Abstract
Purpose
Sepsis affects both macro- and micro-circulatory transport of oxygen to tissues, causing regional hypoxia. However, this relationship is poorly characterized with respect to inter-organ variability, disease severity and the evolution to organ dysfunction. We hypothesized that an early circulatory insult precedes the development of organ dysfunction, and is more severe in predicted non-survivors. Consequently, we assessed temporal changes in myocardial function and regional tissue oxygenation in peripheral and deep organs in a rat model of faecal peritonitis. We also examined the utility of a dynamic oxygen challenge test to assess the microcirculation.
Methods
Awake, tethered, fluid-resuscitated male Wistar rats were randomized to receive intraperitoneal injection of faecal slurry, or to act as controls. At either 6 or 24 h post insult, rats were anaesthetized and underwent echocardiography, arterial cannulation and placement of tissue oxygen probes in peripheral (muscle, bladder) and deep (liver and renal cortex) organ beds. Measurements were repeated during fluid loading and an oxygen challenge test (administration of high oxygen concentrations).
Results
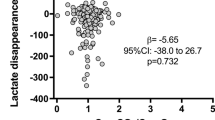
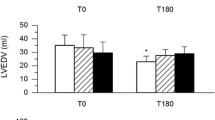
Early sepsis (6 h) was characterized by a fall in global oxygen delivery with concurrent decreases in muscle, renal cortical and, especially, liver tissue PO2. By contrast, during established sepsis (24 h), myocardial and circulatory function had largely recovered despite increasing clinical unwellness, hyperlactataemia and biochemical evidence of organ failure. O2 challenge revealed an early depression of response that, by 24 h, had improved in all organ beds bar the kidney.
Conclusions
This long-term septic model exhibited an early decline in tissue oxygenation, the degree of which related to predicted mortality. Clinical and biochemical deterioration, however, progressed despite cardiovascular recovery. Early circulatory dysfunction may thus be an important trigger for downstream processes that result in multi-organ failure. Furthermore, the utility of tissue PO2 monitoring to highlight the local oxygen supply–demand balance, and dynamic O2 challenge testing to assess microcirculatory function merit further investigation.




Similar content being viewed by others
References
Rudiger A, Singer M (2007) Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35:1599–1608
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med 166:98–104
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360:219–223
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Hayes MA, Timmins AC, Yau EH, Palazzo M, Watson D, Hinds CJ (1997) Oxygen transport patterns in patients with sepsis syndrome or septic shock: influence of treatment and relationship to outcome. Crit Care Med 25:926–936
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 333:1025–1032
Rudiger A, Dyson A, Taylor V, Stidwill R, Protti A, Hughes S, Pellerin D, Singer M (2011) Cardiac dysfunction during early sepsis predicts outcome in a rat model of fecal peritonitis. Shock (in press)
Dyson A, Stidwill R, Taylor V, Singer M (2007) Tissue oxygen monitoring in rodent models of shock. Am J Physiol Heart Circ Physiol 293:H526–H533
Dyson A, Stidwill R, Taylor V, Singer M (2009) The impact of inspired oxygen concentration on tissue oxygenation during progressive haemorrhage. Intensive Care Med 35:1783–1791
Boekstegers P, Weidenhofer S, Pilz G, Werdan K (1991) Peripheral oxygen availability within skeletal muscle in sepsis and septic shock: comparison to limited infection and cardiogenic shock. Infection 19:317–323
Sair M, Etherington PJ, Peter WC, Evans TW (2001) Tissue oxygenation and perfusion in patients with systemic sepsis. Crit Care Med 29:1343–1349
Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE (2005) Relation between muscle Na + K + ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365:871–875
Rosser DM, Stidwill RP, Jacobson D, Singer M (1995) Oxygen tension in the bladder epithelium rises in both high and low cardiac output endotoxemic sepsis. J Appl Physiol 79:1878–1882
Rosser DM, Stidwill RP, Jacobson D, Singer M (1996) Cardiorespiratory and tissue oxygen dose response to rat endotoxemia. Am J Physiol 271:H891–H895
Singer M, De Santis V, Vitale D, Jeffcoate W (2004) Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 364:545–548
Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M (2004) Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 286:R491–R497
Slama M, Susic D, Varagic J, Ahn J, Frohlich ED (2003) Echocardiographic measurement of cardiac output in rats. Am J Physiol Heart Circ Physiol 284:H691–H697
Iwase M, Yokota M, Kitaichi K, Wang L, Takagi K, Nagasaka T, Izawa H, Hasegawa T (2001) Cardiac functional and structural alterations induced by endotoxin in rats: importance of platelet-activating factor. Crit Care Med 29:609–617
Hollenberg SM, Dumasius A, Easington C, Colilla SA, Neumann A, Parrillo JE (2001) Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med 164:891–895
Yu M, Morita SY, Daniel SR, Chapital A, Waxman K, Severino R (2006) Transcutaneous pressure of oxygen: a noninvasive and early detector of peripheral shock and outcome. Shock 26:450–456
Yu M, Chapital A, Ho HC, Wang J, Takanishi D Jr (2007) A prospective randomized trial comparing oxygen delivery versus transcutaneous pressure of oxygen values as resuscitative goals. Shock 27:615–622
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
Levy RJ, Piel DA, Acton PD, Zhou R, Ferrari VA, Karp JS, Deutschman CS (2005) Evidence of myocardial hibernation in the septic heart. Crit Care Med 33:2752–2756
Ellrodt AG, Riedinger MS, Kimchi A, Berman DS, Maddahi J, Swan HJ, Murata GH (1985) Left ventricular performance in septic shock: reversible segmental and global abnormalities. Am Heart J 110:402–409
Kontani M, Izumiya Y, Shimizu M, Yasuma K, Inada A, Ohta H, Ikeda T (2003) Acute reversible myocardial depression associated with sepsis. Intern Med 42:60–65
VanderMeer TJ, Wang H, Fink MP (1995) Endotoxemia causes ileal mucosal acidosis in the absence of mucosal hypoxia in a normodynamic porcine model of septic shock. Crit Care Med 23:1217–1226
James PE, Madhani M, Roebuck W, Jackson SK, Swartz HM (2002) Endotoxin-induced liver hypoxia: defective oxygen delivery versus oxygen consumption. Nitric Oxide 6:18–28
James PE, Bacic G, Grinberg OY, Goda F, Dunn JF, Jackson SK, Swartz HM (1996) Endotoxin-induced changes in intrarenal pO2, measured by in vivo electron paramagnetic resonance oximetry and magnetic resonance imaging. Free Radic Biol Med 21:25–34
Anning PB, Sair M, Winlove CP, Evans TW (1999) Abnormal tissue oxygenation and cardiovascular changes in endotoxemia. Am J Respir Crit Care Med 159:1710–1715
Sair M, Etherington PJ, Curzen NP, Winlove CP, Evans TW (1996) Tissue oxygenation and perfusion in endotoxemia. Am J Physiol 271:H1620–H1625
Vallet B, Lund N, Curtis SE, Kelly D, Cain SM (1994) Gut and muscle tissue PO2 in endotoxemic dogs during shock and resuscitation. J Appl Physiol 76:793–800
Dahn MS, Wilson RF, Lange P, Stone A, Jacobs LA (1990) Hepatic parenchymal oxygen tension following injury and sepsis. Arch Surg 125:441–443
Dahn MS, Mitchell RA, Lange MP, Smith S, Jacobs LA (1995) Hepatic metabolic response to injury and sepsis. Surgery 117:520–530
Rahal L, Garrido AG, Cruz RJ Jr, Rocha e Silva M, Poli-de-Figueiredo LF (2006) Systemic and regional hemodynamic effects of enalaprilat infusion in experimental normotensive sepsis. Braz J Med Biol Res 39:1205–1215
Subramanian RM, Chandel N, Budinger GR, Schumacker PT (2007) Hypoxic conformance of metabolism in primary rat hepatocytes: a model of hepatic hibernation. Hepatology 45:455–464
De Backer D, Ortiz JA, Salgado D (2010) Coupling microcirculation to systemic hemodynamics. Curr Opin Crit Care 16:250–254
Ellis CG, Jagger J, Sharpe M (2005) The microcirculation as a functional system. Crit Care 9:S3–S8
Ince C (2002) The microcirculation unveiled. Am J Respir Crit Care Med 166:1–2
Ince C, Sinaasappel M (1999) Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med 27:1369–1377
Goeckenjan G, Strasser K (1977) Relation of transcutaneous to arterial pO2 in hypoxaemia, normoxaemia and hyperoxaemia. Investigations in adults with normal circulation and in patients with circulatory insufficiency. Biotelemetry 4:77–87
Tremper KK, Waxman K, Shoemaker WC (1979) Effects of hypoxia and shock on transcutaneous PO2 values in dogs. Crit Care Med 7:526–531
Thurau K, Boylan JW (1976) Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med 61:308–315
Ince C (2005) The microcirculation is the motor of sepsis. Crit Care 9:S13–S19
Acknowledgments
A.D. and M.S. were funded by the Medical Research Council, UK. A.R. was supported by grants from the Swiss National Science Foundation, the Stiefel Zangger Foundation (Zurich, Switzerland) and the Siegenthaler Foundation (Zurich, Switzerland). The echocardiography equipment was funded by the British Heart Foundation, UK. This work was undertaken at UCL Hospitals/UCL, who receive support from the UK NIHR Biomedical Research Centre funding scheme. We thank Oxford Optronix for kindly providing the tPO2 probes.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-011-2229-x.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2011_2227_MOESM1_ESM.ppt
Supplementary Fig. 1 Experimental protocol. Fluid infusion: 10 ml/kg/h infusion of 1:1 ratio of 6% hydroxyethyl starch and 5% glucose in normal saline. FB Fluid bolus, 6 h bolus; 25 ml/kg, 24 h bolus; 10 ml/kg of hydroxyethyl starch. Thereafter, the fraction of inspired oxygen (FiO2) was changed at 15 min intervals. Instrumentation, stabilization, fluid loading and oxygen challenge took approximately 2 h. (PPT 169 kb)
Rights and permissions
About this article
Cite this article
Dyson, A., Rudiger, A. & Singer, M. Temporal changes in tissue cardiorespiratory function during faecal peritonitis. Intensive Care Med 37, 1192–1200 (2011). https://doi.org/10.1007/s00134-011-2227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2227-z