Abstract
Objective
To report on the recurrent release of charcoal from an intrapulmonary cavern in a case of acute respiratory failure after charcoal aspiration.
Design
Case report.
Setting
Anaesthesiological ICU, university hospital.
Patient
An 18-year-old ethanol intoxicated comatose patient regurgitated and aspirated activated charcoal during orotracheal intubation.
Treatment
After 2 days of mechanical ventilation, the patient was transferred to a tertiary care university hospital. On admission, acute respiratory distress syndrome with bilateral pulmonary infiltrations was diagnosed. The patient’s recovery was hampered by recurrent release of charcoal from an intrapulmonary cavern. Sophisticated ventilatory support, prone positioning, secretolytics, repetitive bronchoscopy, and antibiotic therapy may have facilitated bronchoalveolar clearance and weaning after 18 days.
Conclusion
Aspiration may be a dramatic complication if charcoal is administered in comatose patients without airway protection. In this case report, advanced intensive care measures were necessary to tackle the special feature of charcoal release from an intrapulmonary cavern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case
A physician-staffed emergency medical service team was called to treat an 18-year-old (190 cm, 75 kg) male patient who had ingested 0.75 l of vodka (270 g of ethanol, ethanol concentration in blood 3.2‰) and presented with Glasgow Coma Scale 3. Suspecting acute ethanol intoxication, the emergency physician placed a gastric tube and applied an unknown dose of activated charcoal. During subsequent endotracheal intubation, inadvertent regurgitation and aspiration of charcoal occurred. The patient was mechanically ventilated and transferred to a primary care hospital.
Case discussion
Activated charcoal should only be considered if a patient has ingested a toxic dose of a drug known to be adsorbed to charcoal [1, 2], e.g., carbamazepine, dapsone, phenobarbital, quinine, theophylline [3, 4], or citalopram [5] among others. Due to its high microporosity, activated charcoal has an exceptionally high surface area (>500 m2/g) and binds to the toxin and respective metabolites to prevent gastrointestinal absorption and/or interrupt the enterohepatic cycle. However, activated charcoal does not bind significantly to alcohols, glycols, ammonia, strong acids and bases, metals and most inorganics [6]. It is contraindicated after acid, alkali, or petroleum ingestion. Since misadventures with activated charcoal do mostly result from unprotected airways, excessive charcoal doses, and inappropriate aqueous dilution, therapy should strictly adhere to predefined safety recommendations [7].
Case
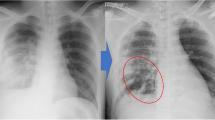
The patient developed acute respiratory distress syndrome and was transferred to our tertiary care university medical center on day 2 after aspiration in the following condition: mechanical ventilation via an orotracheal size 8.5-mm cuffed tube, peak inspiratory pressure (PIP) 22 cmH2O, positive end-expiratory pressure (PEEP) 14 cmH2O, I/E ratio 1:2, respiratory rate 16/min, respiratory minute volume 8 l/min, PaO2/FiO2 200 mmHg, PaCO2 59 mmHg, base excess 5 mmol/l, arterial pH 7.34, cardiac index 5.9 l/(min m2). Chest X-ray (Fig. 1) and thoracic CT scan (Fig. 2) revealed extensive bilateral pulmonary infiltrations, atelectases, diffuse alveolar damage, and mediastinal, dermal and soft tissue emphysema stretching out from the patient’s neck to his scrotum. In the lower left lobe a cavern of 107 × 42 × 85 mm containing an air–fluid level was noticed. Since the cavern was present on the day of admission, it is conceivable that it was pre-existent rather than formed by de novo genesis. Alpha1-antitrypsin plasma concentrations (258 mg/dl) were within normal limits. During bronchoscopy, the tracheobronchial systems appeared entirely coated with charcoal, and highly viscous bronchial secretions clogged the suction channel of the bronchoscope repeatedly. At this point, it was impossible to judge on the integrity of the tracheobronchial mucosa. A tracheal lesion could neither be confirmed, nor be ruled out as a potential origin of the soft tissue emphysema.
High-resolution thoracic CT scan on admission after acute charcoal aspiration. Two CT slices demonstrating diffuse pulmonary infiltrations and dorsobasal atelectases (arrows), mediastinal and soft tissue emphysema (dotted arrows), funnel chest, and a cavern (circle) of 107 × 42 × 85 mm in size (largest diameters) with a visible air-liquid level. Bronchoscopy verified that the cavern served as an intrapulmonary charcoal depository recurrently releasing its contents into the bronchoalveolar system, prolonging the exposure of bronchial mucosa to activated charcoal
Case discussion
Although activated charcoal is an inert substance, aspiration of charcoal is associated with pulmonary compromise due to increased microvascular permeability [8] with concomitant lung edema, surfactant depletion, atelectasis, and non-specific airway inflammation nurturing obliterative bronchiolitis [9, 10]. In addition, aspiration pneumonia is associated with increased ICU and total in-hospital stay [11]. Treatment of charcoal aspiration should include immediate intubation, mechanical ventilation with PEEP, and tracheal suctioning [12–15]; bronchodilators such as salbutamol may enhance mucociliary clearance and alveolar epithelial repair [16–18]. However, in the present case we were alarmed by the existence of a cavern in which charcoal was stored and recurrently released yielding a high viscosity bronchial secretion. Hence, we reckoned with prolonged pulmonary compromise with a high risk of persistent deterioration and the development of obliterative bronchiolitis.
Case
Pulmonary gas exchange deteriorated substantially within a few hours after admission. Peak inspiratory pressure was raised to 29 cmH2O, and PEEP to 20 cmH2O in a pressure-controlled biphasic positive airway pressure (BIPAP) ventilator mode: I/E ratio 1:2, respiratory rate 17/min, respiratory minute volume 10 l/min. This yielded a PaO2/FiO2 of 145 mmHg, PaCO2 of 59 mmHg, base excess of 6.6 mmol/l, and arterial pH of 7.36 at 12 h after admission.
We performed percutaneous dilative tracheostomy (tracheal tube size 9.0 mm) on the day of admission. During daytime, sedation was reduced [Richmond Agitation Sedation Scale (RASS) between 0 and −2] to permit assisted spontaneous breathing (BIPAP-ASB, spontaneous respiratory rate 12–17/min), to promote mucosal clearance and encourage voluntary expectoration in the conscious patient. Prone positioning [19] with pressure-controlled ventilation was carried out in the analgosedated patient every night (RASS −4 or −5). This was combined with abundant use of inhaled salbutamol and secretolytics, and daily bronchoscopic suctioning (for 8 days). Calculated antibiotic treatment was initiated on the day of admission and was switched once, as fever and plasma C-reactive protein (19 mg/dl) peaked on day 4 and declined successively thereafter. Repetitive microbiological evaluations of bronchial secretions, serum, urine and blood cultures were negative. Soft tissue emphysema regressed rapidly. Peak inspiratory pressure and PEEP were reduced as pulmonary gas exchange improved. A biopsy of the bronchial mucosa taken during bronchoscopy displayed dispersed charcoal deposition, vascular and fibroblastic proliferations and a dense inflammatory infiltrate (Fig. 3), compatible with severe organizing bronchitis. As of day 12, the patient was breathing via a T-tube for several hours a day, and was finally weaned on day 18. Contrast media bronchoscopy on day 20 located the cavern in segment 10 of the left lung and proved communication with the airway tract, supporting the notion of a charcoal depository. The patient continued to perform respiratory exercise and physiotherapy, was discharged on day 27 to a rehabilitation center, and made a good recovery thereafter.
Biopsy of bronchial mucosa demonstrating charcoal deposition. a Low power view of inflamed bronchial mucosa with deposition of finely dispersed charcoal fragments (arrows). Note mucosal covering by metaplastic squamous epithelium. b Medium power view of mucosal stroma with fibroblastic and vessel proliferations as well as interspersed mixed inflammatory infiltrate (arrows) corresponding to a granulating bronchial wall inflammation. Note small fragment of charcoal in the inflamed area (circle). c High power view of inflamed squamous epithelium with charcoal deposit (circle)
Case discussion
We present a case of prolonged exposure of the bronchoalveolar system to aspirated charcoal, which was recurrently released from an intrapulmonary cavern, resulting in acute respiratory distress syndrome with histological signs of organizing bronchitis. Specific treatment aimed at promoting bronchoalveolar mucosal clearance in the face of recurrent charcoal exposure and included
-
assisted spontaneous breathing,
-
pressure-controlled ventilation with prone positioning at night,
-
inhaled salbutamol and secretolytics,
-
regular bronchoscopic suctioning,
-
and calculated antibiotic therapy.
This case recalls that activated charcoal is not indicated to treat acute ethanol intoxication and must be used judiciously with adherence to specific safety measures.
References
Chyka PA, Seger D, Krenzelok EP, Vale JA (2005) Position paper: single-dose activated charcoal. Clin Toxicol (Phila) 43:61–87
Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, Hittarage A, Azher S, Jeganathan K, Jayamanne S, Sheriff MR, Warrell DA (2008) Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet 371:579–587
Heard K (2006) The changing indications of gastrointestinal decontamination in poisonings. Clin Lab Med 26:1–12 vii
American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists (1999) Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. J Toxicol Clin Toxicol 37:731–751
Isbister GK, Friberg LE, Duffull SB (2006) Application of pharmacokinetic–pharmacodynamic modelling in management of QT abnormalities after citalopram overdose. Intensive Care Med 32:1060–1065
Bansal RC, Goyal M (2005) Activated carbon adsorption. Taylor and Francis Ltd, London
Mauro LS, Nawarskas JJ, Mauro VF (1994) Misadventures with activated charcoal and recommendations for safe use. Ann Pharmacother 28:915–924
Arnold TC, Willis BH, Xiao F, Conrad SA, Carden DL (1999) Aspiration of activated charcoal elicits an increase in lung microvascular permeability. J Toxicol Clin Toxicol 37:9–16
Elliott CG, Colby TV, Kelly TM, Hicks HG (1989) Charcoal lung. Bronchiolitis obliterans after aspiration of activated charcoal. Chest 96:672–674
Visscher DW, Myers JL (2006) Bronchiolitis: the pathologist’s perspective. Proc Am Thorac Soc 3:41–47
Christ A, Arranto CA, Schindler C, Klima T, Hunziker PR, Siegemund M, Marsch SC, Eriksson U, Mueller C (2006) Incidence, risk factors, and outcome of aspiration pneumonitis in ICU overdose patients. Intensive Care Med 32:1423–1427
Arnold TC, Zhang S, Xiao F, Conrad SA, Carden DL (2003) Pressure-controlled ventilation attenuates lung microvascular injury in a rat model of activated charcoal aspiration. J Toxicol Clin Toxicol 41:119–124
Golej J, Boigner H, Burda G, Hermon M, Trittenwein G (2001) Severe respiratory failure following charcoal application in a toddler. Resuscitation 49:315–318
Pollack MM, Dunbar BS, Holbrook PR, Fields AI (1981) Aspiration of activated charcoal and gastric contents. Ann Emerg Med 10:528–529
Schreiber T, Hueter L, Gaser E, Schmidt B, Schwarzkopf K, Rek H, Karzai W (2006) PEEP has beneficial effects on inflammation in the injured and no deleterious effects on the noninjured lung after unilateral lung acid instillation. Intensive Care Med 32:740–749
Perkins GD, Gao F, Thickett DR (2008) In vivo and in vitro effects of salbutamol on alveolar epithelial repair in acute lung injury. Thorax 63:215–220
Bennett WD, Almond MA, Zeman KL, Johnson JG, Donohue JF (2006) Effect of salmeterol on mucociliary and cough clearance in chronic bronchitis. Pulm Pharmacol Ther 19:96–100
Broadley KJ (2006) Beta-adrenoceptor responses of the airways: for better or worse? Eur J Pharmacol 533:15–27
Easby J, Abraham BK, Bonner SM, Graham S (2003) Prone ventilation following witnessed pulmonary aspiration: the effect on oxygenation. Intensive Care Med 29:2303–2306
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Francis, R.C.E., Schefold, J.C., Bercker, S. et al. Acute respiratory failure after aspiration of activated charcoal with recurrent deposition and release from an intrapulmonary cavern. Intensive Care Med 35, 360–363 (2009). https://doi.org/10.1007/s00134-008-1259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1259-5