Abstract
Objectives
To determine the effect of a change in the “Dutch Directive on Medical Research Involving Human Subjects” (DD) on the number of eligible intensive care unit (ICU) patients for medical research. In addition, we determined how family members experience their role as acting representative for giving informed consent, and in turn whether patients feel their representatives would do well representing them.
Design and setting
Prospective observational study in three Dutch ICUs.
Participants
714 consecutive ICU patients. Analysis was restricted to 211 patients who were incapacitated for more than 24 h after ICU admission.
Measurements and results
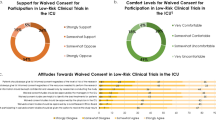
The old DD left 45.5% of patients without a legal representative; with the new DD this figure declines to 8.1%. Older age was significantly associated with the impossibility of obtaining informed consent in the old DD; after the change there was no effect of age. The median grade of confidence that representatives had in giving informed consent for incapacitated patients was 8.0 (IQR 7.0–9.0) on a scale from 0 to 10. Patients gave an equal median grade to their representatives.
Conclusion
When patients' adult children are not legally allowed to give informed consent, older patients are excluded from medical research, causing selection bias. The change in the DD has increased the number of surrogates allowed to give informed consent. Representatives felt very confident in their ability to represent the patients. In turn patients were equally confident that their representatives were able to represent them.

Similar content being viewed by others
References
Anonymous (2001) Directive 2001/20/EC of the European Parliament and Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Communities L121:34–44
Singer EA, Mullner M (2002) Implications of the EU directive on clinical trials for emergency medicine. BMJ 324:1169–1170
Kompanje EJ, Maas AI (2004) 'Treat first, ask later?' Emergency research in acute neurology and neurotraumatology in the European Union. Intensive Care Med 30:168–169
Lemaire F, Bion J, Blanco J, Damas P, Druml C, Falke K, Kesecioglu J, Larsson A, Mancebo J, Matamis D, Pesenti A, Pimentel J, Ranieri M (2005) The European Union Directive on Clinical Research: present status of implementation in EU member states' legislations with regard to the incompetent patient. Intensive Care Med 31:476–479
Dijk Y van, van der Voort PH, Kuiper MA, Kesecioglu J (2003) Research on subjects incapable of giving informed consent: the situation in Dutch intensive care departments. Intensive Care Med 29:2100–2101
Silverman HJ, Druml C, Lemaire F, Nelson R (2004) The European Union Directive and the protection of incapacitated subjects in research: an ethical analysis. Intensive Care Med 30:1723–1729
Druml C, Singer EA (2004) The European Directive: a further blow to science in intensive care medicine in Austria. Intensive Care Med 30:335
Visser HK (2001) Non-therapeutic research in the EU in adults incapable of giving consent? Lancet 357:818–819
Druml C (2004) Informed consent of incapable (ICU) patients in Europe: existing laws and the EU Directive. Curr Opin Crit Care 10:570–573
Schultz MJ, Veelo DP, Spronk PE, Kuiper ME, van der Voort PH, Korevaar JC (2006) The effect of a change in the Dutch Directive on Medical Research Involving Human Subjects on acquiring informed consent for incompetent intensive care unit (ICU)-patients. Proc Am Thorac Soc 3:A379
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
de Jonge E , Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, Kesecioglu J (2003) Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 362:1011–1016
Larsson A, Tonnesen E (2006) ICU research in Denmark: difficult but possible. Intensive Care Med (in press)
Klepstad P, Dale O (2006) Further restrictions for ICU research. Intensive Care Med 32:175
Coppolino M, Ackerson L (2001) Do surrogate decision makers provide accurate consent for intensive care research? Chest 119:603–612
Sulmasy DP, Terry PB, Weisman CS, Miller DJ, Stallings RY, Vettese MA, Haller KB (1998) The accuracy of substituted judgments in patients with terminal diagnoses. Ann Intern Med 128:621–629
Perez-San Gregorio MA, Blanco-Picabia A, Murillo-Cabezas F, Dominguez-Roldan JM, Sanchez B, Nunez-Roldan A (1992) Psychological problems in the family members of gravely traumatised patients admitted into an intensive care unit. Intensive Care Med 18:278–281
Jones C, Skirrow P, Griffiths RD, Humphris G, Ingleby S, Eddleston J, Waldmann C, Gager M (2004) Post-traumatic stress disorder-related symptoms in relatives of patients following intensive care. Intensive Care Med 30:456–460
Acknowledgements
We thank our research nurses José Hofhuis Msc (Gelre Hosiptals) and Matty Koopmans (Medical Center Leeuwarden) for their valuable contribution to this study in interviewing many of the patients and their relatives whose results were included.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-006-0386-0
Rights and permissions
About this article
Cite this article
Veelo, D.P., Spronk, P.E., Kuiper, M.A. et al. A change in the Dutch Directive on Medical Research Involving Human Subjects strongly increases the number of eligible intensive care patients: an observational study. Intensive Care Med 32, 1845–1850 (2006). https://doi.org/10.1007/s00134-006-0384-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0384-2