-GNBs were most common due to A. baumannii (32 patients, 37.6 %) and P. aeruginosa (31 patients, 36.5 %), (Fig. 2).
Demographic and clinical characteristics of all ICU patients included in the study and results of the univariate analysis are shown in Table 1. Exposure to antimicrobial agents is shown in Table 2 and Fig. 3. Results of the multiple analysis are shown in Table 3.
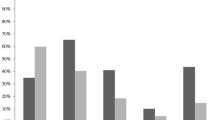
Box plots for the duration (days) of antimicrobial agents administered to patients with carbapenem-resistant (n = 85) and to patients with carbapenem-susceptible (n = 84) Gram-negative bacteremia: horizontal bars represent median values, boxes represent interquartile ranges, whiskers show ranges, circles outliers, and asterisks extreme values. The differences were statistically significant for carbapenems (p < 0.001), colistin (p < 0.001), glycopeptides (p < 0.05), and antifungals (p < 0.05), Kruskal–Wallis test
Respiratory tract infection was the most common source in both CS- and CR- GNB, observed in 38 (45 %) and in 40 (47 %) patients respectively, followed by multiple sources in 16 (19 %) and in 11 (13 %) patients respectively. A focus of infection was not identified in 22 (26 %) patients with CS- GNB and in 24 (28 %) patients with CR- GNB, Table 1.
Patients with CS-GNB versus control patients without bacteremia
According to univariate analysis (Table 1), patients with CS-GNB had a longer length of ICU stay and a higher severity of illness on admission than patients without bacteremia. They presented more often history of diabetes mellitus and renal failure. Also, compared to control patients, they were more likely to have additional intravascular devices, ARDS and VAP development. As shown in Table 2, aminoglycosides, carbapenems, quinolones, monobactams, metronidazole, oxazolidinones and antifungals were administrated for significantly longer period in patients with CS-GNB than in patients without bacteremia. By multiple analysis, independent risk factors for CS-GNB acquisition were the presence of VAP (OR 2.93, 95 % CI 1.74–4.93, p < 0.001), the presence of additional intravascular devices (OR 2.10, 95 % CI 1.23–3.60, p = 0.007) and the SOFA score on ICU admission (OR 1.11, 95 % CI 1.03–1.20, p = 0.006), Table 3.
Patients with CR-GNB versus control patients without bacteremia
According to univariate analysis, (Table 1), patients with CR-GNB had a longer length of ICU stay and a higher severity of illness on admission than those without bacteremia. Also, they were more likely to have additional intravascular devices, to developed VAP and ARDS, and they presented more often history of diabetes mellitus and renal failure. As shown on Table 2, compared to control patients, patients with CR-GNB had exposure for longer period to almost all classes of antimicrobial agents, until the first episode of bacteremia. Multiple analysis revealed that independent risk factors for CR-GNB acquisition were the development of VAP (OR 7.59, 95 % CI 4.54–12.69, p < 0.001) and the presence of additional intravascular devices (OR 3.69, 95 % CI 2.20–6.20, p < 0.001), Table 3.
Patients with CS- versus those with CR-GNB
As shown on Table 1, patients with CR-, as compared to those with CS-GNB, had longer hospitalization and longer length of ICU stay prior to bacteremia a significantly longer duration of mechanical ventilation and a longer total length of ICU stay. In addition, patients with CR-GNB were more likely to have acute lung injury and VAP, than were patients with CS-GNB. Finally, patients with CR-GNB had a significantly longer prior exposure to carbapenems than did patients with CS-GNB, to colistin, to glycopeptides, and to antifungals (Table 2; Fig. 3).
Since the length of ICU stay influences the antibiotic regimen administration, the influence of antibiotics was tested after correction for the exposure time. A significant relation was found between duration of exposure to carbapenems (OR 1.079, 95 % CI 1.022–1.139, p = 0.006) and colistin (OR 1.113, 95 % CI 1.046–1.184, p = 0.001), with the acquisition of CR Gram-negative isolates, whereas the length of ICU stay before the bacteremia, was not found significant.
During the process of analysis, a statistically significant interaction was noted between carbapenem and colistin administration, and VAP: by applying multiple logistic regression analysis including VAP, carbapenems, colistin administration, and their interaction on CR-GNB development, a statistically significant interaction was detected between carbapenems and VAP: (OR 0.904, 95 % CI 0.82–0.99, p = 0.048), Table 4. To further examine the above interaction, an additional analysis was undertaken within patients with GNB by using two statistical models of multiple analysis. The first model included the patients with both VAP and GNB (n = 82), and the second, the patients with GNB in the absence of VAP as a source (n = 87). In the first subgroup, none of the variables was significantly associated with the acquisition of CR-GNB whereas within the second subgroup previous treatment with carbapenems was the only independent risk factor for the development of CR-GNB (OR 1.09, 95 % CI 1.003–1.177, p = 0.042).
Discussion
This study assessed the risk factors for CR-GNB among patients in a multidisciplinary ICU. The main findings are: (i) among ICU patients, the development of CR-GNB, was independently associated with the presence of VAP as a source of bacteremia and excess use of intravascular devices, whereas the development of CS-GNB was associated with the same risk factors plus the severity of organ failure on ICU admission; (ii) among patients with GNB, the presence of VAP and prior use of carbapenems and colistin were independently associated with carbapenem resistance. In the absence of VAP as a source of bacteremia, the only independent risk factor for CR isolate development was the prior carbapenem use.
In the past decade publications, risk factors specific methodological issues were raised. The importance of control group selection on the results of risk factor analysis for antibiotic-resistant isolates has been shown [11, 14–16]. Accordingly, in the present study two groups of case patients, those with CS- and those with CR-GNB, were compared with the same control group, i.e. ICU patients without bacteremia development, admitted to the ICU during the study period. This double case design was used to avoid overestimation of the risk factors found in studies comparing patients with resistant bacteria only to those with susceptible, as it has been pointed out. To our knowledge, only few studies have included double case patients in similar studies [20, 21].
There is little information about the direct effect of patient associated factors on resistance acquisition. In contrast to a traditional thinking, increased severity of illness may not necessarily be a predisposing factor of infection with antibiotic resistant organisms [14]. Indeed, the findings of the present study clearly demonstrate the absence of an independent relationship between illness severity at ICU admission and subsequent acquisition of CR-GNB, confirming that the resistance development does not obligatorily occur to the more severely ill patients. However, the severity of illness was independently associated with CS-GNB development.
Apart from the illness severity, antimicrobial resistance is thought to be more common in patients who have had a prolonged ICU stay, advanced age, prior therapy with antibiotics and therapy with invasive devices [22]. Indeed, in the present study, patients with CR- as compared to those with CS-GNB did have a longer length of hospital and ICU stay before the development of bacteremia in the univariate analysis. However, this variable did not remain significant in the multiple analysis. The incidence of documented catheter related bacteremia was low during the study period. However, the presence of additional intravascular devices was an independent risk factor for both CS- and CR-GNB development, probably reflecting the illness severity and the more often health care personnel contacts. Regarding the patients’ age, we did not find any relation between it and the acquisition of either CS- or CR-GNB.
The increase in resistance among Gram-negative bacteria is frequently related to the high selective pressure of antimicrobials commonly used in hospitalized patients [2, 23–26]. In accordance, in the present study, extensive use of carbapenems, colistin, glycopeptides and antifungals seemed related to the carbapenem resistance in the univariate analysis. However, after controlling for confounding factors and interaction, prior prolonged exposure to carbapenems was the only independent risk factor for ICU acquired CR-GNB. Carbapenems have been identified as a risk factor for CR Gram-negative isolates in previous studies [27–30]: carbapenem use was independently associated with imipenem-resistance [27]; imipenem exposure was the major risk factor for imipenem-resistant P. aeruginosa [21, 28], and also for CR K. pneumoniae isolation [29]. However, there are exceptions to these findings: fluoroquinolones and antipseudomonal penicillins (and not carbapenems) were independent risk factors for CR K. pneumoniae infection elsewhere [31]. Notably, none of these studies, except one [27], have focused on ICU patients and none of them on bacteremia exclusively; therefore, data on the risk factors for CR-GNB in the ICU are still limited.
Apart from antibiotic pressure, the acquisition of antibiotic-resistant bacteria within the ICU also represents the result of the horizontal transfer, usually via the hands of personnel and inanimate objects. Therefore, any antibiotic use may play a minor role in those patients who have acquired the organism by horizontal transfer [14]. This study, as other clinical studies [28, 31, 32], cannot separate these two modes of CR Gram-negative pathogens acquisition because clonality of strains was not evaluated. During two earlier epidemic outbreaks of multidrug resistant A. baumannii [33] and pandrug-resistant P. aeruginosa [34] in our unit, cross-transmission had been confirmed. However, regardless of any mode of transmission, this study clearly shows the impact of carbapenem exposure on carbapenem resistance acquisition in ICU and confirms the common knowledge in an unquestionable infection, such as bacteremia.
In the present study, among the entire number of ICU patients, the presence of VAP was found to be the most important risk factor for both CS- and CR-GNB development. Interestingly, among the patients with GNB, those with VAP had an increased likelihood of having a CR isolate (Table 1). This variation emphasize the importance of defining the control group before we proceed to interpretation of the results in risk factor analysis studies, according to the above mentioned epidemiological principles [11, 14–16]. In accordance, previous studies have shown the respiratory system to be the most frequent site of infections due to CR isolates: Raymond et al. [35] found that, the risk of CR bacteremia was about eight times higher for patients with recent prior VAP, as compared to other sites of infection. Similarly, in a previous study from our ICU, comparing CS versus CR A. baumannii bacteremia, the presence of VAP was the most important risk factor for CR A. baumannii acquisition [10].
There are certain limitations to our study. First, since the clonality of the isolates was not investigated, possible cross-transmission events could not been distinguished from within-host resistance development. Second, previous colonization that might have influenced the antibiotic prescription, was not recorded because active surveillance of patient’s floras was not performed in our unit as a routine. Finally, the single center design of the present study and the high proportion of multidrug resistant pathogens in our unit probably limit the generalizability of our findings.
In summary, our results suggest that VAP development and the presence of additional intravascular devices promote the acquisition of CR-GNB. When bacteremia occurred in the absence of VAP, prior use of carbapenems was the only factor independently related to carbapenem resistance, indicating the need for prudent and rational use of carbapenems, along with compliance with basic control measures for nosocomial infections.
References
Gaynes R, Edwards JR, The National Nosocomial Infections Surveillance System (2005) Overview of Nosocomial Infections caused by Gram-negative bacilli. Clin Infect Dis 41:848–854
Clark NM, Patterson J, Lynch JP (2003) Antimicrobial resistance among gram negative organisms in the intensive care unit. Curr Opin Crit Care 9:413–423
Paterson DL (2006) Resistance in gram-negative bacteria: enterobacteriaceae. Am J Infect Control 34:S20–S28
Flournoy DJ, Reinert RL, Bell-Dixon C et al (2000) Increasing antimicrobial resistance in Gram-negative bacilli isolated from patients in intensive care units. Am J Infect Control 28:244–250
Kollef MH, Fraser VJ (2001) Antibiotic resistance in the intensive care unit. Ann Intern Med 134:298–314
Jones RN, Phaller MA (1998) Bacterial resistance: a worldwide problem. Diagn Microbiol Infect Dis 31:379–388
Fridkin SK (2001) Increasing prevalence of antimicrobial resistance in intensive care units. Crit Care Med 29:N64–N68
Grundmann H, Livermore DM, Giske CG, Canton R, Rossolini GM, Campos G, Vatopoulos A, Gniadkowski M, Toth A, Pfeifer Y, Jarlier V, Carmeli Y; CNSE Working Group 2010) Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euroserveill 15(46). pii 19711
Pratikaki M, Platsouka E, Sotiropoulou C, Vassilakopoulos T, Paniara O, Roussos C, Routsi C (2009) Risk factors for and influence of bloodstream infections on mortality: a 1-year prospective study in a Greek intensive care unit. Epidemiol Infect 137:727–735
Routsi C, Pratikaki M, Platsouka E, Sotiropoulou C, Nanas S, Markaki V, Vrettou C, Paniara O, Giamarellou H, Roussos C (2010) Carbapenem-resistant versus carbapenem-susceptible Acinetobacter baumannii bacteremia in a Greek intensive care unit: risk factors, clinical features and outcomes. Infection 38:173–180
Kaye KS, Harris AD, Samore M, Carmeli Y (2005) The case–case–control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect Control Hosp Epidemiol 26:346–351
Knaus WA, Draper EA, Wagner DP, Zimmerman JA (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, De Mendoka A, Cantraine F et al (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units. Results of a multicenter, prospective study. Crit Care Med 26:1793–1800
Paterson DL (2002) Looking for risk factors for the acquisition of antibiotic resistance: a 21st-century approach. Clin Infect Dis 34:1564–1567
Harris AD, Samore M, Lipsitch Kaye KS, Perencevich E, Carmeli Y (2002) Control-group selection importance in studies with antimicrobial resistance: examples applied to Pseudomonas aeruginosa, enterococci, and Escherichia coli. Clin Infect Dis 34:1558–1563
Harris AD, Karchmer TB, Carmeli Y, Samore MH (2001) Methodological principles of case-control studies that analyzed risk factors for antibiotic resistance: a systematic review. Clin Infect Dis 32:1055–1061
Levy MM, Fink MP, Marshall JC et al (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538
Garner JS, Jarvis WR, Emori TG et al (1998) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
Clinical and Laboratory Standards Institute (2012) Performance standards for antimicrobial susceptibility testing. Twenty-second Informational Supplement M100—S22. 32(3)
Kritsotakis EI, Tsioutis C, Roumbelaki M, Christidou A, Gikas A (2011) Antibiotic use and the risk of carbapenem-resistant extended-spectrum-β lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case–control study. J Antimicrob Chemother 66:1383–1391
Harris AD, Smith D, Johnson JA, Bradham DD, Roghmann M-C (2002) Risk factors for imipenem-resistant Pseudomonas aeruginosa among hospitalized patients. Clin Infect Dis 34:340–345
Niedreman MS (2001) Impact of antibiotic resistance on clinical outcomes and the cost of care. Crit Care Med 29(Suppl):N114–N120
Gaynes R (1997) The impact of antimicrobial use on the emergence of antimicrobial-resistant bacteria in hospitals. Infect Dis Clin North Am 11:757–765
Loeffler JM, Garbino J, Lew D et al (2003) Antibiotic consumption, bacterial resistance and their correlations in a Swiss university hospital and its adult intensive care units. Scand J Infect Dis 35:843–850
Friedrich LV, White RL, Bosso JA (1999) Impact of use of multiple antimicrobials on changes in susceptibility of gram-negative aerobes. Clin Infect Dis 28:1017–1024
Hsueh PR, Chen WH, Luh KT (2005) Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int J Antimicrob Agents 26:463–472
Akinci E, Colpan A, Bodur H, Balaban N, Erbay A (2005) Risk factors for ICU-acquired imipenem-resistant Gram-negative bacterial infections. J Hosp Infect 59:317–323
Troillet N, Samore MH, Carmeli Y (1997) Imipenem-resistant Pseudomonas aeruginosa: risk factors and antibiotic susceptibility patterns. Clin Infect Dis 25:1094–1098
Kwak YG, Choi SH, Choo EJ, Chung JW, Jeong JW, Kim NJ, Woo JH, Ryu J, Kim YS (2005) Risk factors for the acquisition of carbapenem-resistant Klebsiella pneumoniae among hospitalized patients. Microb Drug Resist 11:165–169
Ong DSY, Jongerden IP, Buiting AG, Leverstein-van Hall MA, Speelberg B, Kesecioglu J, Bonten MJM (2011) Antibiotic exposure and resistance development in Pseudomonas aeruginosa and Enterobacter species in intensive care units. Crit Care Med 39:2458–2463
Falagas ME, Rafailidis PI, Kofteridis D, Virtzili S, Chelvatzoglou FC, Papaioannou V, Maraki S, Samonis G, Michalopoulos A (2007) Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case-control study. J Antimicrobial Chemother 60:1124–1130
Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y (2008) Predictors of carbapenem-resistant Klebsiella pneumonia acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 52:1028. doi:10.1128/AAC01020-07
Kraniotaki E, Manganelli R, Platsouka E, Grossato A, Paniara O, Palu G (2006) Molecular investigation of an outbreak of multidrug-resistant Acinetobacter baumannii, with characterization of class 1 integrons. Int J Antimicrob Agents 28:193–199
Mentzelopoulos S, Pratikaki M, Platsouka E, Kraniotaki H, Zervakis D et al (2007) Prolonged use of carbapenems and collistin predisposes to ventilator-associated pneumonia by pandrug-resistant Pseudomonas aeruginosa. Intensive Care Med 33:1524–1532
Raymond DP, Pelletier SJ, Crabtree TD, Evans HL, Pruett TL, Sawyer RG (2003) Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 31:1035–1104
Acknowledgments
We would like acknowledge the physicians and allied health staff at the ICU and the Department of Clinical Microbiology for their contributions.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Routsi, C., Pratikaki, M., Platsouka, E. et al. Risk factors for carbapenem-resistant Gram-negative bacteremia in intensive care unit patients. Intensive Care Med 39, 1253–1261 (2013). https://doi.org/10.1007/s00134-013-2914-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2914-z