Abstract
Purpose
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is used to treat severe forms of acute respiratory distress syndrome (ARDS). VV-ECMO management may be confusing due to the lack of information about the interplay between the determinant parameters and their impact on oxygenation. We found a relationship between arterial oxygen saturation (SaO2) and its relevant parameters. The aim of this study was to assess the validity of this model.
Methods
We report our experience in 17 patients under VV-ECMO for severe ARDS. We compared, at two different levels of pump flow, SaO2 and the oxygen saturation measured in the pulmonary artery (SpaO2) with the predicted saturation using the formula: SpaO2 = (EF/CO)SmO2 + (1 − EF/CO)SvO2 + 10−2PmO2, where PF is pump flow, R is recirculation, EF is effective flow [= (1 − R)PF], SmO2 is saturation of the oxygenator outgoing blood, CO is cardiac output, SvO2 is saturation of mixed venous blood, and PmO2 is oxygen partial pressure of the oxygenator outgoing blood.
Results
There was no significant difference between predicted and measured SpaO2: the mean predicted and measured SpaO2 values were 90.7 ± 2.8 % and 90.4 ± 2.7 % , respectively (p = 0.696, r = 0.966). Bland-Altman analysis showed good agreement between predicted and measured SpaO2. Predicted SpaO2 and SaO2 was well correlated (r = 0.80).
Conclusions
We have presented an explicit relationship between SaO2 and its direct determinants during VV-ECMO. Good agreement was found with the measured values of SaO2, but the model remains to be fully validated before its use in clinical practice.
Similar content being viewed by others
Introduction
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is used to treat the most severe forms of acute respiratory distress syndrome (ARDS) [1–5]. The objective of the VV-ECMO technique is to correct severely impaired gaseous exchange due to lung injury and to allow pulmonary recovery [6–8]. Normocarbia is easy to achieve but sufficient oxygenation is not always possible. The greater solubility of carbon dioxide (CO2), coupled with the fact that the CO2 dissociation curve is more linear and more steep than the oxygen (O2) dissociation curve, imply that CO2 clearance (and PaCO2) are largely determined by the rate of fresh gas flow. But blood oxygenation is controlled by blood flow (or pump flow, PF) which depends on the patient’s cardiac output (CO) and the recirculation rate (R). Whereas effective oxygenation requires high PF values from 50–60 to 80–100 mL/kg/min, effective CO2 clearance can be reached with values as small as 10–15 mL/kg/min [9, 10].
R is defined as the fraction of oxygenated blood that exits the infusion port and is immediately drained back into the drainage port, instead of being delivered to the patient’s circulation. Recirculation is a dynamic event and R is influenced by a variety of factors, such as cannula position, volume status, PF rate, and CO. In the absence of recirculation, PF only needs to be equal to CO to achieve complete oxygenation. R increases with PF and is a recognized limiting factor in VV-ECMO [11, 12]. Moreover, there are other possible causes of decreased blood oxygenation during ECMO: for example, variation in a patient’s CO, and an increase in a patient’s O2 consumption (e.g. sepsis, hyperthermia etc.).
This difficulty in achieving sufficient oxygenation explains: (1) saturation targets recommended by the VV-ECMO guidelines (for example, Extracorporeal Life Support Organization guidelines define adequate support as support allowing an arterial saturation greater than 80 % [13]), and (2) research to improve oxygenation by reducing recirculation (bicaval drainage [14] and the χ-configuration [15]).
The physiology of VV-ECMO is complex [9] and the absence of an explicit relationship between the determinants of oxygenation and the consequences of their interactions makes the management of oxygenation during VV-ECMO quite intricate [11, 16]. As in a similar mathematical description of the mixing of venous blood [17], we believe that mathematical modelling may be a valuable tool for exploring oxygenation during VV-ECMO, particularly the interplay between blood oxygenation and its delivery parameters. After validation, such a model could be useful in clinical practice: firstly, it could enable these parameters to be optimized and better adjusted when starting an ECMO procedure in order to achieve sufficient oxygenation, and secondly, these parameters could be corrected if hypoxaemia did indeed occur during VV-ECMO. From the physiology of transport and delivery of O2 to tissues, we established a relationship between arterial O2 saturation (SaO2) and its determinants. The aim of this study was to validate this formula.
Patients and methods
Patient characteristics
From August 2007 to January 2012, 17 patients with ARDS were treated with VV-ECMO. All patients failed to reach an acceptable blood oxygenation level with optimized ventilation settings (inspired O2 fraction >90 % and plateau airway pressure >30 cmH2O) combined with supportive treatment (inhaled NO, prone positioning, etc.) as appropriate for each patient. The protocol was approved by the central institutional review board. All patients or their relatives signed informed consent forms. We applied the Extracorporeal Life Support Organization indications and contraindications to VV-ECMO [13].
ECMO management and patient interventions
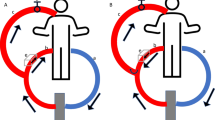
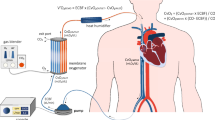
We set up a standard two-cannula femorojugular VV-ECMO circuit (Fig. 1). Deoxygenated blood was drained from the right atrium through a cannula inserted into the right femoral vein. The oxygenated blood was restored via a short cannula inserted into the right internal jugular vein reaching the distal part of the superior vena cava. The sizes of cannulas were in the range 21–28F depending on the patient’s anatomical features. The ECMO equipment used was a centrifugal pump (Rotaflow; Maquet, Hirrlingen, Germany) and a hollow fibre oxygenator (Quadrox-D; Maquet) connected with tubes treated with Bioline Coating (Maquet). The oxygenator’s FiO2 was set at 100 % and gas flow was set 1:1 with blood flow. Once optimal PF had been achieved (equal to 80 % of the patient’s CO), ventilation was gradually reduced for protective ventilation [7]. Protective ventilation [18] settings were: pressure control, inspiratory plateau pressure of less than 30 cm H2O, and positive end-expiratory pressure between 10 and 15 cm H2O, depending on the pressure/volume curves. Respiratory frequency was reduced to 4–10/min. FiO2 was reduced to 50 % or lower, whenever possible.
VV-ECMO configuration: PdO 2 partial pressure of O2 in the drainage cannula, SdO 2 O2 saturation in the drainage cannula, PF pump flow, PmO 2 partial pressure of O2 in the blood exiting the oxygenator (membrane), SmO 2 O2 saturation in the blood exiting the oxygenator (membrane), ScvO 2 central venous O2 saturation, CO cardiac output, SGF sweep gas flow, FmO 2 fractional inspired O2 sweep gas, PaO 2 partial pressure of O2 in the radial artery, SaO 2 O2 saturation in the radial artery, PpaO 2 partial pressure of O2 in the pulmonary artery, SpaO 2 O2 saturation in the pulmonary artery
Patient monitoring
A pulmonary artery catheter was inserted in order to measure CO and to take pulmonary blood samples for gas analysis. Central venous blood gas analysis was obtained through a central venous catheter. Circuit monitoring included pre-oxygenator and post-oxygenator blood gas analysis and pressure analysis.
Formula for SaO2 calculation
From physiology of transport and delivery of O2 to tissues, we established a relationship between SaO2 and its determinants:
where SpaO2 is the O2 saturation in a pulmonary artery (%), EF = (1 − R) PF; EF is the effective flow rate (L/min), PF is the pump flow (L/min), R is the recirculation rate (%), SmO2 is the O2 saturation of the blood leaving the oxygenator (mm Hg), CO is the cardiac output (L/min), SvO2 is the O2 saturation of mixed venous blood (%), and ΔSaO2 (≈10−2 PmO2; PmO2 is the partial O2 pressure of the blood leaving the oxygenator, mm Hg) is the increase in SaO2 due to dissolved O2 (%). Figure 1 shows the VV-ECMO configuration.
SvO2 measurement
SvO2 is the percentage of haemoglobin saturated with O2 in mixed venous blood. Mixed venous blood is the mixture of blood from all regions of the body in the right cavities, before being oxygenated in the lung capillaries. SvO2 can be obtained by taking a blood sample via a pulmonary artery catheter [19]. Under ECMO, the mixed venous blood is the last point in the circulation before oxygenation. Some of the mixed venous blood is withdrawn through the drainage cannula and passes through the oxygenator. The mixed venous blood that is not drained by the circuit flows through the pulmonary arteries without being oxygenated. Consequently, it is impossible to measure SvO2 under VV-ECMO by sampling blood from the pulmonary artery, because the O2 content of the blood entering the pulmonary circulation is a mixture of the deoxygenated venous return and the oxygenated blood from the circuit.
In all the calculations we replaced SvO2 with ScvO2 (superior vena cava O2 saturation) which has a similar value to SvO2, by sampling blood from a major vein that was not affected by the recirculation or by the blood resulting from the ECMO circuit. Central venous blood samples for gas analysis were obtained through a central venous catheter positioned in the distal portion of the superior vena cava [15, 19].
Recirculation calculation
For determination of R, we applied a simple formula, using the evaluation of blood O2 saturation obtained from different sites according to the formula [15]: R (%) = (SdO2 − SvO2)/(SmO2 − SvO2) × 100, where SdO2 is the O2 saturation in the drainage cannula, SmO2 is the O2 saturation level of the blood exiting the oxygenator (membrane), and SvO2 is the O2 saturation of the mixed venous blood.
Data collection and statistical analysis
Blood samples were collected from the patient’s radial artery, pulmonary artery, central venous catheter, pre-oxygenator and post-oxygenator lines when the ECMO PF was 80 % of CO and protective mechanical ventilation was used. A second measurement was obtained when the ECMO PF was reduced to 50 % of CO during weaning from the support. Every measurement was repeated three times. Any individual measurement at any site that differed by more than 10 % from the other two measurements was discarded and another measurement was obtained. The respective mean values were analysed. R was calculated in all patients from the gasometric data. SpaO2 was inferred from the formula and then compared to the measured value from the blood samples. Data collection and statistical analysis were conducted with XLSTAT. The normality of the distribution parameters was verified with the Kolmogorov-Smirnov test. The t test was used to compare data between the two groups. Data were analysed using Bland-Altman plots. The results are presented as means ± standard deviations and p values <0.05 were considered significant.
Results
Pre-ECMO data are shown in Table 1, and include demographic data, body surface area, ARDS aetiology, mechanical ventilatory settings, arterial blood gas values, haemodynamic values, survival at intensive care unit discharge and on hospital discharge. VV-ECMO data are presented in Table 2.
VV-ECMO assessment started after the patient had been stabilized (9.59 ± 6.2 h). We obtained measurements in each patient when the following conditions were fulfilled: PF was 80 % of the patient’s CO, SaO2 was ≥90 % and protective pulmonary ventilation was sufficient (mean peak inspiratory pressure 29.3 ± 7.1 cm H2O, respiratory minute volume 1945 ± 685 mL, tidal volume 182 ± 112 mL, FiO2 36.8 ± 12.6 %). A second set of measurements was obtained during weaning from ECMO, when PF was 50 % of CO. The results of the SpaO2 calculation are presented in Table 3.
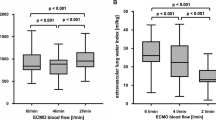
When PF was 80 % of CO, the mean PF was 5.68 ± 1.04 L/min and R was 28.8 ± 7.9 %. SpaO2 values were in the range 88.7–96.8 %. Mean measured SpaO2 was 92.3 ± 2.4 % and mean predicted SpaO2 was 92.8 ± 2.3 % (not significantly different, p = 0.513). The O2 saturation was significantly lower in the pulmonary artery than in the radial artery (SaO2; 92.8 ± 2.4 versus 95.7 ± 1.7 %, p < 0.002), and the predicted SpaO2 and SaO2 were well correlated (r = 0.80). When PF was 50 % of CO, the mean PF was 3.25 ± 0.44 L/min and R was 9.2 ± 2.5 %. SpaO2 values were in the range 85.1–91.1 %. Mean measured SpaO2 was 88.5 ± 1.38 % and mean predicted SpaO2 was 88.6 ± 1.5 % (not significantly different, p = 0.888).
Figure 2 shows the statistical analysis. In two series (34 measurements), SpaO2 values were in the range 85.1–98 %. Mean predicted SpaO2 was 90.7 ± 2.8 % and mean measured SpaO2 was 90.4 ± 2.7 % (not significantly different, p = 0.696). So predicted SpaO2 values and measured SpaO2 values were well correlated (r = 0.966). Bland-Altman analysis showed that predicted SpaO2 value were close to measured SpaO2 values with a low bias (0.271 ± 0.736 %) and the limits of agreement at 95 % were in the clinically acceptably range (−1.17 % to +1.71 %). Thus, when oxygenation is adequate, measured and predicted SpaO2 values are in good agreement.
Discussion
VV-ECMO is used to treat the most severe forms of acute respiratory failure. However, sufficient blood oxygenation with VV-ECMO remains difficult to achieve [10, 11, 16, 20]. Oxygenation depends on many parameters including CO and R [9, 10]. We established a new relationship between SaO2 and the relevant parameters under VV-ECMO. The focus of the study was to validate this relationship in relation to the treatment of ARDS.
Our main finding was that there is a good match between SpaO2 as predicted by the formula and SpaO2 as measured at two levels of PF (predicted SpaO2 90.7 ± 2.8 versus measured SpaO2 90.4 ± 2.7 %, p = 0.696. SaO2 is mainly determined by the saturation of the blood before passing through the lungs (SaO2≈SpaO2). In our study, SpaO2 was lower than SaO2 because the residual pulmonary function helps increase the oxygenation level. However, we were able to demonstrate a good correlation between predicted SpaO2 and SaO2 (r = 0.80).
Two approximations may cause a lack of precision when predicting SpaO2. First, ScvO2 is an approximation of SvO2, and the relationship between these two variables changes with catheter placement and CO, and can be influenced by relative changes in superior and inferior vena cava flow and coronary sinus flow. Placement of the tip of the central line near the right atrium (in our study, in the distal portion of the superior vena cava) increases the accuracy of ScvO2 in reflecting SvO2 [15, 19]. Second, ΔSaO2 is an approximate value which depends on many parameters, including haemoglobin saturation in the right heart cavities (mixture of saturated haemoglobin from the oxygenator and unsaturated haemoglobin), CO, PF, R, PmO2, haemoglobin concentration, and haemoglobin affinity for O2. Increased accuracy is possible, but the formula becomes too complex for clinical use.
Moreover, SaO2 can be lower than SpaO2 for the following reasons: (1) in a normal lung, some of the bronchial artery blood is collected by the pulmonary veins after perfusing the bronchi and its O2 is partly depleted, (2) a small amount of coronary venous blood drains directly into the cavity of the left ventricle through the Thebesian veins, and (3) some patients have an abnormal vascular connection between the small pulmonary artery and vein (pulmonary arteriovenous fistula) [21]. The role of each relationship determinant (SmO2, PmO2, PF, R, CO and SvO2) in the oxygenation was confirmed. According to current recommendations, recapitulated in guidelines and troubleshooting charts [11, 16] and also deduced from the formula, hypoxaemia may occur for the following reasons: low SmO2 and low PmO2 (failure of oxygenator), high R (decrease in EF), elevated CO (decrease in the EF/CO ratio), and low SvO2 (increase in the relative contribution of deoxygenated blood to oxygenation).
Although the information contained in this formula corroborates all the current recommendations in the event of insufficient oxygenation under ECMO [11, 16], we are aware of its limitations. It has only been tested over a normal oxygenation range. If these results are reproducible in situations of insufficient oxygenation, this formula may be useful in the clinical context. In practical terms, if blood oxygenation is insufficient or if hypoxaemia occurs during VV-ECMO, collecting all the parameters of the equation might allow the clinician to analyse the cause and address the deficiency promptly.
Conclusions
The use of VV-ECMO in patients suffering from severe acute respiratory failure has increased in advanced critical care medicine. However, in a certain number of cases, it is impossible to obtain a sufficient SaO2, and VV-ECMO management may be confusing. In this paper we have presented a new and explicit relationship between SaO2 and its direct determinants. This model sheds new light on oxygenation during VV-ECMO and allows a good grasp of oxygenation physiology when using this technique. To the best of our knowledge, there are no explicit results in the literature regarding how the important determinant parameters interact and affect oxygenation. Previous work has been limited to recommendations recapitulated in guidelines or troubleshooting charts. We validated the relationship under conditions of sufficient oxygenation, and a greater oxygenation range needs to be examined to complete the validation of the model. Despite this, we believe our work could be a starting point for a new way to use VV-ECMO.
References
Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL (2009) Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med 35:2105–2114
Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, Terragni P, Antonelli M, Gattinoni L, Oleari F, Pesenti A (2011) The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 37:1447–1457
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomized controlled trial. Lancet 374:1351–1363
Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, Dizier S, Forel JM, Guervilly C, Gariboldi V, Collart F, Michelet P, Perrin G, Charrel R, Papazian L (2010) Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med 36:1899–1905
Forrest P, Ratchford J, Burns B, Herkes R, Jackson A, Plunkett B, Torzillo P, Nair P, Granger E, Wilson M, Pye R (2011) Retrieval of critically ill adults using extracorporeal membrane oxygenation: an Australian experience. Intensive Care Med 37:824–830
Linko R, Okkonen M, Pettilä V, Parviainen I, Ruokonen E, Tenhunen J, Ala-Kokko T, Varpula T, The FINNALI-study group (2009) Acute respiratory failure in intensive care units. FINNALI: a prospective cohort study. Intensive Care Med 35:1352–1361
Hemmila MR, Napolitano LM (2006) Severe respiratory failure: advanced treatment options. Crit Care Med 34:278–290
Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD (2009) NIH NHLBI ARDS network. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 37:1574–1579
Barlett RH (2012) Physiology of extracorporeal life support. In: Annich GM, Lynch WR, MacLaren G, Wilson JM, Barlett RH JB (eds) ECMO, extracorporeal cardiopulmonary support in critical care, 4th edn. Extracorporeal Life Support Organization, Ann Arbor, pp 11–31
MacLaren G, Combes A, Bartlett RH (2012) Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 38:210–220
Barlett RH (2012) Management of blood flow and gas exchange during ECLS. In: Annich GM, Lynch WR, MacLaren G, Wilson JM, Barlett RH JB (eds) ECMO, Extracorporeal cardiopulmonary support in critical care, 4th edn. Extracorporeal Life Support Organization, Ann Arbor, pp 149–156
Rais-Bahrami K, Walton DM, Sell JE, Rivera O, Mikesell GT, Short BL (2002) Improved oxygenation with reduced recirculation during venovenous ECMO: comparison of two catheters. Perfusion 17:415–419
Extracorporeal Life Support Organization (2009) Patient specific supplements to the ELSO general guidelines. http://www.elso.med.umich.edu/WordForms/ELSO%20Pt%20Specific%20Guidelines.pdf. Accessed 21 Nov 2012
Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB (2008) Wang-Zwische double lumen cannula – toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J 54:606–611
Bonacchi M, Hamelin G, Peris A, Sani G (2011) A novel strategy to improve systemic oxygenation in venovenous extracorporeal membrane oxygenation: the “X-configuration”. J Thorac Cardiovasc Surg 142:1197–1204
Sidebotham D, McGeorge A, McGuiness S, Edwards M, Willcox T, Beca J (2010) Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: Part 2 – technical considerations. J Cardiothorac Vasc Anesth 24:164–172
Rees ES, Klæstrup E, Handy J, Andreassen S, Kristensen SR (2010) Mathematical modelling of the acid-base chemistry and oxygenation of blood: a mass balance, mass action approach including plasma and red blood cells. Eur J Appl Physiol 108:483–494
Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM (2011) The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 37:1932–1941
Walley KR (2011) Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med 184:514–520
Körver EPJ, Ganushchak YM, Simons AP, Donker DW, Maessen JG, Weerwind PW (2012) Quantification of recirculation as an adjuvant to transthoracic echocardiography for optimization of dual-lumen extracorporeal life support. Intensive Care Med 38:906–909
West JB (2004) Ventilation-perfusion relationships. In: West JB (ed) Respiratory physiology – the essentials, 7th edn. Lippincott, pp 54–73
Conflicts of interest
None of the authors has a source of support or a relationship with a commercial entity that has an interest in the subject of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Messaï, E., Bouguerra, A., Harmelin, G. et al. A new formula for determining arterial oxygen saturation during venovenous extracorporeal oxygenation. Intensive Care Med 39, 327–334 (2013). https://doi.org/10.1007/s00134-012-2756-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2756-0