Abstract
Purpose
To evaluate the efficacy and safety of intravenous sildenafil for immediate postoperative pulmonary hypertension (PH) in pediatric patients undergoing congenital heart surgery.
Methods
A double-blind, multicenter, placebo-controlled, dose-ranging, parallel-group trial was conducted. Patients were randomized to one of three doses of intravenous sildenafil, or placebo, for a minimum of 24 h.
Results
The study was heavily underpowered. Whereas enrollment of 228 patients (57 per treatment arm) was required to achieve the sample size estimate to detect difference between arms, the sponsor terminated the study after 15 months owing to slow patient accrual. Seventeen patients (median age 5 months) experiencing postoperative PH were randomized and treated, five with placebo and four each with low-, medium-, and high-dose sildenafil. In the first 24 h, 40% of placebo and 17% of sildenafil patients required additional therapy (p = 0.330). Median time to extubation (3 versus 8 days, p = 0.023) and intensive care unit stay (6 versus 15 days, p = 0.008) were shorter for sildenafil patients. Mean ± standard deviation systolic pulmonary artery pressure was reduced with sildenafil (46 ± 11 to 35 ± 6 mmHg, p = 0.027 versus placebo). No adverse events or systemic hypotension were attributed to sildenafil.
Conclusion
Intravenous sildenafil reduced pulmonary artery pressure and shortened time to extubation and intensive care unit stay in children with postoperative PH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children with many forms of congenital heart disease (CHD) are prone to develop postoperative elevation in pulmonary vascular resistance (PVR) [1]. Resultant pulmonary hypertension (PH) may complicate the postoperative course [2]. PH may develop after cardiopulmonary bypass due to injury or transient dysfunction of the pulmonary endothelium [3]. Several therapies are currently used for postoperative PH [sedation, alkalosis, inhaled nitric oxide (iNO), intravenous prostacyclins, adenosine, and others] [4]. However, intravenous prostacyclins [5] may cause systemic hypotension, and although iNO appears selective for the pulmonary vasculature, the drug is costly, deliverable only as a gas, and may be associated with rebound PH during withdrawal [6]. A fast-acting, pulmonary-selective, easily administered agent that is safe and without a rebound effect is needed.
Sildenafil acts by inhibiting phosphodiesterase type 5 (PDE5), an enzyme that hydrolyzes intracellular cyclic guanosine monophosphate (cGMP) to 5′-GMP [7] and is highly expressed in the lungs [8]. Oral sildenafil received approval for treatment of PH after a double-blind, placebo-controlled trial showed it to be an effective treatment of chronic PH in adults [9]. In pediatric patients undergoing heart surgery, oral sildenafil attenuated rebound PH after iNO withdrawal during the early postoperative period [10, 11]. However, when administered enterally, the bioavailability of sildenafil is only about 40% in healthy subjects [12]. Critically ill children in the postoperative setting may have unpredictable enteral absorption [13], such that intravenous sildenafil may be more appropriate. Although preliminary studies in children with intravenous sildenafil report lower pulmonary arterial pressure (PAP) and PVR after cardiac surgery or during cardiac catheterization [14, 15], the optimal dose and the potential adverse effects remain undetermined, and little information is available on the effect of sildenafil on mortality and morbidity.
A clinical trial was designed to assess the efficacy, safety, and resource utilization of intravenous sildenafil in PH that developed during the postoperative period in children undergoing corrective congenital heart surgery. Although the trial was stopped due to slow accrual of patients, 17 patients were randomized and treated. The results from these patients are reported herein.
Materials and methods
Study design
From October 2003 to January 2005, a randomized, multicenter, double-blind, placebo-controlled, dose-ranging, parallel-group study was conducted in children undergoing corrective cardiac surgery who developed postoperative PH. Patients were randomized to one of three doses of intravenous sildenafil, or placebo, for a minimum of 24 h.
The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practices guidelines. The study protocol was reviewed and approved by the local institutional ethics committees. Patients believed to be at high risk for developing postoperative PH were identified before surgery. Written informed consent was obtained prior to initiation of protocol-specified procedures from each patient’s parent or legal guardian, and patient consent was obtained (when applicable) within the 2 weeks before surgery.
Eligibility
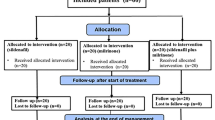
All infants or children aged 0 (≥34 weeks gestational age) to 17 years undergoing corrective cardiac surgery with a clinical diagnosis of postoperative PH within 48 h of the end of surgery and systolic PAP >50% of systolic arterial blood pressure (>75% for neonates ≤28 days) were eligible (Fig. 1). PH was confirmed by Doppler echocardiogram using the modified Bernoulli equation to the peak velocity of tricuspid regurgitation [p = 4v 2, where p = peak pressure drop from the right ventricle to the right atrium and v = peak velocity of tricuspid regurgitation (m/s)] that has been correlated with invasive transcatheter measurements [8], or pulmonary artery (PA) catheter at baseline. The exclusion criteria included: use of postoperative treatment solely for the purpose of treating or preventing PH, including agents used at relatively high doses for the purpose of deep/heavy sedation, such as fentanyl; agents used for continued paralysis, such as pancuronium (except when used to manage clinical situations such as open chest or critical airway); alkalinization by methods such as hyperventilation (pCO2 <30 mmHg) or bicarbonate (HCO3) infusion; vasodilators; meeting the criteria for extracorporeal membrane oxygenation (ECMO); receipt of concomitant nitrates or NO donors, open-label sildenafil within 48 h prior to surgery or any time postoperatively; supplemental arginine, long-acting α-blockers, endothelin antagonists (e.g., bosentan), or potent cytochrome P450 3A4 inhibitors such as ritonavir or nicorandil. Additional exclusion criteria were: occurrence of postoperative complications that resulted in hypoxemia (other than PH) due to lung disease; serious postoperative bleeding resulting in hypotension; impaired renal function [serum creatinine >2.5 times the upper limit of normal (ULN)] or hepatic function (alanine transaminase or aspartate transaminase >3.0 ULN; conjugated bilirubin >2.0 ULN; total bilirubin >2.0 ULN); and leukopenia (WBC <2,500/μl). Permitted medications included inotropes, milrinone, and other medication used to treat congestive heart failure.
Randomization
The patients, parents, all clinical staff, and the investigators were blinded to study drug allocation. An automated interactive voice response system was used to assign patients to treatment. Randomization was stratified by age (neonate or non-neonate) and by study center to ensure a balance across treatment groups. The minimization approach with biased coin assignment was used for stratification. The sponsor (Pfizer Inc.) provided 50-ml vials containing sildenafil 1.0 mg/ml. Placebo was provided as bags of 5% dextrose in water. The research pharmacist or other qualified individual received the randomized treatment assignment and prepared the bolus dose and maintenance infusion. The dilution was calculated according to the patient’s weight such that the assigned concentration (low, medium, or high) was infused at the same rate for all patients of a particular weight so that the staff remained blinded to the study dose.
Protocol
Baseline echocardiograms were performed to verify the presence of PH. Baseline hemodynamic parameters (in patients with a pulmonary artery, left atrial, and/or right atrial/central venous catheter), inotrope score [16], and vital signs were measured, and blood samples were collected for serum lactate measurement. Final study assessments were performed at discharge or 7 days after study drug infusion, whichever occurred first. There was a follow-up telephone call 28 days after study drug infusion.
Three intravenous sildenafil dosage regimens were selected to achieve target sildenafil plasma concentrations of approximately 40, 120, and 360 ng/ml in the low-, medium-, and high-dose groups, respectively. Selection of sildenafil doses for neonates and infants <60 days of age was to be based on pharmacokinetic data in neonates obtained from a clinical trial that was ongoing at the time [17, 18]. Each intravenous sildenafil dose regimen consisted of a bolus loading dose infused over 5 min followed immediately by a maintenance infusion over 24–72 h (Supplementary Table 2). After 30 min of study drug infusion, additional therapy for PH was to be initiated if clinically indicated based on protocol-defined rules (see Supplementary Material). If the patient was judged clinically stable at 24 h or longer after randomization, the infusion was discontinued. The infusion of study drug continued for a minimum of 24 and maximum of 72 h.
Pharmacokinetics
Plasma sample extracts were analyzed using liquid chromatography and tandem mass spectrometry for separation and detection of the analytes. The overall method imprecision values for the analysis of plasma quality control samples at concentrations of 3, 30, and 350 ng/ml were 6.2%, 4.5%, and 6.1%, respectively, for sildenafil.
Statistics
Data are expressed as mean ± standard deviation (SD) if normally distributed or as median (range). Comparisons between groups were made with independent t tests and Wilcoxon tests for continuous variables, exact log-rank tests for times to event, and chi-square tests for frequencies. A p value <0.05 was considered significant.
Assuming that 75% of the placebo patients and 40% of patients at the highest sildenafil dose would require additional therapy within 24 h of the start of infusion, a sample size of 228 patients (57 per treatment arm) was required to detect this difference with 90% power with a one-sided type I error rate of 0.025 (corresponding to a two-sided level of 0.05), using a Hochberg adjustment for multiple comparisons that results in the minimum one-sided per-comparison error rate of 0.0085. Allowing for a postrandomization withdrawal rate of 10%, 252 patients were required to be randomized.
Endpoints
The primary endpoint was the receipt of any additional therapy for treatment of postoperative PH within 24 h of start of study drug infusion. The secondary endpoints were duration of mechanical ventilation and length of postoperative hospital stay. Tertiary endpoints included total duration of administration of additional therapy for PH, change from baseline in postoperative inotrope scores at postbaseline assessment times, length of stay in an intensive care unit (ICU), and deaths within 28 days of follow-up or during hospital stay. Also, change from baseline in serum lactate levels and change from baseline in hemodynamic parameters (PAP, left atrial, right atrial/central venous pressure) were determined.
Results
Twenty-seven centers worldwide participated in the study, but only six centers in France and the USA randomized patients. The sponsor terminated the study after 15 months owing to slow patient accrual. Of 87 patients screened who provided informed consent, 18 (21%) had postoperative PH and were randomized. No neonates and infants less than 60 days old were enrolled because sildenafil dosing for these patients remained undetermined. One patient was withdrawn before treatment. The subsequent analysis was performed on the remaining 17 treated patients (Fig. 1).
Patient characteristics
The median age and weight were 5 months (range 3 months–14 years), and 5.2 kg (range 3.9–60 kg), respectively. There were eight females (Table 1). Seven had Down syndrome. Five patients received placebo, and four patients each received low-, medium-, and high-dose intravenous sildenafil (Fig. 1). The types of lesions are presented in Table 1.
Efficacy of sildenafil
Additional therapy after 30 min of protocol infusion and within the first 24 h was required by 2 of 5 (40%) placebo-treated and 2 of 12 (17%) sildenafil-treated patients (all doses combined, p = 0.330). No patient from the intravenous sildenafil high-dose group received additional therapy within 24 h of start of study drug. Nitric oxide was the most frequently received additional therapy. Median time to extubation was shorter for patients on sildenafil compared with placebo: 3 days (range 1–11 days) versus 8 days (range 4–11 days, p = 0.023; log rank) (Fig. 2a). Median time to first discharge from the hospital was 12 days in the sildenafil group and 21 days in the placebo group (p = 0.135) (Fig. 2b). Median time to first discharge from the ICU was shorter for sildenafil patients: 6 days (range 1–12 days) versus 15 days (range 5–15 days, p = 0.008) (Fig. 2c).
During the first 4 h of treatment, reduction in systolic PAP and mean PAP (mean ± SD) was greater with sildenafil than placebo: 46 ± 11 to 35 ± 6 mmHg versus 49 ± 12 to 49 ± 17 mmHg (p = 0.027) and 33 ± 10 to 23 ± 5 mmHg versus 37 ± 10 to 36 ± 15 mmHg (p = 0.055), respectively (Fig. 3, Supplementary Fig. 4). Reduction in diastolic PAP did not differ by treatment (21 ± 12 to 13 ± 7 mmHg versus 27 ± 11 to 15 ± 5 mmHg, p = 0.306) (Supplementary Fig. 4). Only minor reductions in systemic systolic and diastolic blood pressure were observed: 82 ± 14 to 79 ± 13 mmHg versus 72 ± 15 to 73 ± 11 mmHg (p = 0.658) and 49 ± 9 to 45 ± 7 mmHg versus 47 ± 9 to 43 ± 14 mmHg (p = 0.813), respectively (Fig. 3, Supplementary Fig. 4).
Other outcome measures
Eight of 17 treated patients, 5 on sildenafil (42%) and 3 on placebo (60%), received additional therapy up to day 28 of the follow-up. Last postbaseline serum lactate values ranged from 0.5 to 1.92 mmol/l (median 1.02 mmol/L) in the three sildenafil groups, and from 1.0 to 2.31 mmol/l (median 1.2 mmol/l) in the placebo group (p = 0.874). Similarly, the last postbaseline total inotrope score did not differ between sildenafil groups (median 10, range 5–32) and placebo (median 11.5, range 5–24.5; p = 0.909).
Safety
Adverse events were reported in 15 (88.2%) patients; none were treatment related.
No patients experienced significant hemodynamic compromise while on sildenafil infusion. There were no differences in mean arterial, left atrial, and central venous pressure between placebo and sildenafil patients. No patient experienced a clinically relevant decrease in oxygenation as measured by pulse oximetry and reported as an adverse event during infusion of study drug.
Four patients died. Two patients died before randomization and the cause of death was related to their congenital heart disease. Two additional patients from the placebo group died after randomization. One 6-month-old female with Down syndrome and ventricular septal defect experienced respiratory distress 19 days after surgical closure of the defect. The second postoperative death occurred in a 14-year-old male who experienced a complicated postoperative course after mitral valve repair with refractory PH and eventually died from fungal sepsis on postoperative day 64.
Discussion
In the present study, intravenous sildenafil lowered PAP and decreased morbidity by shortening the time to extubation and length of ICU stay in a small pediatric population sample undergoing corrective heart surgery. Despite the benefit of early correction and improvements with intra- and postoperative care, postoperative PH still occurs. In a recent survey, an estimated 2% of pediatric patients undergoing congenital heart surgery experienced severe postoperative PH [19]. In our study, we found that, in a selected pediatric population considered at risk for PH, 21% experienced postoperative PH.
Interestingly, a high proportion (7 out of 17) of our study patients had Down syndrome. It is well known that patients with Down syndrome exhibit an abnormal propensity for postoperative PH after congenital heart surgery. Patients with Down syndrome are readily overrepresented in studies of postoperative PH, accounting for 44–60% of cases [15, 19]. Specific features associated with PH in Down syndrome include alveolar hypoplasia and thickness of small pulmonary arteries [20]. To date, no specific study has addressed use of sildenafil in postoperative PH associated with Down syndrome. However, management and treatment of such patients with pulmonary vasodilators do not differ and carry results similar to those of comparable patients without chromosomal aberrations [19]. Finally, the true incidence of postoperative PH may be underestimated in this study for the following reasons: first, some patients with life-threatening postoperative PH were emergently treated with pulmonary vasodilators or ECMO and could not be randomized to the study; second, neonates and infants aged <60 days, traditionally accounting for the majority of postoperative PH cases (particularly following repair of transposition of the great arteries, truncus arteriosus, and total anomalous pulmonary venous drainage) [19, 20], were not included.
Four deaths were reported, two before randomization and two in the placebo group, suggesting that children potentially at risk for postoperative PH in the present study represent an at-risk population for congenital heart surgery. However, the risk of postoperative PH cannot be ascertained from this small and selected population sample. Nevertheless, postoperative PH still carries significant morbidity and even mortality. In a recent study, among 100 patient deaths after congenital heart surgery, PH was the fifth most frequent etiology, accounting for 8% of the deaths [21]. This is also in agreement with the results of Lindberg and colleagues, who reported 7.4% mortality within 30 days postoperatively, and 11% mortality within 1 year in children who underwent cardiac operations and had severe postoperative PH, despite the use of specific therapy, including iNO [19]. This further emphasizes the need for an optimal strategy to anticipate and treat postoperative PH.
Our results show that intravenous sildenafil effectively decreased systolic PAP. However, oral sildenafil is also often effective [9–11], despite unpredictable bioavailability [12]. Diminished visceral blood flow and gastrointestinal motility as well as use of opioids can reduce the absorption of sildenafil, especially after cardiopulmonary bypass. Whether the intravenous form is potentially more effective than oral administration remains undetermined. Similarly, we cannot conclude from the present study if the intravenous form is associated with more frequent adverse events.
Unlike previous reports investigating intravenous sildenafil after pediatric cardiac surgery [14, 15, 22], this is the first study to explore sildenafil as an initial treatment for nonacute postoperative PH. The use of a bolus followed by a maintenance dose for a maximum of 72 h was specifically designed to treat postoperative PH. The administration of intravenous sildenafil at any dose was not associated with any clinically relevant adverse events. No significant systemic vasodilatation occurred compared with placebo. The results indicate that intravenous sildenafil, administered as a bolus dose followed by a maintenance dose, seems well tolerated in this patient population.
The major limitation of this study was the premature closure of the trial, prompted by slow accrual of patients with demonstrable PH. Contributing to the slow accrual was the exclusion of patients who were emergently treated with a pulmonary vasodilator or ECMO and of neonates and infants aged <60 days, who were not included. Of the patients enrolled preoperatively, 21% had significant untreated PH upon reaching the postoperative ICU. Consequently, 1,086 patients would have needed to be enrolled to achieve the sample size estimate of 228. Given the known difficulties in executing pediatric trials and the slow accrual rate (18 patients in 15 months), the original assumptions made the target enrollment number appear unattainable. However, in retrospect, the treatment effect was larger than anticipated, so that a positive trial may have been seen with far fewer patients. These observations may have important bearing on planning future studies on postoperative PH. Nonetheless, with such a limited number of patients, we could not show convincing efficacy, nor could we determine the optimal intravenous sildenafil dose. However, the absence of any clinically relevant adverse events associated with its efficacy on PAP (including any important change in continuously monitored pulse oximetry) suggests that use of intravenous sildenafil is relatively safe at the three doses we used. The absence of clinically relevant hypoxemia suggests that the potential adverse impact on intrapulmonary shunt was not clinically significant, although the fact that blood gas measurements and arterial PO2 were not regularly recorded represents a significant study limitation in this regard. No firm conclusion can be made about the impact of intravenous sildenafil on gas exchange. Further studies are needed to determine the optimal dose needed to decrease pulmonary vascular resistance while still avoiding significant adverse events.
References
Wessel DL (2006) Postoperative treatment of pulmonary hypertension. In: Beghetti M, Barst RJ, Naeije R, Rubin LJ (eds) Pulmonary arterial hypertension related to congenital heart disease. Munich, Elsevier, Urban & Fischer, pp 143–176
Hopkins RA, Bull C, Haworth SG, de Leval MR, Stark J (1991) Pulmonary hypertensive crises following surgery for congenital heart defects in young children. Eur J Cardiothorac Surg 5:628–634
Wessel DL, Adatia I, Giglia TM, Thompson JE, Kulik TJ (1993) Use of inhaled nitric oxide and acetylcholine in the evaluation of pulmonary hypertension and endothelial function after cardiopulmonary bypass. Circulation 88:2128–2138
Wessel DL (2001) Current and future strategies in the treatment of childhood pulmonary hypertension. Prog Pediatr Cardiol 12:289–318
Barnett CF, Machado RF (2006) Sildenafil in the treatment of pulmonary hypertension. Vasc Health Risk Manag 2:411–422
Atz AM, Adatia I, Wessel DL (1996) Rebound pulmonary hypertension after inhalation of nitric oxide. Ann Thorac Surg 62:1759–1764
Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM (1998) Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol 159:2164–2171
Sanchez LS, de la Monte SM, Filippov G, Jones RC, Zapol WM, Bloch KD (1998) Cyclic-GMP-binding, cyclic-GMP-specific phosphodiesterase (PDE5) gene expression is regulated during rat pulmonary development. Pediatr Res 43:163–168
Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G (2005) Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353:2148–2157
Atz AM, Lefler AK, Fairbrother DL, Uber WE, Bradley SM (2002) Sildenafil augments the effect of inhaled nitric oxide for postoperative pulmonary hypertensive crises. J Thorac Cardiovasc Surg 124:628–629
Atz AM, Wessel DL (1999) Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology 91:307–310
Nichols DJ, Muirhead GJ, Harness JA (2002) Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol 53:5S–12S
Lammers AE, Haworth SG, Pierce CM (2006) Intravenous sildenafil as an effective treatment of pulmonary hypertensive crises during acute intestinal malabsorption. Cardiol Young 16:84–86
Schulze-Neick I, Hartenstein P, Li J, Stiller B, Nagdyman N, Hubler M, Butrous G, Petros A, Lange P, Redington AN (2003) Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation 108:II167–II173
Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS (2003) Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Med 29:1996–2003
Wernovsky G, Wypij D, Jonas RA, Mayer JE Jr, Hanley FL, Hickey PR, Walsh AZ, Chang AC, Castaneda AR, Newburger JW, Wessel DL (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235
Mukherjee A, Dombi T, Wittke B, Lalonde R (2009) Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther 85:56–63
Steinhorn RH, Kinsella JP, Pierce C, Butrous G, Dilleen M, Oakes M, Wessel DL (2009) Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr 155:841–847 (e841)
Lindberg L, Olsson AK, Jogi P, Jonmarker C (2002) How common is severe pulmonary hypertension after pediatric cardiac surgery? J Thorac Cardiovasc Surg 123:1155–1163
Bando K, Turrentine MW, Sharp TG, Sekine Y, Aufiero TX, Sun K, Sekine E, Brown JW (1996) Pulmonary hypertension after operations for congenital heart disease: analysis of risk factors and management. J Thorac Cardiovasc Surg 112:1600–1607; discussion 1607–1609
Ma M, Gauvreau K, Allan CK, Mayer JE, Jenkins KJ (2007) Causes of death after congenital heart surgery. Ann Thorac Surg 83:1438–1445
Nagdyman N, Fleck T, Bitterling B, Ewert P, Abdul-Khaliq H, Stiller B, Hubler M, Lange PE, Berger F, Schulze-Neick I (2006) Influence of intravenous sildenafil on cerebral oxygenation measured by near-infrared spectroscopy in infants after cardiac surgery. Pediatr Res 59:462–465
Acknowledgments
The authors would like to acknowledge Dr. Robyn J. Barst, for her advice during the conduct of the study; the participating investigators, Dr D. Dunbar Ivy, Denver, CO, USA; Dr. John R. Charpie, Ann Arbor, MI, USA; Dr. George E. Reynolds, Omaha, NE, USA; and Dr. Phillipe Pouard, Paris, France for their involvement with the study; and Dr. Camilla Chong, Dr. Colin Ewen, Dr. Marjana Serdarevic-Pehar for their role in the daily management of the study. This study was funded by Pfizer Inc. Editorial support was provided by Janet E. Matsuura, PhD at Complete Healthcare Communications, Inc., and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Fraisse, A., Butrous, G., Taylor, M.B. et al. Intravenous sildenafil for postoperative pulmonary hypertension in children with congenital heart disease. Intensive Care Med 37, 502–509 (2011). https://doi.org/10.1007/s00134-010-2065-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2065-4