Abstract
Purpose
Ventilation problems are common in critically ill patients with intra-abdominal hypertension. The aim of this study was to investigate the effects of preserved spontaneous breathing during mechanical ventilation on hemodynamics, gas exchange, respiratory function and lung injury in experimental intra-abdominal hypertension.
Methods
Twenty anesthetized pigs were intubated and ventilated for 24 h with biphasic positive airway pressure without (BIPAPPC) or with additional, unsynchronized spontaneous breathing (BIPAPSB). In 12 animals, intra-abdominal pressure was increased to 30 mmHg for two 9 h periods followed by a 3 h pressure relief each. Eight animals served as controls and were ventilated for 24 h. Hemodynamics, gas exchange and respiratory mechanics were measured and lung injury was determined histologically.
Results
Intra-abdominal hypertension caused significant impairment of hemodynamics and respiratory mechanics in both modes. In the presence of intra-abdominal hypertension, BIPAPSB did not demonstrate superior respiratory mechanics and cardiovascular stability as compared to BIPAPPC. Although the decrease of dynamic compliance and the increase of airway pressures were mitigated, BIPAPSB failed to lower pulmonary vascular resistance and caused increased dead space ventilation (p = 0.007). Blood pressures and cardiac output increased in BIPAPSB, caused by an increase in heart rate (p < 0.001), but not in stroke volume (p = 0.06). BIPAPSB was associated with an increased breathing effort, decreased transpulmonary pressure during inspiration and lower lobe diffuse alveolar damage (p = 0.002).
Conclusions
In the presence of severe intra-abdominal hypertension, the addition of unsupported spontaneous breaths to BIPAP did not improve hemodynamic and respiratory function and caused greater histopathologic damage to the lungs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intra-abdominal hypertension (IAH) is an under-recognized condition in critically ill patients. IAH may result from increased intra-abdominal volume or a decreased abdominal wall compliance in the course of abdominal bleeding, peritonitis or trauma and may lead to abdominal compartment syndrome [1], a life-threatening complication with a mortality rate up to 50% [2–5]. The most severe form of IAH grade 3 consistent with abdominal compartment syndrome includes a sustained intra-abdominal pressure (IAP) > 20 mmHg associated with newly developed organ dysfunction or failure [3, 6–8]. IAH does not only impair intestinal organ perfusion but also compromises chest wall movement [9], causing worsening of lung mechanics and gas exchange. Post mortem histological examinations have revealed tissue necrosis and loss of organ integrity after untreated IAH [10]. Depending on the severity of IAH, only decompressive laparotomy can save organ function [4, 5, 11, 12], but might induce reperfusion injury [13, 14].
Respiratory mechanics and gas exchange are influenced by the way mechanical ventilation is applied. It has been demonstrated in various models of acute lung injury [15–17] that preserved spontaneous breathing activity during mechanical ventilation improves gas exchange as compared to controlled mechanical ventilation. The addition of unsupported spontaneous breathing during biphasic positive pressure ventilation (BIPAP) had resulted in an increase of transpulmonary pressure without increasing airway pressures, in recruitment of collapsed lung and in improvement of ventilation/perfusion distributions [17] [16]. Further beneficial effects of BIPAP ventilation were decreased intrathoracic pressures, better perfusion of the intestine and reduced sedation requirements contributing to hemodynamic stabilization [17, 18]. The diaphragm is relaxed during controlled mechanical ventilation, leading to abdominal displacement predominately in the ventral regions. Active contractions of the diaphragm during spontaneous breathing thus improve ventilation to the dorsal regions. The deleterious effects of IAH have been shown to potentiate dorsal atelectasis formation [19]. We tested the hypothesis that preserved spontaneous breathing activity during BIPAP attenuates the sequelae of IAH on hemodynamics, respiratory function and lung injury and improves gas exchange in a porcine model of repeated IAH. Some results of this investigation have been previously reported in the form of an abstract [20].
Methods and materials
After approval by the Institutional Animal Care Committee, the study was conducted in compliance with international guidelines [21].
Anesthesia was induced in 20 male domestic pigs (46.3 ± 3.3 kg) and maintained with continuous infusions of ketamine and pentobarbital. Ten animals each were randomized to receive biphasic intermittent positive airway pressure without (BIPAPPC) or with unsupported spontaneous breathing (BIPAPSB). In principle, BIPAP without spontaneous breathing activity is equal to pressure controlled ventilation. The high pressure level serves as pressure controlled and time-cycled mechanical breath, the low pressure level as positive end-expiratory pressure (PEEP). During BIPAPSB, unsupported breathing is possible on both levels. In each group, six animals were submitted to increased abdominal pressure and four animals served as controls. The absence of respiratory effort during BIPAPPC was confirmed by monitoring the esophageal pressure trace.
After orotracheal intubation with an 8.0 mm ID cuffed tube, the inspiratory oxygen fraction (FiO2) was set to 0.3 and the lower pressure level to 8 cmH2O. The high pressure was set to achieve a tidal volume of 8 ml/kg BW with an I:E ratio of 1:1.4 and the respiratory rate adjusted to a PaCO2 of 40–45 mmHg (Evita IV, Draeger Medical, Luebeck, Germany). The sedation in BIPAPSB was then reduced to allow for interfaced unsupported spontaneous breathing as seen on the esophageal pressure trace and titrated against spontaneous movements of the limbs or head. After induction of IAH, the upper level was adjusted in both groups to maintain tidal volume. Intravenous fluids were administered at 10 ml/kg/h, as this was necessary to maintain stable hemodynamics in a 24-h model of IAH found in previous investigations [22]. Blood pressures were transduced via catheters inserted into the femoral vessels. Cardiac output and stroke volume were calculated from a pulmonary artery catheter inserted via the jugular vein by pulmonary artery thermodilution (A/S3; Datex-Ohmeda, Duisburg, Germany). Arterial and mixed venous blood samples were analyzed immediately (Abl510; Radiometer, Copenhagen, Denmark) and venous admixture calculated according to standard formulas [23]. Expired gases were collected and dead space ventilation (Vd/Vt) calculated from the Enghoff equation.
A balloon catheter (Bicore Inc. Irvine, USA) was placed in the esophagus as described previously [24]. Airway pressures and flow were measured by a differential pressure transducer (CP100; Bicore). Flow and pressure signals were recorded and tidal volume, respiratory rate and airway pressures were averaged over ten breaths. Dynamic compliance was measured from the curves as described previously [25]. The total work of breathing (WOBt) was calculated by the pulmonary monitor as described by the manufacturer (Bicore). Transpulmonary pressures were calculated during inspiration and expiration as the difference between airway and esophageal pressures. IAH was controlled by insufflation of carbon dioxide by a CO2-insufflator (electronik-pneu, Storz, Tutlingen, Germany). IAP was defined as the superimposed pressure provided by the insufflator. Urine output was monitored by a Foley catheter inserted in the urinary bladder.
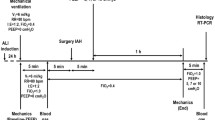
Experimental protocol
Baseline measurements were performed after instrumentation; then IAP was increased to 30 mmHg in the IAH- groups and kept constant for 9 h followed by a 3 h pressure relief to simulate decompression and reperfusion injury. The procedure was repeated once. Controls were ventilated for 24 h with unchanged settings.
Measurements were performed at 1, 5, 9, 11, 13, 17, 21 and 24 h after baseline measurements. Afterwards, animals were killed with a thiopentone overdose and biopsies were taken from dorsal and ventral lung, fixated in 10% buffered formaldehyde and stained with hematoxylin-eosin (HE). Histological damage, identified by a pathologist blinded to the underlying treatment (MA), was classified as none, mild, moderate, or severe (grade 1–4) for each of the following criteria: alveolar and interstitial edema, granulocytes, lymphocytes and erythrocytes, fibrinous exudate and microthrombi. An overall score (maximum 40) was calculated as the sum of sub-scores [26].
Statistical analysis
All data are reported as means ± SD. The data were normally distributed. The effects of IAH over time (within group) were tested with repeated measures analysis of variance (ANOVA). In case of significant results, a post hoc analysis was performed by Scheffe’s procedure. To test differences between groups, controls and IAH animals were analyzed separately. In the IAH groups, if no time effect was found, cumulative data from the zero pressure phases (hours 0,11,24) were compared with the pressure phases (hours 1,5,9,13,17,21) between groups by independent t-test. Statistical significance was accepted for p values less than 0.05 (SPSS WIN 10.0; SPSS Inc, Chicago, IL, USA).
Results
At baseline, there were no differences in hemodynamics, gas exchange or respiratory mechanics between controls and IAH animals and between BIPAPPC and BIPAPSB animals.
Controls without elevated abdominal pressure
Animals were stable over time and no significant changes could be observed in ventilation parameters and lung mechanics during 24 h of mechanical ventilation (supplementary material). BIPAPSB resulted in lower airway pressures and higher dynamic compliance (p < 0.001) (supplementary material, Table 1). Gas exchange remained unchanged in both groups, but PaCO2 and oxygen delivery were higher during BIPAPSB (supplementary material, Table 2). During BIPAPSB, cardiac output was higher and stroke volume increased (supplementary material, Table 3; Fig. 1). Also, pulmonary vascular resistance was lower in BIPAPSB (p = 0.019).
IAH group
Sedation medication was less during BIPAPSB (ketamine 3 ± 1 mg/kg/h; pentothal 3 ± 1 mg/kg.h) than during BIPAPPC (ketamine 5 ± 2 mg/kg.h; pentothal 8 ± 3 mg/kg.h) to facilitate spontaneous breathing (p < 0.001). All animals completed the end of the first phase of IAH. Hemodynamic instability in two cases and technical problems with the insufflator in one case precluded three animals in the BIPAPSB group from receiving the second phase of IAH. Their hemodynamic and respiratory data were excluded from the analysis of the respective period, but animals were ventilated until the end of the 24 h period. The nine remaining animals exhibited no differences between the changes in the first and second pressurization phase.
Hemodynamic results
Fluid administration was equal. Animals received 6 ± 1 ml/kg/h of crystalloid and 5 ± 1 ml/kg/h of colloid solutions in BIPAPPC and 6 ± 2 ml/kg/h of crystalloid and 5 ± 2 ml/kg/h of colloid solutions in BIPAPSB ventilation (p = 0.708 and p = 0.083, respectively).
With the onset of IAH, mean arterial and pulmonary pressures, central venous, pulmonary artery occlusion pressure and pulmonary vascular resistance increased significantly in all animals (Table 1). Only during BIPAPSB, cardiac output increased with IAH, caused by an increase in heart rate, but not in stroke volume (supplementary material, Fig. 2). No reduction in pulmonary vascular resistance was observed for BIPAPSB (Table 1). An increasing variability of cardiac output and heart rate at the end of both IAH phases was observed during BIPAPSB (supplementary material, Fig. 2). During the phases of pressure relief, BIPAPSB presented with more favourable pressures, but no difference in cardiac output (Table 1).
Respiratory and gas exchange results
Gas exchange parameters worsened during the IAH phases in both modes (Table 2) and were fully reversible after pressure relief. BIPAPSB was characterized by a smaller decrease in PaO2 and a greater increase in PaCO2 than BIPAPPC, but exhibited an equal increase in the venous admixture.
During the IAH phases, BIPAPSB ventilated animals demonstrated higher respiratory rates, minute ventilation and dynamic compliance than animals without spontaneous breathing. Additional spontaneous breathing accounted for 7% (during zero pressure phase) to 17% (during IAH phases) of total minute ventilation during BIPAPSB, contributing to an increased work of breathing (supplementary material, Fig. 4), which was not seen in controls (supplementary material, Fig. 3). Although spontaneous breathing was encountered, a considerable amount of this activity was interfaced, and not superimposed, with the mechanical breaths (Figs. 1, 2). During the IAH phase, the transpulmonary pressures were decreased with BIPAPSB (21 ± 11 vs. 29 ± 10, p = 0.003) in inspiration, and plateau pressures were higher with BIPAPPC (Table 3). The actual PEEP levels were higher with BIPAPSB during IAH (9.6 ± 0.8 vs. 8.2 ± 0.7, p < 0.05), but not during zero pressure phases. No differences were observed for the expiratory transpulmonary pressure (p = 0.224).
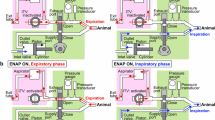
Representative tracings of airway pressure (P AW ), esophageal pressure (P ES ), flow and tidal volume (V T ) of an animal ventilated with biphasic positive airway pressure without spontaneous breathing (BIPAP PC ) during intra-abdominal hypertension with an intra-abdominal pressure of 30 mmHg. Respiratory rate 30 min−1 (P TP transpulmonary pressure. Flow and VT arbitrary units)
Representative tracings of airway pressure (P AW ), esophageal pressure (P ES ), flow and tidal volume (V T ) of an animal ventilated with biphasic positive airway pressure with spontaneous breathing (BIPAP SB ) during intra-abdominal hypertension with an intra-abdominal pressure of 30 mmHg. The mechanical respiratory rate is 31 min−1, additional spontaneous breaths are present with almost every machine breath. Interfaced spontaneous breathing activity accounts for additional minute ventilation (hatched areas). (P TP transpulmonary pressure. Flow and V T arbitrary units)
Histopathological analysis
In control animals without IAH, only minor histologic changes were observed with total lung damage scores of 20.8 ± 6.2 (BIPAPPC) and 21.4 ± 6.5 (BIPAPSB) (p = 0.803).
In contrast, phases of IAH caused significant histological damage, which was more profound in all six BIPAPSB ventilated animals (p < 0.002 vs. BIPAPPC) (Fig. 3; Table 4). The histological damage was higher graded in the lower lobe than in the upper lobe (p = 0,015) after BIPAPSB ventilation, but not after BIPAPPC (p = 0.131) (supplementary material, Fig. 5).
Discussion
In this study the effects of preserved spontaneous breathing activity on respiratory function and hemodynamics were investigated in the presence of severely elevated abdominal pressure. The addition of spontaneous breathing to BIPAP during experimental IAH had no beneficial effects on hemodynamics, gas exchange and lung protection, in contrast to previous investigations in acute lung injury.
IAH caused by peritonitis, pancreatitis, bowel obstruction, sepsis, burns or trauma is a frequent, but often under-recognized problem in critically ill patients. Furthermore, elevated abdominal pressures exert adverse effects on cardiac, pulmonary and renal function. Serial measurements of IAP in patients at risk of developing abdominal compartment syndrome have been recommended [1–3, 27–29] but no recommendations as to ventilatory management have been made. We have used a model of hyperacute IAH, which is rarely seen in patients, where IAH develops over several hours as a consequence of excess fluid in the abdomen. However, temporary relief by surgery and secondary relapse of IAH is a condition not infrequently observed. IAP > 25 mmHg has been reported to happen in 5–32% of trauma or liver transplant patients [3]. We could demonstrate that the consequences of IAH on respiratory mechanics were fully reversible after 9 h and after 24 h, but damage occurred to the lungs. It is not known whether the consequences of IAH on respiratory mechanics are different whether a model of excess fluid or gas insufflation is used. There is a difference in the way pressure is relieved: Gas can be evacuated, whereby laparostomy only is able to decrease IAP rapidly in abdominal edema. From our clinical experience, patients with IAH react to laparostomy with improvements of respiratory mechanics in a way similar to the observations made in this study.
We used a model of capno-peritoneum, since it is easy to deploy, does not interfere with fluid status and has been studied in animals and humans undergoing laparoscopic operations [30]. Similar to clinical conditions, the CO2 will be re-absorbed, although this mechanism might be impaired in conditions of increased abdominal pressure [31]. In contrast to previous investigations without elevated IAP [17], BIPAP ventilation with the addition of spontaneous breathing did not facilitate better CO2 elimination than controlled ventilation. In the presence of IAH, despite an increase in minute ventilation, dead space ventilation and PaCO2 were significantly higher during BIPAPSB. Previous investigations from our group performed in a porcine lung injury model showed similar results [16].
Oxygenation deteriorated during IAH with controlled ventilation, which can also be observed in clinical situations. Basal compression atelectasis, leading to an increase in venous admixture, is the likely reason. During BIPAPSB, no such deterioration in oxygenation occurred, despite increased venous admixture. We have struggled to explain improved oxygenation without reduction in intrapulmonary shunt during BIPAP ventilation before. Using the MIGET methodology to calculate ventilation-perfusion distributions, we were able to show that the improvements in oxygenation during BIPAPSB were mostly attributed to an increase of blood flow through non-shunted lung regions [18]. This was associated by an increase in cardiac output facilitating higher oxygen delivery.
We speculate that increased cardiac output as represented by a higher oxygen delivery caused the better oxygenation during BIPAPSB ventilation. Although it is likely that atelectasis formation was more pronounced in BIPAPSB, the resolution of atelectasis as found on CT correlates only poorly with oxygenation improvement [32, 33]. Instead, respiration mechanics better represented the condition of the lungs in acute lung injury.
During BIPAPSB, the decrease of dynamic compliance in the phases of IAH was less dramatic than with BIPAPPC. Importantly, this was associated with an increase of total work of breathing and inspiratory pressures in both groups. Mechanical ventilation in critically ill patients is associated with increased intrathoracic pressures, impaired hemodynamics and the need for deep sedation. As it has been shown in experimental and clinical studies, these adverse effects are less if spontaneous breathing efforts during mechanical ventilation are present. The blood flow through the intestine is improved [34, 35]. The reduced need for sedation will mitigate the direct circulatory depression caused by sedative drugs [36].
These beneficial attributes of preserved spontaneous breathing during mechanical ventilation have stimulated us to investigate BIPAPSB in intra-abdominal hypertension, a condition itself associated with decreased organ perfusion and altered respiratory mechanics [22]. Interestingly, the behaviour of hemodynamic changes in the presence of IAH was different from those found during normal intra-abdominal pressures. In fact, despite higher blood pressures and cardiac output, BIPAPSB ventilated animals were more unstable, as characterized by a higher heart rate and a greater variability of cardiac output and arterial pressures. We speculate that the increased work of breathing, which is considerably higher during BIPAPSB than during other modes of partial ventilatory assistance [16, 37, 38] has caused this. The complex mechanical interactions at the diaphragm are greatly influenced by abdominal pressure. If the chest wall is regarded as a parallel of spring resistors, the chest wall elastance (EW) is increasingly determined by the diaphragmal elastance (ED) with rising abdominal pressure, since the elastance of the thoracic cage (ETH) is low:
With increasing abdominal pressures, the total chest wall compliance becomes mainly dependent on the diaphragmal compliance [39]. The increasing trans-diaphragmal work necessary to facilitate diaphragmal movements might therefore outweigh the benefits from preserved spontaneous breathing. Direct interactions of diaphragmal activity with the heart, being in close proximity, are also possible [29]. The increased oxygen consumption in BIPAPSB and the mechanical irritation to the breathing apparatus were sufficient to destabilize the animals, not in the short-term, but during prolonged and repeated IAH. The histopathological analysis revealed more injury and collapse after BIPAPSB (Table 4), which has been demonstrated in similar experiments with histological organ damage as a result of disturbed microcirculation [22]. Dorsal diaphragmal movement was not able to attenuate atelectasis formation and lung damage in the lower lobe. This resulted in more inflammation and lung damage than in animals without preserved spontaneous breathing. A major mechanism seems to be a decrease in transpulmonary pressure during inspiration, which might have been caused by paradoxical movements of the diaphragm towards the chest, but not the abdomen. Direct visualization of the diaphragm by fluoroscopy would have been an advantage to explain the unanticipated results of this study and should be used for future investigations.
Could BIPAPSB have been employed differently? Most investigations, including those from our group, have demonstrated improved gas exchange and hemodynamics with the addition of spontaneous breathing to BIPAP in the way it was done. It is questionable, whether the small addition of breathing effort would exert such a huge effect on lung stretch and strain. More likely, the massive pressure from the abdomen could not be counterbalanced by muscular effort, resulting in fatigue, de-synchronization and instability. We designed the experiment with comparable tidal volumes and pressures, but failed to balance minute ventilation. Comparable minute ventilation could only be achieved by further reducing respiratory rate, leaving more room for spontaneous breathing and destabilization and the potential for worsening respiratory acidosis. We speculate that in doing so, the outcome would have been even worse. It seems that reducing the (intra-thoracic) airway pressure support for the diaphragm against intra-abdominal pressure is contra-productive in severe IAH.
In conclusion, IAH of 30 mmHg resulted in impairment of respiratory mechanics, gas exchange and hemodynamics in BIPAP-ventilated animals with and without spontaneous breathing. Although respiratory mechanics and gas exchange seemed less affected during BIPAPSB ventilation, adverse effects counterfeited the potential benefits of added spontaneous breathing. BIPAPSB was characterized by a higher work of breathing, decreased transpulmonary pressures and deteriorating hemodynamics towards the end of the intra-abdominal hypertension phases and a higher degree of histopathologic lung injury. This study could not demonstrate a superiority of adding unsupported spontaneous breathing to BIPAP in the presence of severe intra-abdominal hypertension.
References
Vidal MG, Ruiz Weisser J, Gonzalez F, Toro MA, Loudet C, Balasini C, Canales H, Reina R, Estenssoro E (2008) Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med 36:1823–1831
Reintam A, Parm P, Kitus R, Kern H, Starkopf J (2008) Primary and secondary intra-abdominal hypertension-different impact on ICU outcome. Intensive Care Med 34:1624–1631
Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Frank G, de Souza P, Cesana B, Gattinoni L (2005) Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 33:315–322
Ivatury RR, Diebel L, Porter JM, Simon RJ (1997) Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am 77:783–800
De Waele JJ, Hoste EA, Malbrain ML (2006) Decompressive laparotomy for abdominal compartment syndrome: a critical analysis. Crit Care 10:R51
Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Johansson K, Kolkman K, Wilmer A (2006) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome, I: definitions. Intensive Care Med 32:1722–1732
Moore AF, Hargest R, Martin M, Delicata RJ (2004) Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg 91:1102–1110
Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, D’Amours S, Wendon J, Hillman K, Wilmer A (2007) Results from the international conference of experts on intra-abdominal hypertension and abdominal compartment syndrome, II: recommendations. Intensive Care Med 33:951–962
Quintel M, Pelosi P, Caironi P, Meinhardt JP, Luecke T, Herrmann P, Taccone P, Rylander C, Valenza F, Carlesso E, Gattinoni L (2004) An increase of abdominal pressure increases pulmonary edema in oleic acid-induced lung injury. Am J Respir Crit Care Med 169:534–541
Toens C, Schachtrupp A, Hoer J, Junge K, Klosterhalfen B, Schumpelick V (2002) A porcine model of the abdominal compartment syndrome. Shock 18:316–321
Balogh Z, McKinley BA, Cox CS Jr, Allen SJ, Cocanour CS, Kozar RA, Moore EE, Miller CC III, Weisbrodt NW, Moore FA (2003) Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock 20:483–492
Ertel W, Oberholzer A, Platz A, Stocker R, Trentz O (2000) Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med 28:1747–1753
Schein M, Ivatury R (1998) Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg 85:1027–1028
Diebel LN, Dulchavsky SA, Brown WJ (1997) Splanchnic ischemia and bacterial translocation in the abdominal compartment syndrome. J Trauma 43:852–855
Wrigge H, Zinserling J, Neumann P, Muders T, Magnusson A, Putensen C, Hedenstierna G (2005) Spontaneous breathing with airway pressure release ventilation favors ventilation in dependent lung regions and counters cyclic alveolar collapse in oleic-acid-induced lung injury: a randomized controlled computed tomography trial. Crit Care 9:R780–R789
Henzler D, Pelosi P, Bensberg R, Dembinski R, Quintel M, Pielen V, Rossaint R, Kuhlen R (2006) Effects of partial ventilatory support modalities on respiratory function in severe hypoxemic lung injury. Crit Care Med 34:1738–1745
Putensen C, Rasanen J, Lopez FA (1994) Ventilation-perfusion distributions during mechanical ventilation with superimposed spontaneous breathing in canine lung injury. Am J Respir Crit Care Med 150:101–108
Henzler D, Dembinski R, Bensberg R, Hochhausen N, Rossaint R, Kuhlen R (2004) Ventilation with biphasic positive airway pressure in experimental lung injury: influence of transpulmonary pressure on gas exchange and haemodynamics. Intensive Care Med 30:935–943
Quintel M, Pelosi P, Caironi P, Meinhardt JP, Luecke T, Herrmann P, Taccone P, Rylander C, Valenza F, Carlesso E, Gattinoni L (2004) An increase of abdominal pressure increases pulmonary edema in oleic acid-induced lung injury. Am J Respir Crit Care Med 169:534–541
Bensberg R, Henzler D, Fackeldey V, Dembinski R, Rossaint R, Kuhlen R (2004) Influence of elevated abdominal pressure on lung mechanics and gas exchange during PCV with and without spontaneous breathing. Crit Care 8(Suppl):86
Drummond JC, Todd MM, Saidman LJ (1996) Use of neuromuscular blocking drugs in scientific investigations involving animal subjects, the benefit of the doubt goes to the animal. Anesthesiology 85:697–699
Schachtrupp A, Lawong G, Afify M, Graf J, Toens C, Schumpelick V (2005) Fluid resuscitation preserves cardiac output but cannot prevent organ damage in a porcine model during 24 h of intraabdominal hypertension. Shock 24:153–158
Berggren SM (1942) The oxygen deficit of arterial blood caused by non-ventilated parts of the lung. Acta Physiol Scand Suppl 4:4–92
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126:788–791
Bates JH, Rossi A, Milic-Emili J (1985) Analysis of the behavior of the respiratory system with constant inspiratory flow. J Appl Physiol 58:1840–1848
Dembinski R, Hochhausen N, Terbeck S, Uhlig S, Dassow C, Schneider M, Schachtrupp A, Henzler D, Rossaint R, Kuhlen R (2007) Pumpless extracorporeal lung assist for protective mechanical ventilation in experimental lung injury. Crit Care Med 35:2359–2366
Sugrue M, Jones F, Deane SA, Bishop G, Bauman A, Hillman K (1999) Intra-abdominal hypertension is an independent cause of postoperative renal impairment. Arch Surg 134:1082–1085
Schachtrupp A, Graf J, Tons C, Hoer J, Fackeldey V, Schumpelick V (2003) Intravascular volume depletion in a 24-hour porcine model of intra-abdominal hypertension. J Trauma 55:734–740
Kashtan J, Green JF, Parsons EQ, Holcroft JW (1981) Hemodynamic effect of increased abdominal pressure. J Surg Res 30:249–255
Sprung J, Whalley DG, Falcone T, Wilks W, Navratil JE, Bourke DL (2003) The effects of tidal volume and respiratory rate on oxygenation and respiratory mechanics during laparoscopy in morbidly obese patients. Anesth Analg 97:268–274
Blobner M, Bogdanski R, Kochs E, Henke J, Findeis A, Jelen-Esselborn S (1999) Visceral resorption of intra-abdominal insufflated carbon dioxide in swine. Anasthesiol Intensivmed Notfallmed Schmerzther 34:94–99
Henzler D, Pelosi P, Dembinski R, Ullmann A, Mahnken AH, Rossaint R, Kuhlen R (2005) Respiratory compliance but not gas exchange correlates with changes in lung aeration after a recruitment maneuver: an experimental study in pigs with saline lavage lung injury. Crit Care 9:R471–R482
Henzler D, Mahnken AH, Wildberger JE, Rossaint R, Günther RW, Kuhlen R (2006) Multislice spiral computed tomography to determine the effects of a recruitment maneuver in experimental lung injury. Eur Radiol 16:1351–1359
Santak B, Radermacher P, Sandmann W, Falke KJ (1991) Influence of SIMV plus inspiratory pressure support on VA/Q distributions during postoperative weaning. Intensive Care Med 17:136–140
Hering R, Peters D, Zinserling J, Wrigge H, von Spiegel T, Putensen C (2002) Effects of spontaneous breathing during airway pressure release ventilation on renal perfusion and function in patients with acute lung injury. Intensive Care Med 28:1426–1433
Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, Von Spiegel T, Mutz N (2001) Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 164:43–49
Calzia E, Lindner KH, Witt S, Schirmer U, Lange H, Stenz R, Georgieff M (1994) Pressure-time product and work of breathing during biphasic continuous positive airway pressure and assisted spontaneous breathing. Am J Respir Crit Care Med 150:904–910
Gama de Abreu M, Spieth PM, Pelosi P, Carvalho AR, Walter C, Schreiber-Ferstl A, Aikele P, Neykova B, Hübler M, Koch T (2008) Noisy pressure support ventilation: a pilot study on a new assisted ventilation mode in experimental lung injury. Crit Care Med 36:818–827
Henzler D, Hochhausen N, Dembinski R, Orfao S, Rossaint R, Kuhlen R (2007) Parameters derived from the pulmonary pressure volume curve, but not the pressure time curve, indicate recruitment in experimental lung injury. Anesth Analg 105:1072–1078
Acknowledgments
Institution where the work was performed: Department of Anesthesiology, University Hospital Aachen, Pauwelsstr. 30, D-52074 Aachen. We thank Dr. Mahmdouh Afifi, University Hospital Aachen, for the histopathologic analysis. The study was supported by the Medical Research Division of the German Armed Forces and the Faculty of Medicine, RWTH Aachen.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Henzler, D., Hochhausen, N., Bensberg, R. et al. Effects of preserved spontaneous breathing activity during mechanical ventilation in experimental intra-abdominal hypertension. Intensive Care Med 36, 1427–1435 (2010). https://doi.org/10.1007/s00134-010-1827-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1827-3