Abstract
Objective
To test the effects of high-frequency percussive ventilation (HFPV) compared with high-frequency oscillatory ventilation (HFOV) and low-volume conventional mechanical ventilation (LVCMV), on lung injury course in a gastric juice aspiration model.
Design
Prospective, randomized, controlled, in-vivo animal study.
Setting
University animal research laboratory.
Subjects
Forty-three New Zealand rabbits.
Interventions
Lung injury was induced by intratracheal instillation of human gastric juice in order to achieve profound hypoxaemia (PaO2/FIO2 ≤ 50). Animals were ventilated for 4 h after randomization in one of the following four groups: HFPV (median pressure 15 cmH2O); LVCMV (VT 6 ml kg–1 and PEEP set to reach 15 cmH2O plateau pressure); HFOV (mean pressure 15 cmH2O); and a high-volume control group HVCMV (VT 12 ml kg–1 and ZEEP).
Measurements and results
Static respiratory compliance increased after the ventilation period in the HFPV, LVMCV and HFOV groups, in contrast with the HVCMV group. PaO2/FIO2 improved similarly in the HFPV, LVCMV and HFOV groups, and remained lower in the HVCMV group than in the three others. Lung oedema, myeloperoxidase and histological lung injury score were higher in the HVCMV group, but not different among all others. Arterial lactate markedly increased after 4 h of ventilation in the HVCMV group, while lower but similar levels were observed in the three other groups.
Conclusion
HFPV, like HFOV and protective CMV, improves respiratory mechanics and oxygenation, and attenuates lung damage. The HFPV provides attractive lung protection, but further studies should confirm these results before introducing HFPV into the clinical arena.
Similar content being viewed by others
Introduction
Conventional mechanical ventilation (CMV) represents life-threatening supportive care in the setting of acute respiratory distress syndrome (ARDS), but it can also promote harmful ventilator-associated lung injury. Since tidal volume is preferentially delivered to the most compliant units, large tidal ventilation could overdistend the less injured alveoli. On the other hand, severely injured alveoli with surfactant dysfunction are unstable and exposed to cyclic tidal opening and closing phenomenon when positive end-expiratory pressure (PEEP) is below critical alveolar closing pressure [1–4]. Both volutrauma and atelectrauma include a pro-inflammatory response to associated lung injury [5]. By minimizing alveolar distension (using tidal volume reduction) and by maintaining high end-expiratory lung volume (using adequately adjusted PEEP level), protective CMV decreases lung and systemic inflammatory responses and improves the outcome of ARDS patients [2, 6, 7].
Other ventilator strategies have emerged on the basis of an infraphysiological tidal volume delivered at high frequency. High-frequency oscillatory ventilation (HFOV) has been largely investigated in experimental and clinical settings. In animal studies, HFOV attenuates lung injury, improves oxygenation and appears to be as beneficial as CMV when compared with an open lung positive pressure ventilation [8–14]. In ARDS patients, the outcome was not modified with HFOV in two prospective, but underpowered, randomized controlled trials [15, 16].
Providing a unique respiratory pattern, high-frequency percussive ventilation (HFPV) is administered with a pneumatically powered volumetric diffusive respirator (VDR). A piston (Phasitron, Percussionaire, Bird Space Technologies, Sandpoint, Idaho), interposed before tracheal tube, pulses high-pressure gas at high frequency (200–900 cycle min–1. The injector/exhalation valve employs a sliding Venturi to deliver pulsed repetitive subtidal volume with adjustable inspiratory (i) and expiratory (e) duration. The maximum airway pressure varies cyclically, at low rate (10–15 cycle min–1), between two different levels. During the high-pressure cycle, named inspiratory cycle (I), the maximum value corresponds to the peak inspiratory pressure (PIP) and the minimum value is called continuous positive pressure ventilation (CPAP). During the low-pressure cycle, where gas washout from the lungs, so-called expiratory cycle (E), positive end expiratory pressure (PEEP) is the maximum pressure and CPAP is the minimal one. Differences between those two phases of a cycle depend on the variation of both the maximum and the amplitude pressure (maximum minus minimum), providing mainly for convective ventilation; thus, HFPV combines both high-frequency ventilation and convective MV potential advantages [17]. A number of clinicians have now reported their successful experience using HFPV, mainly in burn patients with smoke inhalation lung injury or ARDS patients [18–22]; however, both in experimental and human subjects, the morphological lung consequences of this ventilation are still not clearly defined.
The purpose of this study was therefore to investigate the effects of HFPV on lung parenchyma during the acute phase of gastric aspiration-induced lung injury. The HFPV was compared with two classically recognized protective ventilation strategies (low-volume CMV and HFOV) and with a ventilated lung-injured control group (high-volume CMV). End points of interest were oxygenation, pulmonary mechanics, lung pathology and cellular inflammatory response.
Materials and methods
Animal preparation
This study was approved by the animal ethics committee of the Université de la Méditerranée, Marseille, France, and the animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals [23]. Fifty-four pathogen-free New Zealand rabbits (Charles Rivers Laboratories, L'Abresles, France) weighing 2.1–3.5 kg were anaesthetized with intravenous ethylcarbamate (1 g kg–1). Oxygen supplementation by a nose cone and spontaneous breathing were maintained during tracheotomy procedure. The anterior neck was dissected after local anaesthesia (Lidocaine 1%) and a 4.0-mm internal diameter tracheal tube was inserted. Anaesthesia was sustained with ethylcarbamate (0.3 g kg–1 h–1) and muscle was paralyzed with cisatracurium (0.5 mg kg–1 followed by 1 mg kg–1 h–1). A 3-F catheter was inserted into the right femoral artery for monitoring and blood sampling. Central body temperature was monitored with a rectal probe and maintained at 38°C by a thermostatically controlled heating pad. Sterile conditions were maintained throughout the experiment. Fluid maintenance was provided by a continuous infusion of 0.9% saline solution containing 5% dextrose (6 ml kg–1 h–1). All animals were ventilated first with HFOV. Main initial settings were FIO2 of 0.21, frequency (f) of 15 Hz, inspiratory time (Ti) of 33%, pressure amplitude (Δ P) of 40 cmH2O and mean airway pressure (mPaw) of 7 cmH2O. These settings were assumed to minimize lung damage in rabbits prior to randomization [9].
Gastric juice preparation
Human gastric content was collected via a nasogastric tube from a single patient during the first 24-h period following initiation of mechanical ventilation. No anti-acid medication was administrated before gastric content collection. The collected volume was dispersed in 20-ml aliquots and immediately frozen at –80°C for storage. Bacteriological analyses of gastric juice, performed at collection and at the time of the experiment, were all negative. One hour before each experiment, a single aliquot was thawed. After homogenization, pH measurement was performed twice and averaged (Ecoscan, Eutech Instruments, Nijkerk, The Netherlands).
Induction of lung injury
After preparation, the first 1.5 ml kg–1 tracheal instillation was administered (half of the volume in each lung) with a single-lumen 4-F catheter. Animals were connected again to HFOV while FIO2 was increased to 1 and Δ P to 60 cmH2O. The other settings remained unchanged. Twenty minutes later, an arterial blood gas sample was obtained. If PaO2 was > 50 mmHg, subsequent 0.5 ml kg–1 gastric juice boluses were administered, using the same protocol, until injury end point was reached (i. e., PaO2/FIO2 ≤ 50 mmHg).
Experimental groups
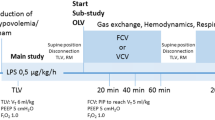
The study design is presented in Fig. 1. Thirty minutes after the last gastric juice instillation, the animals were randomly assigned to one of the following four groups, with initial settings as follows:
-
1.
HFPV: continuous positive airway pressure (CPAP) at 4 cmH2O; PEEP at 8 cmH2O; peak inspiratory pressure (PIP) at 25 cmH2O; frequency at 15 Hz with i/e of 1/1; and initial respiratory rate (RR) at 10 cycle min–1 with I/E of 1/1.
-
2.
LVCMV: tidal volume at 6 ml kg–1; PEEP set to achieve a 15-cmH2O plateau pressure; inspiratory to expiratory ratio (I/E) of 1/2 with an inspiratory pause of 10%; and RR at 60 cycle min–1.
-
3.
HFOV: f at 15 Hz; Ti at 33%; mPaw at 15 cmH2O; and Δ P at 60 cmH2O.
-
4.
HVCMV: tidal volume at 12 ml kg–1; zero end-expiratory pressure (ZEEP); I/E at 1/2 with an inspiratory pause of 10%; and RR at 30 cycle min–1.
Prior to initiating the assigned ventilation, a sustained inflation at 40 cmH2O was applied for 40 s. Animals which received CMV were ventilated with a Servo 900 C (Siemens Elema, Solna, Sweden). The HFOV was delivered with an OHF-1 (Dufour, Villeneuve d'Asc, France) and HFPV with a volumetric diffusive respirator (VDR-4, Percussionaire Corporation, Sandpoint, Idaho).
Haemodynamic and respiratory measurements
Heart rate and systemic arterial blood pressure were continuously monitored with a calibrated pressure transducer. Mean arterial pressure (MAP) was maintained above 65 mmHg with up to five 20 ml kg–1 rapid infusions of 0.9% saline solution, if required. Persistent hypotension, despite fluid loading, was treated by a continuous infusion of norepinephrine. Arterial blood gases were analysed with an ABL330 (Radiometer, Copenhagen, Denmark). Arterial lactate was determined using an YSI 1500 Sport (Yellow Springs Instruments, Yellow Springs, Ohio). Air flow was measured with a number 00 Fleisch pneumotacograph and tidal volume was obtained through integration of the air-flow signal. Plateau pressure (Pplat) was defined as the tracheal pressure measured at the end of a 3-s end-inspiratory pause in CMV. Total PEEP was measured after a 3-s end-expiratory pause at the tracheal cannula. In the HFPV group, median pressure was defined as follows: \( \text{Pmed}\,=\,\{[(\text{PIP}\,+\,\text{CPAP})/2]\,\times\,(\text{Ti}/\text{Ttot})\}\,+\,\{[(\text{PEEP}\break +\,\text{CPAP})/2]\,\times\,(\text{Te/Ttot})\}\;. \)
We consider the median pressure, corresponding at the mean pressure of both cycle corrected by the duration of the phase, to determine the continuous distending pressure. The characteristics of airway pressure, flow and volume waveforms observed with HFPV are represented in Fig. 2.
A static airway pressure-volume (PV) curve was obtained after the lung injury period (before randomization) and at the end of the experiment (after 4 h of ventilation). Briefly, a 50-ml syringe filled with pure oxygen was connected to the trachea after lung emptying to functional residual capacity. Repeated 5-ml inflated volume steps completed the PV relationship, each prolonged by a 2-s pause. Inspiratory compliance of the respiratory system (Crs) was calculated by linear regression analysis from the chord slope of the curve.
Tissue removal and lung processing
The animals were killed with 5% pentobarbital and midline sternotomy was performed. The intra-thoracic trachea was clamped at end inspiration before the heart and lungs were removed en bloc from the thoracic cavity. The general appearance of the lungs was graded according to the Kolobow scoring system (Table 1) [24]. The right lung was filled with formaldehyde 10%, at a transpulmonary pressure of 15 cmH2O, via a 3-F catheter and stored in formaldehyde 10% for delayed histopathology examination. The left lung was weighed before being processed for myeloperoxidase determination.
Measurement of myeloperoxidase activity
Myeloperoxidase (MPO) activity in lung tissue homogenates was measured spectrophotometrically. Briefly, 100 μl of left lung homogenate was added to 2.9 ml of a 1% weight-volume dimethoxybenzine solution and 1 mM hydrogen peroxide solution. Thirty minutes after incubation, the reaction was stopped by addition of 200 μl of 3 M HCL. Myeloperoxidase activity was measured by the change in absorbance at 410 nm and 37°C with a Spectronic Genesys 2 spectrophotometer (Milton Roy Company, Rochester, New York).
Histological examination
The right lung was serially sectioned in a coronal fashion from apex to base and four random sections were processed for morphometric qualitative analysis and embedded in paraffin. Each section was sliced to 5 μm and was stained with hematoxylin-eosin and Masson's trichrome before being examined blindly by two lung pathologists. Lung pathology was assessed on a five-point scale, according to the severity of alveolar exudates, alveolar haemorrhage, polymorphonuclear neutrophil (PMN) infiltrates in the air space and/or in alveolar wall, PMN interstitial infiltrates, interstitial oedema, and hyaline membrane formation. The following scale was used for grading: 0 = no or minimal damage; 1+ = mild damage; 2+ = moderate damage; 3+ = severe damage; and 4+ = maximum damage [25]. A composite lung injury score was calculated and expressed as the percentage of maximum damage corrected for the group's sample size.
Statistical analysis
Data were analysed with SigmaStat software (version 2.03, SPPS, Chicago, Ill.). Categorical variables were compared with chi-square test or Fischer's exact test. Distribution of continuous variables was assessed using a Kolmogorov–Smirnoff test. Continuous data are expressed as mean ± standard deviation (SD). One-way analyses of variance or non-parametric equivalent (Kruskal–Wallis test) were performed to compare data between groups with Dunn's comparison. Respiratory mechanics data were compared with a paired t-test before and after the ventilation period. Two-way analyses of variance for repeated measures (ANOVA RM) were performed to compare the data from treatment groups and time. Post-hoc analysis and all pair-wise comparisons were performed with a Tuckey test. Statistical significance was fixed at p < 0.05.
Results
Population
Eleven animals died of refractory shock before completing the study and were excluded from analysis (2 in HFPV, 2 in LVCMV, 4 in HFOV and 3 in HVCMV). Finally, each group included 11 animals except for HVCMV with 10.
Gastric juice requirements
The volume of gastric juice required to achieve lung injury end point did not differ between groups (animal body weight 2.3 ± 0.5ml kg–1 in HFPV, 2.3 ± 0.5 ml kg–1 in LVCMV, 2.1 ± 0.5 ml kg–1 in HFOV and 2.2 ± 0.6 ml kg–1 in HVCMV groups). Gastric juice pH was the equivalent in all aliquots (pH = 4.1 ± 0.3).
Gas exchange
The time course of PaO2/FIO2 is presented in Fig. 3. One hour after the initiation of ventilator strategies, PaCO2 was lower in the HVCMV group (35 ± 5 mmHg) than in both the HFPV (47 ± 11 mmHg) and LVCMV (51 ± 9 mmHg) groups (p < 0.01 and p < 0.001, respectively). Arterial pH decreased similarly in all four groups throughout the experiment and was lower after 4 h of ventilation when compared with baseline and lung-injury times (data not shown).
Time course of the PaO2/FIO2 ratio in the four groups; HFPV, high-frequency percussive ventilation; LVCMV, low-volume conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; HVCMV, high-volume conventional mechanical ventilation; Data are expressed as mean ± SEM. * p < 0.05 vs. baseline, # p < 0.05 vs. lung injury time, † p < 0.05 HVCMV vs. other three groups
Respiratory system mechanics
Respiratory compliance decreased in the HVCMV group (from 1.2 ± 0.2 ml kg–1 cmH2O–1 after lung injury to 1 ± 0.6 after 4 h of ventilation, p = NS). In contrast, respiratory compliance improved in all other groups (from 1 ± 0.3 to 1.5 ± 0.6 ml kg–1 cmH2O–1 for HFPV, from 1.2 ± 0.9 to 1.3 ± 1.1 ml kg–1 cmH2O–1 for LVCMV and from 1.1 ± 0.3 to 1.3 ± 0.5 ml kg–1 cmH2O–1 for HFOV, p < 0.05 for all comparisons). Post-ventilation respiratory compliances were not different in the three protective groups (Fig. 4). Respiratory settings are presented in Table 2.
Static inspiratory pressure-volume relationships after lung injury (white circles) and at the end of the study protocol (black circles). Values are expressed as mean ± SD. Note the shift to the left part of the curve after 4 h of ventilation for high-frequency percussive ventilation (HFPV), low-volume conventional mechanical ventilation (LVCMV), and high-frequency oscillatory ventilation (HFOV) groups. Inversely, a shift to the right was observed in the high-volume conventional mechanical ventilation (HVCMV) group between the two periods
Haemodynamic parameters
The MAP declined significantly in all groups when compared with baseline and post-injury values (data not shown); however, at the end of the study period, perfusion was greater in the HFPV (80 ± 17 mmHg) and the HFOV (77 ± 14 mmHg) groups than in the HVCMV (62 ± 22 mmHg) group (p < 0.02 and p < 0.05, respectively). There was no difference with the LVCMV group (76 ± 17 mmHg). Arterial lactate markedly increased at the fourth hour in the HVCMV group (7.8 ± 7.1 mmol l–1) and was significantly higher compared with the HFPV (3.7 ± 3.2 mmol l–1) and LVCMV (4.1 ± 3.2 mmol l–1) groups (p < 0.02 and p < 0.03, respectively), whereas the difference did not reach significance with the HFOV group (4.7 ± 2.1 mmol l–1). Norepinephrine requirement did not differ between groups: 8 animals in HFPV group (73%); 7 animals in LVCMV (64%); 9 animals in HFOV (82%); and 7 animals in HVCMV (70%). Fluid loading was also equivalent.
Lung weight and myeloperoxidase
Left lung weight was lower in the HFPV, LVCMV and HFOV groups than in the HVCMV group (3 ± 0.6, 2.7 ± 0.5, 3 ± 1.2 and 4.5 ± 1.4 g kg–1 body weight, respectively, p < 0.02 on ANOVA). MPO activity was reduced in the three protective groups as compared with the HVCMV group (Fig. 5).
General appearance and lung histopathology
Most of the animals in the HVCMV group presented very severe damage, whereas the animals in the three protective groups tended to present moderate or severe damage (Fig. 6; Table 1). The composite lung injury score was lower in the HFPV group than in the HVCMV group, as was the case for the two other protective strategies (Fig. 7).
Macroscopic posterior lung photographs (left panel) and corresponding right lung hematoxylin and eosin × 100 microphotographs (right panel) of representative animals; HFPV, high-frequency percussive ventilation; LVCMV, low-volume conventional mechanical ventilation; HFOV, high-frequency oscillatory ventilation; HVCMV, high-volume conventional mechanical ventilation. Note the importance of atelectasis (A) and/or congestion area (C) on macroscopic photographs and the polymorphonuclear cells infiltration (arrow) on microphotographs in the HVCMV group
Discussion
The present study shows that HFPV attenuates the severity of gastric aspiration-induced lung injury. The HFPV, as well as protective CMV and HFOV, enhanced pulmonary mechanics and oxygenation, and led to less histological damage when compared with high-volume CMV.
The HFPV-associated lung improvement observed in this study is not support by a solid physiopathological framework. Indeed, little is known about the gas-transport mechanism during HFPV. We speculated that it involved many phenomena, as in HFOV [26], including intrapulmonary diffusion during percussive high-velocity mixing and convection during time-cycled ventilation. Basically, tidal volume is the result of both the convective and non-convective parts of ventilation, but it remains below the physiological dead space [27]; therefore, we hypothesized that, as in HFOV, reducing tidal volume would minimize the proportion of overdistended alveoli and limit the cyclic tidal-associated stretch variation.
Obviously, what happens at the alveolar level is currently unknown, but it can be supposed that there is a considerable drop in airway pressure between the trachea and alveoli. The magnitude of intrathoracic percussive shock waves decrease throughout the bronchial airways with resistive loads. As a consequence, PIP and mPaw vary differently. Lucangelo et al., when increasing the impedance on a lung simulator, demonstrated larger variation in PIP (+ 70%) than in mPaw (+ 30%) [28]; hence, even if PIP was higher in our HFPV group than in both CMV groups, it could provide less barotrauma because of greater pressure attenuation and, significantly, it did not result in greater lung injury. In a 7-day primate model of moderate smoke injury, Cioffi et al. compared HFPV, HFOV and non-protective CMV with PIP values that were similar to those used in our study [29]. They reported that HFPV was associated with a decrease in lung damage and barotrauma. In the present study, we also demonstrated that HFPV attenuates lung oedema, histopathology and PMN infiltrates. Moreover, we noted limited haemodynamic side effects with regard to the arterial lactate course.
All of the tested protective strategies improved Crs when compared with post-injury measurements. Similar Crs increase may have reflected similar lung injury [30]. In contrast, Crs worsened in the HVCMV group. Myeloperoxidase, a marker of PMN activation, was reduced in the HFPV group, as well as in the HFOV and LVCMV groups, when compared with the HVCMV group. Similarly, histological results showed greater damage in the HVCMV group, again without differences in the other three groups; therefore, our HFPV setting was able to prevent worsening of lung injury after gastric aspiration. Our findings also corroborate previous studies which have emphasized the protective role of CMV and HFOV [8–14, 31]. Within the framework of lung injury, while ZEEP and high-tidal ventilation provided alveolar instability [32], limited alveolar stretch and/or overdistension, irrespective of ventilator modes, prevented further lung impairment [1, 4]. Nevertheless, given the lack of additional advantages of one of those three protective ventilations, neither of them should be prioritized.
The gastric aspiration-induced lung injury model is not widely used in the field of acute lung injury but offers many advantages. Aspiration pneumonitis is associated with heterogeneously dispersed injuries. Most other direct lung injury models have failed to achieve such a situation, providing rather diffuse alveolar collapse. In this study, all of the animals exhibited at least moderate damage, with heterogeneous macroscopic injuries prominent in the posterior and dependent areas. We previously reported a sustained oxygenation decrease using this model and characterized its haemodynamic profile [33]. It constitutes a robust and clinically relevant model of “first-hit” lung injury which could emphasize the influence of a “second hit”, represented by mechanical ventilation [25].
Some limitations must be pointed out. Firstly, the short duration of this trial may have limit the emergence of discrepancies between ventilator strategies; thus, extrapolation to clinical settings of this small animal study should be made with caution. Secondly, we decided to limit plateau pressure and to set tidal volume. Accordingly, PEEP level, directly dependent of respiratory compliance, could be inappropriate for providing an optimal open-lung approach. Nevertheless, limiting plateau pressure to minimize lung overdistension represents a major recommendation in ARDS patient management. Finally, given the complexity to reconcile such different ventilator modes, we attempted to harmonize them, but that was not necessarily the optimal way to efficiently administer each one.
Conclusion
In conclusion, this study provides new evidence to consider HFPV as an effective protective ventilation strategy. Both high-frequency ventilator modes (HFOV and HFPV), and protective CMV, set with a common distending pressure objective, attenuated lung injury and improved pulmonary function indexes in a short period. Further investigations are required to confirm these results before transposing HFPV to the clinical arena.
References
Halter JM, Steinberg JM, Gatto LA, Dirocco JD, Pavone LA, Schiller HJ, Albert S, Lee HM, Carney D, Nieman GF (2007) Effect of positive end-expiratory pressure and tidal volume on lung injury induced by alveolar instability. Crit Care DOI 10.1186/cc5695
Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF (2003) Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167:1620–1626
Ito Y, Veldhuizen RA, Yao LJ, McCaig LA, Bartlett AJ, Lewis JF (1997) Ventilation strategies affect surfactant aggregate conversion in acute lung injury. Am J Respir Crit Care Med 155:493–499
Steinberg JM, Schiller HJ, Halter JM, Gatto LA, Lee HM, Pavone LA, Nieman GF (2004) Alveolar instability causes early ventilator-induced lung injury independent of neutrophils. Am J Respir Crit Care Med 169:57–63
Pinhu L, Whitehead T, Evans T, Griffiths M (2003) Ventilator-associated lung injury. Lancet 361:332–340
ARDSNet (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Ranieri VM, Suter PM, Tortorella C, Tullio R de, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. J Am Med Assoc 282:54–61
Hamilton PP, Onayemi A, Smyth JA, Gillan JE, Cutz E, Froese AB, Bryan AC (1983) Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 55:131–138
Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K (2001) Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 91:1836–1844
Kolton M, Cattran CB, Kent G, Volgyesi G, Froese AB, Bryan AC (1982) Oxygenation during high-frequency ventilation compared with conventional mechanical ventilation in two models of lung injury. Anesth Analg 61:323–332
Van Kaam AH, de Jaegere A, Haitsma JJ, Van Aalderen WM, Kok JH, Lachmann B (2003) Positive pressure ventilation with the open lung concept optimizes gas exchange and reduces ventilator-induced lung injury in newborn piglets. Pediatr Res 53:245–253
Van Kaam AH, Haitsma JJ, De Jaegere A, van Aalderen WM, Kok JH, Lachmann B (2004) Open lung ventilation improves gas exchange and attenuates secondary lung injury in a piglet model of meconium aspiration. Crit Care Med 32:443–449
Vazquez de Anda GF, Gommers D, Verbrugge SJ, De Jaegere A, Lachmann B (2000) Mechanical ventilation with high positive end-expiratory pressure and small driving pressure amplitude is as effective as high-frequency oscillatory ventilation to preserve the function of exogenous surfactant in lung-lavaged rats. Crit Care Med 28:2921–2925
Vazquez de Anda GF, Hartog A, Verbrugge SJ, Gommers D, Lachmann B (1999) The open lung concept: pressure-controlled ventilation is as effective as high-frequency oscillatory ventilation in improving gas exchange and lung mechanics in surfactant-deficient animals. Intensive Care Med 25:990–996
Bollen CW, van Well GT, Sherry T, Beale RJ, Shah S, Findlay G, Monchi M, Chiche JD, Weiler N, Uiterwaal CS (2005) High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial. Crit Care doi:10.1186/cc3737
Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J (2002) High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 166:801–808
Salim A, Martin M (2005) High-frequency percussive ventilation. Crit Care Med 33:S241–S245
Eastman A, Holland D, Higgins J, Smith B, Delagarza J, Olson C, Brakenridge S, Foteh K, Friese R (2006) High-frequency percussive ventilation improves oxygenation in trauma patients with acute respiratory distress syndrome: a retrospective review. Am J Surg 192:191–195
Gallagher TJ, Boysen PG, Davidson DD, Miller JR, Leven SB (1989) High-frequency percussive ventilation compared with conventional mechanical ventilation. Crit Care Med 17:364–366
Paulsen SM, Killyon GW, Barillo DJ (2002) High-frequency percussive ventilation as a salvage modality in adult respiratory distress syndrome: a preliminary study. Am Surg 68:852–856
Reper P, Wibaux O, Van Laeke P, Vandeenen D, Duinslaeger L, Vanderkelen A (2002) High frequency percussive ventilation and conventional ventilation after smoke inhalation: a randomised study. Burns 28:503–508
Velmahos GC, Chan LS, Tatevossian R, Cornwell EE, Dougherty WR, Escudero J, Demetriades D (1999) High-frequency percussive ventilation improves oxygenation in patients with ARDS. Chest 116:440–446
Institute of Laboratory Animal Ressources Commission on Life Sciences (1996) Guide for the Care and Use of Laboratory Animals. National Academy Press, Washington, D.C.
Kolobow T, Moretti MP, Fumagalli R, Mascheroni D, Prato P, Chen V, Joris M (1987) Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation. An experimental study. Am Rev Respir Dis 135:312–315
Bregeon F, Delpierre S, Chetaille B, Kajikawa O, Martin TR, Autillo-Touati A, Jammes Y, Pugin J (2005) Mechanical ventilation affects lung function and cytokine production in an experimental model of endotoxemia. Anesthesiology 102:331–339
Chang HK (1984) Mechanisms of gas transport during ventilation by high-frequency oscillation. J Appl Physiol 56:553–563
Allan PF, Thurlby JR, Naworol GA (2007) Measurement of pulsatile tidal volume, pressure amplitude, and gas flow during high-frequency percussive ventilation, with and without partial cuff deflation. Respir Care 52:45–49
Lucangelo U, Antonaglia V, Zin WA, Fontanesi L, Peratoner A, Bird FM, Gullo A (2004) Effects of mechanical load on flow, volume and pressure delivered by high-frequency percussive ventilation. Respir Physiol Neurobiol 142:81–91
Cioffi WG, deLemos RA, Coalson JJ, Gerstmann DA, Pruitt BA (1993) Decreased pulmonary damage in primates with inhalation injury treated with high-frequency ventilation. Ann Surg 218:328–335
Sibilla S, Tredici S, Porro A, Irace M, Guglielmi M, Nicolini G, Tredici G, Valenza F, Gattinoni L (2002) Equal increases in respiratory system elastance reflect similar lung damage in experimental ventilator-induced lung injury. Intensive Care Med 28:196–203
Rotta AT, Gunnarsson B, Fuhrman BP, Hernan LJ, Steinhorn DM (2001) Comparison of lung protective ventilation strategies in a rabbit model of acute lung injury. Crit Care Med 29:2176–2184
Steinberg J, Schiller HJ, Halter JM, Gatto LA, Dasilva M, Amato M, McCann UG, Nieman GF (2002) Tidal volume increases do not affect alveolar mechanics in normal lung but cause alveolar overdistension and exacerbate alveolar instability after surfactant deactivation. Crit Care Med 30:2675–2683
Fraisse A, Bregeon F, Delpierre S, Gaudart J, Payan MJ, Pugin J, Papazian L (2007) Hemodynamics in experimental gastric juice induced aspiration pneumonitis. Intensive Care Med 33:300–307
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allardet-Servent, J., Bregeon, F., Delpierre, S. et al. High-frequency percussive ventilation attenuates lung injury in a rabbit model of gastric juice aspiration. Intensive Care Med 34, 91–100 (2008). https://doi.org/10.1007/s00134-007-0848-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0848-z