Abstract
Purpose
The changed epidemiology of extended spectrum beta-lactamases (ESBL), the spread to the community and the need for prudent use of carbapenems require updated knowledge of risk factors for colonization with ESBL-producing enterobacteriaceae (ESBL-PE).
Methods
An 8-month prospective study in the medical ICU of an 850-bed general and university-affiliated hospital.
Results
Of 610 patients admitted, 531 (87 %) had a rectal swab obtained at admission, showing a 15 % (82 patients) ESBL-PE carriage rate, mostly of E. coli (n = 51, 62 %); ESBL-PE caused 9 (3 %) infections on admission. By multivariable analysis, transfer from another ICU (OR = 2.56 [1, 22]), hospital admission in another country [OR = 5.28 (1.56–17.8)], surgery within the past year [OR = 2.28 (1.34–3.86)], prior neurologic disease [OR = 2.09 (1.1–4.0)], and prior administration of third generation cephalosporin (within 3–12 months before ICU admission) [OR = 3.05 (1.21–7.68)] were independent predictive factors of colonization by ESBL-PE upon ICU admission. Twenty-eight patients (13 % of those staying for more than 5 days) acquired ESBL carriage in ICU, mostly with E. cloacae (n = 13, 46 %) and K. pneumoniae (n = 10, 36 %). In carriers, ESBL-PE caused 10 and 27 % of first and second episodes of ICU-acquired infections, respectively.
Conclusion
We found a high prevalence of ESBLE-PE colonization on admission to our ICU, even in the subgroup admitted from the community, but few first infections. Identifying risk factors for ESBL-PE colonization may help identifying which patients may warrant empiric ESBL-targeted antimicrobial drug therapy as a means to limit carbapenem use.
Similar content being viewed by others
Introduction
In gram-negative pathogens, beta-lactamase production remains the most important contributing factor to antimicrobial resistance. Cases of infections with extended-spectrum beta-lactamase-producing enterobacteriaceae (ESBL-PE) were first reported during the late 1980s and have subsequently spread worldwide [1–3]. The emergence of CTX-M-type ESBLs has modified the epidemiology of ESBLs since dissemination of these enzymes is not restricted to the healthcare setting but also involves the community, especially among Escherichia coli [4–10]. Since the beginning of the century, the prevalence of infection with ESBL, notably among E.coli and Klebsiella pneumoniae, has increased dramatically [11–13]. Infections caused by ESBL producers have been associated with severe adverse clinical outcomes, leading to increased mortality, prolonged hospital stay, and increased costs [14–17], mostly because of delayed effective therapy. Consequently, carbapenems are increasingly used by intensivists as empiric therapy of hospital-acquired sepsis. This vicious circle of bacterial resistance already leads to a rapid worrying international dissemination of carbapenemase-producing enterobacteriaceae (CPE), especially among K. pneumoniae (KPC) [18–20]. New agents against these multi-drug-resistant bacteria are not going to be available soon, and the intensivist’s armamentarium is close to a dead end without the cautious use of carbapenems [21]. An important risk factor for nosocomial infection is prior colonization [22]. The changed epidemiology, the spread of ESBL to the community, and the need for prudent use of carbapenems require updated studies to identify current risk factors for colonization with ESBL-PE.
The primary objective of our study was to determine which factors are predictive of colonization with ESBL-PE at admission to an intensive care unit (ICU). The secondary objectives were to identify the rates of and risk factors for acquisition of ESBL-PE during the ICU stay. We also examined the occurrence and risk factors of ESBL-PE infections in relation to carriage of ESBL-PE.
Patients and methods
Setting and patients
This 8-month prospective study (1 October 2010–31 May 2011) was conducted in the medical intensive care unit of a French 850-bed general and university-affiliated hospital. The ICU includes 13 beds with 5 single rooms, and a step-down unit of 11 beds with 3 single rooms. No specific isolation precautions were being used during the study for patients with ESBL-producing bacteria recovered from clinical or screening cultures, and contact isolation precautions were applied only for patients with carbapenemase-producing organisms, Clostridium difficile, methicillin-resistant S. aureus, and vancomycin-resistant enterococci. However, an active hand hygiene improvement program with repeated auditing and feedback, as well as twice weekly surveillance of patients for ESBL-producing bacteria using rectal swabs, had been conducted for 2 years as part of ‘MOSAR,’ an ongoing EC-funded (FP-6) study. In addition, daily body washings with chlorhexidine were routinely used for all patients. This study was approved by our Institutional Review Board (CPP Ile de France IX), and informed consent was waived.
Rectal swabs were collected from each patient within 24 h of ICU admission and twice weekly for the duration of hospitalization in the ICU. We excluded readmissions. ESBL-PE acquisition was defined as a positive rectal swab culture after a negative admission swab. Rectal swab samples were screened for ESBL-PE on chromogenic agar (Oxoid Ltd, Cambridge, UK), and ESBL production was confirmed by the double-disk diffusion method using ceftazidime or cefotaxime with clavulanic acid [23] (see the ESM for details).
Demographic, clinical, and laboratory data
A detailed clinical profile of each patient was established from the patient’s medical record, referral documents (e.g., from the family physician or nursing home), interviews with each patient and/or his or her family, and previous hospital admission records. The following data were collected: demographic characteristics, the simplified acute physiology score (SAPS II), days and in hospital location before ICU admission when appropriate, main reason for admission, hospital admission and administration of antibiotics in the previous year (stratified according to within 3 months of admission or earlier), prior antibiotic exposure (class and duration), surgery in the previous year, presence of underlying diseases and Charlson comorbidity index [24], and whether any indwelling tubes were in place for more than 24 h before ICU admission.
We defined colonization pressure as the sum of the daily proportion of patients in the unit colonized with ESBL-PE during the days preceding acquisition or ICU discharge [25].
The clinical impact of ESBL-PE colonization in the ICU was assessed from the rate of positive clinical samples until hospital discharge, mortality rate and length of stay, and comparing ESBL-PE carriers with non-carriers, and the primary outcome was defined as the rate of infection with ESBL-PE, contrasting early and late infection in carriers and non-carriers.
Statistical analysis
Results are reported as medians and interquartile ranges (25th–75th percentiles) or numbers with percentages. Associations between each variable and colonization with ESBL-PE at ICU admission were tested using the χ2 or Fisher’s exact test for categorical data and by the Mann-Whitney U test for continuous data. We used multivariable logistic regression with a backward procedure to identify patients’ characteristics associated with ESBL-PE colonization at ICU admission. Variables selected by bivariate analysis (P < 0.10) and those considered clinically relevant were entered in a logistic regression model. Considering the number of events, a maximum of eight variables was entered in a two-step model, first including baseline characteristics, then antibiotic exposures [26]. Results are expressed as crude and adjusted odds ratios (OR) with their 95 % confidence intervals (CI). A P value <0.05 was considered statistically significant. A similar analysis was conducted for the subgroup of patients admitted from the community, defined as those admitted directly from home or having stayed in the hospital for <48 h before ICU admission.
To examine risk factors for ESBL acquisition, we selected patients without ESBL colonization at admission, staying 5 days or more in the ICU and having at least two screening samples obtained before ICU discharge. Additional analyses were performed to identify risk factors for subsequent culture positivity with ESBL-PE among all the patients and among the subgroup of patients colonized with ESBL-PE. Statistical analyses used the Stata software, version 10.1 (StataCorp, College Station, TX, USA).
Results
ESBL-PE colonization at ICU admission
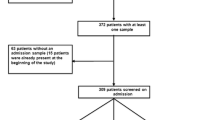
During the study period, 610 patients had at least one ICU admission, of which 531 (87 %) had a screening sample obtained within 24 h of ICU admission. The remaining 79 patients had similar characteristics (data not shown) but a much shorter length of ICU stay (2 days [2, 3] vs. 5 days [3–10]) and were not screened for ESBL carriage.
The median age of the 531 patients was 64 years (50–75); 198 (37 %) had a Charlson comorbidity index of > 2. Within the previous year, 231 (43 %) patients had been hospitalized for more than 24 h and 242 (46 %) had been exposed to antibiotics.
Eighty-two (15 %) patients were detected to be ESBL-PE carriers on the admission screening sample, mostly of E. coli (n = 51; 62 %) or K. pneumoniae (n = 15; 18 %). Table 1 shows the main characteristics of the patients and those associated with colonization at ICU admission. By multivariable analysis (Table 2), transfer from another ICU, previous hospital admission in another country, surgery within the past year, prior neurologic disease, and prior administration of third generation cephalosporin (within 3–12 months before ICU admission) remained associated with colonization by ESBL-PE upon ICU admission; exposure to fluoroquinolones within the past 3 months fell short of statistical significance (P = 0.062). The final model showed a good calibration (Hoshmer-Lemeshow χ2 = 2.13; P = 0.54), but limited discrimination (area under the curve, 0.68).
In the subgroup of 394 (74 %) patients admitted from the community, 49 (12 %) were found colonized by an ESBL-PE on the admission screening sample, 41 (84 %) of which were E. coli. Independent predictive factors of colonization by ESBL-PE at ICU admission in this subgroup included a previous hospital admission within 3–12 months before admission, prior urinary tract disease, and exposure to third generation cephalosporin (Table 2); again treatment with fluoroquinolone within the past 3 months fell short of statistical significance (P = 0.077). The final model again showed good calibration (χ2 = 0.19; P = 0.91) but limited discrimination (area under the curve, 0.69).
ESBL-PE-acquired carriage in ICU
At least two screening samples were obtained in 212 patients without detectable colonization with ESBL-PE on admission and staying at least 5 days in the ICU. Twenty-eight (13 %) acquired ESBL carriage, detected a median of 9 days [8–20] after ICU admission. Thirteen (46 %) were Enterobacter cloacae, ten (36 %) were K. pneumoniae, and only two (7 %) were E. coli (see the ESM Table). Two patients (7 %) had several ESBL-PE species identified (K. pneumoniae with E. coli or C. diversus). No carbapenemase-producing enterobacteriaceae was detected. Factors associated with ESBL acquisition in the ICU found by univariate and multivariable analyses are shown in Tables 3 and 4, respectively. Prior exposure to third generation cephalosporins or to a β-lactam + inhibitor (within 3 months of ICU admission) were both strongly associated with ESBL-PE acquisition. The final model showed both a good calibration (χ2 = 2.32; P = 0.97) and discrimination (area under the curve, 0.89).
Impact of ESBL-PE on infections in the ICU
During the 8-month study period, 210 patients had community-acquired infection on admission (Fig. 1). Only three such patients (1.4 %, or 6.1 % of ESBL-PE carriers) were infected with ESBL-PE on admission (all urinary tract infection), one of whom received a delayed effective therapy. Of 84 patients with hospital-acquired infections identified at ICU admission, 6 (7.1 %) were caused by ESBL-PE, including pulmonary infection (n = 3), and urinary tract infection, catheter-related bloodstream infection or septic arthritis (one each); one received delayed effective therapy. Among the 365 patients staying in the ICU for more than 3 days, we found 108 ICU-acquired infections in 87 patients (Fig. 2). Seven (6.5 %) of these were caused by ESBL-PE, including urinary tract infection (n = 5), or pulmonary infection and intra-abdominal infection with bacteraemia (1 each). None of these patients received delayed effective therapy. All patients with ICU-acquired infection caused by ESBL-PE also had rectal carriage, including 14 (82 %) with the same species; only one patient had infection before detection of carriage.
ESBL colonization and infection on ICU admission. Breakdown of patients by carriage status of extended-spectrum beta-lactamase (ESBL) producing enterobacteriaceae (ESBL-PE) on ICU admission among those admitted from the community (N = 394) or having stayed in the hospital for 2 days or more before ICU admission (N = 137) and corresponding number of patients having infection on ICU admission (or within 48 h of admission) with an ESBL-PE microorganism, including those having bloodstream infection (BSI). CA community-acquired, HAI hospital-acquired infection
ICU-acquired ESBL colonization and infection. Number (%) of patients with first and secondary infections caused by ESBL-PE among those acquiring infection during the ICU stay contrasting carriers and non-carriers and patients colonized on admission or acquiring ESBL-PE carriage. AI acquired infection, BSI bloodstream infection
The median length of stay before the occurrence of ICU-acquired infections caused by ESBL-PE was 10 days [6–11]. Among the 90 patients staying in the ICU for more than 3 days and having ESBL-PE carriage either on admission or acquired during the ICU stay, ESBL-PE caused 4/41 (10 %) of first episodes and 3/11 (27 %) of the second episodes of ICU-acquired infection (Fig. 2). Eight further patients having clinical samples growing ESBL-PE were considered to have colonizations, which were not treated with antibiotics.
The median time elapsed between the detection of ESBL carriage in rectal swabs and detection of ICU-acquired infection was 5 days [3–9]. The few ESBL infections did not allow multivariate analysis of variables associated with infection caused by ESBL-PE.
The overall in-ICU mortality rate was 18 %, and the median length of stay was 5 days [3–10]. The mortality rate of the 110 ESBL-colonized patients and of the 16 patients having ESBL-PE infection was similar (19 %), as was their length of ICU stay (9 days [4–19] and 9 days [7–15], respectively).
Discussion
The main finding from this 8-month study of 531 patients is the high rate of rectal carriage of ESBL-PE on ICU admission (15 % overall and 12 % in patients admitted from the community), with E. coli representing the most common ESBL-PE species recovered (62 %). Second, infections caused by ESBL-PE were rarely (3 %, Fig. 1) observed on ICU admission despite this high carriage rate; conversely, such infection was more common (10 %) among patients with ICU-acquired infections, especially during second episodes (14 %, Fig. 2). This study is, to our knowledge, the first to analyze risk factors for ESBL-PE carriage at ICU admission and acquisition, based on prospectively collected and comprehensive information on patients’ characteristics and exposures to antibiotics and other risk factors prior to and during ICU admission.
Most studies conducted in the past decade report an increasing incidence of ESBL-PE isolates recovered from both clinical and surveillance samples. Recent studies of colonization rates in ICU patients are sparse [27–30], with rates varying from 2 % [28] to as high as 49 % [30]. Although ESBL rates reported differ according to the regional area and patient populations studied, the overall incidence of ESBL-PE has markedly increased in Europe during the past decade, including in the community [3, 5, 10, 12, 13]. Of concern, E.coli has emerged as the most common microorganism recovered from ESBL-PE carriers at admission; however, K. pneumonia and E. cloacae remain the most common ICU-acquired ESBL-PE microorganisms.
The increasing prevalence of ESBL-PE carriage on ICU admission raises important questions on empiric therapy policies in patients presenting with infection, which may include the use of a carbapenem as first-line therapy. However, carbapenemase-producing enterobacteriaceae have now emerged (notably among Klebsiella pneumoniae) as a group of highly drug-resistant gram-negative bacilli causing infections associated with significant morbidity and mortality [32]. Since the emergence of antibiotic resistance is associated with widespread broad-spectrum antibiotic use, intensivists may now be close to a dead end without the cautious use of carbapenems [21]. In this context, examining the incidence and risk factors for colonization and infection with such microorganisms might help better targeting empiric therapy. Our results suggest that, despite the high colonization rate at ICU admission, infections caused by ESBL-PE remain very infrequent (only 3 % of infected patients in our study). Thus, limited use of empiric treatment with carbapenems targeting ESBL-PE can still be recommended, even in a setting with a high endemic rate of ESBL-PE carriage. Restricting their use to selected patients having risk factors for colonization with ESBL-producing bacteria at ICU admission is likely to limit unnecessary exposure to carbapenems in the ICU.
Previous studies have shown that patients infected with ESBL-producing bacteria have identifiable clinical characteristics that can be used readily upon ICU admission [28, 29, 31, 33, 34], such as male gender, being elderly and/or a nursing home resident, recent hospitalization, or exposure to any antibiotic [10]. We identified prior hospital admission in another country, transfer from another ICU, surgery within the past year, and prior neurologic disease as independent risk factors for colonization with ESBL-PE at ICU admission. Predictive factors in the subgroup of patients admitted from the community included a previous hospital admission within 3–12 months before ICU admission and a history of urinary tract disease. Interestingly, our study confirms that exposure to third generation cephalosporin within months before ICU admission was an independent risk factor for colonization with ESBL-PE at ICU admission, irrespective of the patient’s location before ICU admission.
Despite the high colonization pressure with ESBL-PE, the rate of ICU-acquired colonization with these microorganisms was relatively low, at 6 % overall and 9 % in the population staying in the ICU for >3 days. This is probably explained mostly by the high hand hygiene compliance rate (>80 %) of personnel in our unit during the study period resulting from the ongoing hand hygiene improvement program and possibly from the routine use of daily chlorhexidine body washings. Among the 212 patients staying in the ICU for >5 days and non-colonized on admission, only 28 (13 %) acquired ESBL-PE carriage, mostly with E. cloacae (n = 13, 46 %) and K. pneumoniae (n = 10, 36 %), but rarely with E. coli. Acquisition of ESBL-PE during the ICU stay was associated with age >75 years, male gender, colonization pressure, and administration of a third generation cephalosporin or a β-lactam/inhibitor combination within 3 months before ICU admission. Intriguingly, exposure to various antibiotic classes during the ICU stay did not remain associated with ESBL-PE acquisition after multivariable analysis. However, colonization pressure remained associated with acquisitions, and a substantial proportion of these were due to K. pneumoniae and Enterobacter spp, for which horizontal transmission may predominate over selection of resistance [35].
Important features of our study include its prospective design, accounting for antibiotic exposure before and during the ICU stay, in the unique setting of a sustained controlled high hand hygiene compliance rate and absence of additional contact precautions, which helps interpreting the results. First, despite the high level of standard precautions, ESBLE-PE acquisitions still remained substantial and related to colonization pressure. Standard precautions alone thus do not appear sufficient to control the spread of ESBL-PE within ICUs. Second, the use of third generation cephalosporins should be limited, as prior exposure to these drugs was a risk factor both for carriage on admission and for acquisition. Third, a different control policy may be justified against E.coli on one hand and K. pneumoniae and Enterobacter spp. on the other. While there are no universal guidelines concerning infection control measures for ESBL-PE carriers, our data suggest that additional measures may be warranted to control the spread of the latter species. Indeed, K. pneumoniae and Enterobacter are frequently involved in hospital outbreaks; in addition, environmental contamination is more frequent with ESBL-producing Klebsiella than with E coli [36]. Therefore, intensifying control measures might prove useful for these two species.
Overall, there were 294 infections at admission and 108 ICU-acquired infections, of which 3 and 6.5 % were caused by ESBL-PE, respectively. Infections caused by ESBL-PE were thus relatively infrequent in our study despite the high carriage rate. In a prospective study of 455 consecutive episodes of K. pneumoniae bacteremia in 12 hospitals from seven countries conducted in 1996–1997, 85 (19 %) were due to an ESBL-producing strain [37]. This rate was higher among the 253 nosocomial infections (31 %), particularly those acquired in the intensive care unit (43 %). In our patients, ESBL-PE caused 3 % of all ICU-acquired bacteremias and 5 % of ICU-acquired bloodstream infections caused by gram-negative bacilli. Although ventilator-associated pneumonia (VAP) is the main source of ICU-acquired infections [40], only one of our patients developed a VAP caused by an ESBL-PE.
A major risk factor for nosocomial infection is prior colonization [22]. All but one of the patients who had an ICU-acquired infection caused by ESBL-PE were found to have been previously colonized a median of 5 days [3–9] before infection. ESBL-PE caused only 4.6 % of 87 first episodes but 14 % of 21 secondary episodes of ICU-acquired infection. Among the 90 ESBL-PE carriers staying in the ICU for more than 3 days, these organisms caused 10 % of the first episodes and 27 % of the second episodes of ICU-acquired infections. In previous studies, performed in the context of much lower prevalence rates on ICU admissions, 9–25 % of ESBL-colonized patients acquired ESBL-PE infection [28, 31]. In our environment, carbapenems can thus be viewed as drugs of choice for empiric therapy of late ICU-acquired infections, especially in known carriers, thus providing coverage for ESBL-PE, as well as for drug-resistant Pseudomonas aeruginosa, another major cause of late ICU-acquired infection. Indeed, 73 patients received carbapenems during the 8-month period, mostly for empiric therapy of ICU-acquired infections.
Our study has some limitations. Because the study was monocentric, the results cannot be extrapolated to other ICUs with different epidemiologies and infection control policies and standards, especially regarding the acquisition of ESBL-EB. Colonization relied on rectal swabbing, which does not provide 100 % sensitivity for the detection of ESBL carriage, thus possibly resulting in misclassification of patients. Confronting epidemiological information with the results of molecular typing of isolates could have provided a better insight into the risk of cross-transmission between different species. A final limitation of our study is the relatively small number of infections, thus limiting our ability to identify specific risk factors for infection. In addition, despite careful examination of patients’ records, it is likely that we did not retrieve all antimicrobial drugs that patients may have received as outpatients before their hospital admission. However, the information collected in our study reflects the information available to the intensivist in real-life practice when confronted with decisions on antibiotic therapy in patients presenting with sepsis in the ICU.
To conclude, since improving carbapenem use is currently a major challenge for intensivists, our analyses of risk factors for colonization on admission and acquisitions of ESBL-PE may be useful for identifying which patients may warrant empiric therapy targeting these organisms in the context of high endemic rates, even on ICU admission. They may also contribute to future antibiotic stewardship programs and/or interventional studies to help control ESBL-PE [38, 39]. Larger scale studies are needed to identify risk factors for ICU-acquired ESBL infection and to assess patterns of use of empiric therapy with carbapenems according to the knowledge of ESBL colonization status of the patient.
References
Kliebe C, Nies BA, Meyer JF, Tolxdorff-Neutzling RM, Wiedemann B (1985) Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother 28:302–307
Paterson DL, Bonomo RA (2005) Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 18:657–686
Canton R, Novais A, Valverde A, Machado E, Peixe L, Baquero F, Coque TM (2008) Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect 14(Suppl 1):144–153
Rodriguez-Bano J, Alcala JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tortola T, Mirelis B, Navarro G, Cuenca M, Esteve M, Pena C, Llanos AC, Canton R, Pascual A (2008) Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med 168:1897–1902
Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Ruiz M, Pena C, Almela M, Almirante B, Grill F, Colomina J, Gimenez M, Oliver A, Horcajada JP, Navarro G, Coloma A, Pascual A (2010) Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis 50:40–48
Lavigne JP, Marchandin H, Delmas J, Moreau J, Bouziges N, Lecaillon E, Cavalie L, Jean-Pierre H, Bonnet R, Sotto A (2007) CTX-M beta-lactamase-producing Escherichia coli in French hospitals: prevalence, molecular epidemiology, and risk factors. J Clin Microbiol 45:620–626
Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Fishman NO, Bilker WB, Mao X, Lautenbach E (2005) Risk factors for increasing multidrug resistance among extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species. Clin Infect Dis 40:1317–1324
Hsieh CJ, Shen YH, Hwang KP (2010) Clinical implications, risk factors and mortality following community-onset bacteremia caused by extended-spectrum beta-lactamase (ESBL) and non-ESBL producing Escherichia coli. J Microbiol Immunol Infect 43:240–248
Calbo E, Romani V, Xercavins M, Gomez L, Vidal CG, Quintana S, Vila J, Garau J (2006) Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother 57:780–783
Ben-Ami R, Rodriguez-Bano J, Arslan H, Pitout JD, Quentin C, Calbo ES, Azap OK, Arpin C, Pascual A, Livermore DM, Garau J, Carmeli Y (2009) A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49:682–690
Drieux L, Brossier F, Duquesnoy O, Aubry A, Robert J, Sougakoff W, Lecso-Bornet M, Jarlier V (2009) Increase in hospital-acquired bloodstream infections caused by extended spectrum beta-lactamase-producing Escherichia coli in a large French teaching hospital. Eur J Clin Microbiol Infect Dis 28:491–498
Saurina G, Quale JM, Manikal VM, Oydna E, Landman D (2000) Antimicrobial resistance in Enterobacteriaceae in Brooklyn, NY: epidemiology and relation to antibiotic usage patterns. J Antimicrob Chemother 45:895–898
European Centre for Disease Surveillance and Control–EARS-Net Antimicrobial Resistance Surveillance in Europe (2010). http://ecdc.europa.eu/en/publications/Publications/1111_SUR_AMR_data.pdf.pdf
Lee SY, Kotapati S, Kuti JL, Nightingale CH, Nicolau DP (2006) Impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol 27:1226–1232
Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO (2001) Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32:1162–1171
Blot S, Depuydt P, Vogelaers D, Decruyenaere J, De Waele J, Hoste E, Peleman R, Claeys G, Verschraegen G, Colardyn F, Vandewoude K (2005) Colonization status and appropriate antibiotic therapy for nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in an intensive care unit. Infect Control Hosp Epidemiol 26:575–579
de Kraker ME, Wolkewitz M, Davey PG, Koller W, Berger J, Nagler J, Icket C, Kalenic S, Horvatic J, Seifert H, Kaasch A, Paniara O, Argyropoulou A, Bompola M, Smyth E, Skally M, Raglio A, Dumpis U, Melbarde Kelmere A, Borg M, Xuereb D, Ghita MC, Noble M, Kolman J, Grabljevec S, Turner D, Lansbury L, Grundmann H (2011) Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third generation cephalosporins. J Antimicrob Chemother 66:398–407
Karah N, Haldorsen B, Hermansen NO, Tveten Y, Ragnhildstveit E, Skutlaberg DH, Tofteland S, Sundsfjord A, Samuelsen O (2011) Emergence of OXA-carbapenemase- and 16S rRNA methylase-producing international clones of Acinetobacter baumannii in Norway. J Med Microbiol 60:515–521
Chen S, Hu F, Xu X, Liu Y, Wu W, Zhu D, Wang H (2011) High prevalence of KPC-2-type carbapenemase coupled with CTX-M-type extended-spectrum beta-lactamases in carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China. Antimicrob Agents Chemother 55:2493–2494
Munoz-Price LS, De La Cuesta C, Adams S, Wyckoff M, Cleary T, McCurdy SP, Huband MD, Lemmon MM, Lescoe M, Dibhajj FB, Hayden MK, Lolans K, Quinn JP (2010) Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 31:1074–1077
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12
Bonten MJ, Weinstein RA (1996) The role of colonization in the pathogenesis of nosocomial infections. Infect Control Hosp Epidemiol 17:193–200
Clinical Laboratory Standards Institute, Wayne (PA) (2006) Performance standards for antimicrobial susceptibility testing. 16th informational supplement (M100-S16)
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Ajao AO, Harris AD, Roghmann MC, Johnson JK, Zhan M, McGregor JC, Furuno JP (2009) Systematic review of measurement and adjustment for colonization pressure in studies of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and clostridium difficile acquisition. Infect Control Hosp Epidemiol 32:481–489
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48:1503–1510
Thouverez M, Talon D, Bertrand X (2004) Control of Enterobacteriaceae producing extended-spectrum beta-lactamase in intensive care units: rectal screening may not be needed in non-epidemic situations. Infect Control Hosp Epidemiol 25:838–841
Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, Hebden JN, Morris JG Jr (2007) Risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis 13:1144–1149
Friedmann R, Raveh D, Zartzer E, Rudensky B, Broide E, Attias D, Yinnon AM (2009) Prospective evaluation of colonization with extended-spectrum beta-lactamase (ESBL)-producing enterobacteriaceae among patients at hospital admission and of subsequent colonization with ESBL-producing enterobacteriaceae among patients during hospitalization. Infect Control Hosp Epidemiol 30:534–542
Azim A, Dwivedi M, Rao PB, Baronia AK, Singh RK, Prasad KN, Poddar B, Mishra A, Gurjar M, Dhole TN (2010) Epidemiology of bacterial colonization at intensive care unit admission with emphasis on extended-spectrum beta-lactamase- and metallo-beta-lactamase-producing Gram-negative bacteria—an Indian experience. J Med Microbiol 59:955–960
Gardam MA, Burrows LL, Kus JV, Brunton J, Low DE, Conly JM, Humar A (2002) Is surveillance for multidrug-resistant enterobacteriaceae an effective infection control strategy in the absence of an outbreak? J Infect Dis 186:1754–1760
Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, Tsioka A, Roilides E, Sofianou D, Gritsi-Gerogianni N (2010) Bloodstream infections caused by metallo-beta-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 31:1250–1256
Pena C, Pujol M, Ricart A, Ardanuy C, Ayats J, Linares J, Garrigosa F, Ariza J, Gudiol F (1997) Risk factors for faecal carriage of Klebsiella pneumoniae producing extended spectrum beta-lactamase (ESBL-KP) in the intensive care unit. J Hosp Infect 35:9–16
Ben-Ami R, Schwaber MJ, Navon-Venezia S, Schwartz D, Giladi M, Chmelnitsky I, Leavitt A, Carmeli Y (2006) Influx of extended-spectrum beta-lactamase-producing enterobacteriaceae into the hospital. Clin Infect Dis 42:925–934
Harris AD, Perencevich EN, Johnson JK, Paterson DL, Morris JG, Strauss SM, Johnson JA (2007) Patient-to-patient transmission is important in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae acquisition. Clin Infect Dis 45:1347–1350
Guet-Revillet H, Le Monnier A, Breton N, Descamps P, Lecuyer H, Alaabouche I, Bureau C, Nassif X, Zahar JR (2012) Environmental contamination with extended-spectrum beta-lactamases: is there any difference between Escherichia coli and Klebsiella spp? Am J Infect Control
Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG, Yu VL (2004) Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis 39:31–37
Lipworth AD, Hyle EP, Fishman NO, Nachamkin I, Bilker WB, Marr AM, Larosa LA, Kasbekar N, Lautenbach E (2006) Limiting the emergence of extended-spectrum beta-lactamase-producing enterobacteriaceae: influence of patient population characteristics on the response to antimicrobial formulary interventions. Infect Control Hosp Epidemiol 27:279–286
Rice LB, Eckstein EC, DeVente J, Shlaes DM (1996) Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin Infect Dis 23:118–124
American Thoracic Society and Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Acknowledgments
This study was supported in part by funding from the European Community (MOSAR network contract LSHP-CT-2007-037941).
Conflicts of interest
All authors report no conflict of interest relevant to this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
In collaboration with the MOSAR WP3 study team.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Razazi, K., Derde, L.P.G., Verachten, M. et al. Clinical impact and risk factors for colonization with extended-spectrum β-lactamase-producing bacteria in the intensive care unit. Intensive Care Med 38, 1769–1778 (2012). https://doi.org/10.1007/s00134-012-2675-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2675-0