Abstract
Objective
To ascertain outcome, patterns of disease, incidence of concurrent infection, superinfection and penicillin resistance in children requiring intensive care for Streptococcus pneumoniae infection and compare it to a similar disease pattern, namely Neisseria meningitidisb infection.
Design and setting
Prospective cohort study in a regional paediatric intensive care unit (PICU).
Patients and participants
Children with invasive pneumococcal and meningococcal disease requiring intensive care.
Measurements and results
The study included 22 children with invasive pneumococcal disease (IPD), median age 14 months (interquartile range 3–52), median Paediatric Index of Mortality (PIM) 0.051 (0.028–0.066), median length of PICU stay 8.5 days (4–13). Four patients died, three (13.5%) attributable to IPD. Incidence of concurrent infection 27%. There were no superinfections. All S. pneumoniae were sensitive to cefotaxime; one isolate (3.7%) was resistant to penicillin. There were 186 children with meningococcal disease (MD), with a higher PIM (median 0.068, 0.033–0.108), older age (29 months, 10.7–77.9) and shorter length of PICU stay (median 3 days, 2–6). Eight (4.3%) children died from MD. Incidence of concurrent and superinfection was 18% and 6%, respectively in children with MD. All N. meningitidis cases were sensitive to cefotaxime and penicillin. The standardized mortality ratio was considerably higher with IPD (2.0) than with MD (0.52).
Conclusions
In invasive pneumococcal disease preventative measures including early recognition, immediate antibiotic therapy and vaccination need to be taken in the community, similar to the control of meningococcal disease. Invasive pneumococcal disease should command the same respect as meningococcal disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae is common in the mucosa of the nasopharynx and throat of healthy children, at rates of 7–99% depending on health, socio-economic status, study population and the patient’s age, tending to decrease with increasing age [1, 2]. In contrast, carriage rates for Neisseria meningitidis are 10–25% in teenagers and adults, with a decreasing incidence with decreasing age [3]. S. pneumoniae is one of the most pathogenic micro-organisms. It causes not only upper respiratory tract infections such as conjunctivitis, otitis media, sinusitis and bronchitis but also invasive pneumococcal disease (IPD), including pneumonia, septicaemia and meningitis. The pathophysiology of S. pneumoniae is similar to that of N. meningitidis [3, 4, 5]. The mortality rate of IPD in children varies between 1% and 11.8% [6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. This is similar to the experience with meningococcal disease (MD) [16]. Analogous to MD, there is a characteristic seasonable distribution for IPD, with a peak incidence during the winter/spring months [3, 6]. Likewise, viral infections are known to be immunosuppressive and pave the way for bacterial co-infections due to N. meningitidis and especially S. pneumoniae [6, 17]. A recent European surveillance study on resistant S. pneumoniae isolates from blood and cerebrospinal fluid (CSF) shows that penicillin resistance varies widely, with the highest incidence in Spain with 11%, followed by Belgium, Ireland and the United Kingdom with 4%, and less than 1% in The Netherlands, Germany and Scandinavia [18, 19]. The highest incidence of penicillin-resistance is generally observed in children, especially those with upper respiratory tract infections, who have often received multiple courses of antibiotics [18, 20].
A prospective observational cohort study was undertaken in children with IPD requiring intensive care to ascertain outcome, concurrent and superinfection, and penicillin-resistance amongst S. pneumoniae, and compare it to N. meningitidis infection which has a similar disease and clinical pattern.
Patients and methods
Setting
The study was undertaken on a paediatric intensive care unit (PICU) at the Royal Liverpool Children’s Hospital, United Kingdom, a university-affiliated, multi-disciplinary, regional referral centre. The PICU is a 20-bedded facility with an annual admission rate of 1000 children.
Patients
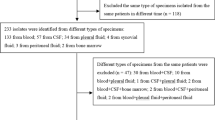
A prospective observational cohort study of children admitted into PICU with IPD or MD between 1 January 1999 and 1 October 2003 (56 months). The study was approved by the Institutional Ethics Review Board. IPD was defined by the isolation of S. pneumoniae from normally sterile body fluids, for example, blood, CSF and lower airway secretions, with laboratory and clinical parameters of severe sepsis [21]. Meningococcal disease was diagnosed on the isolation [culture and/or polymerase chain reaction (PCR)] of N. meningitidis from blood or CSF (rarely) and clinical parameters of infection. Of the 4,710 PICU admissions during the study period 23% fell into the category of septicaemia, meningitis/encephalitis or pneumonia, and no causative pathogen was identified in 3.9% of the total PICU population (46 with septicaemia, 26 with meningitis, 112 with pneumonia).
The demographic and clinical characteristics of patients in the IPD and MD groups are summarized in Table 1. In the IPD group the median length of stay was 8.5 days (IQR 4–13) and that of survivors 9 days (4–16). One IPD patient developed renal failure requiring dialysis due to haemolytic uraemic syndrome as a consequence of IPD. Three children with pneumococcal respiratory infections had underlying neurological co-morbidity (muscular dystrophy, transverse myelitis, subacute necrotizing encephalomyelopathy). S. pneumoniae was isolated from the 22 patients in the following sites: blood (n=7), blood and CSF (n=2), CSF (n=3), lower airway secretions (n=7), pneumococcus and respiratory syncytial virus (RSV) in lower airway secretions (n=3). All the patients fulfilled the 2001 Consensus Conference definition of severe sepsis or septic shock. There were no differences in outcome, inflammatory markers, PIM or inotropic requirement according to the site of positive microbiological culture. In children with MD all microbiological diagnoses were made on blood results, apart from three on CSF. Only two children with MD had underlying neurological co-morbidity (hydrocephalus).
Endpoints
The endpoints were: (a) mortality, (b) inflammation markers [C-reactive protein (CRP), leucocytes and platelets, (c) concurrent and superinfections and (d) antibiotic sensitivity.
Clinical management of severe pneumococcal and meningococcal infections
Corticosteroids were used in patients with meningitis, as well as in children with septic shock who did not respond to the treatment. No novel/experimental therapies, for example, activated protein C and tissue plasminogen activator, were used in either group.
Microbiology
Diagnostic samples of blood, lower airway secretions and CSF were taken on clinical indication. Surveillance samples of throat and rectum were obtained on admission and then twice weekly. The diagnostic samples of blood, lower airway secretions and CSF were inoculated onto universal media of blood and chocolate agar [22]. All diagnostic samples for N. meningitidis were sent for PCR. The sensitivity of all isolates was tested using the E-test [23]. S. pneumoniae isolates were not serotyped unless penicillin-resistant [24]. RSV nasopharyngeal aspirates were tested by the Directigen RSV test (Becton Dickinson, Maryland, USA), an in vitro enzyme-linked immunosorbent assay (ELISA) membrane test for RSV antigen [25]. Surveillance samples of throat and rectal swabs were processed qualitatively and semi-quantitatively to detect the level of carriage of potential pathogens [26].
Antibiotic policies
Patients with signs of infection received intravenous cefotaxime (150 mg/kg per day in four doses for up to 7 days) as first-line therapy for 48 h whilst awaiting culture results. Clinical status on presentation governed whether supplementary intravenous cover with an aminoglycoside, gentamicin (7.5 mg/kg per day in three doses for up to 7 days) was added. Once the causative micro-organism was confirmed as S. pneumoniae or N. meningitidis gentamicin was discontinued. When the surveillance samples revealed abnormal flora including aerobic Gram-negative bacilli (AGNB) selective decontamination of the digestive tract (SDD) using enteral polymyxin, tobramycin and amphotericin B was administered in order to prevent superinfections [27].
Definitions
Internationally accepted definitions were utilized for infections, sepsis and abnormal carriage [21, 26, 27, 28].
Analytic methods
Data were collected prospectively. Prediction of mortality using the Paediatric Index of Mortality (PIM) was obtained on the patient’s first contact with the PICU team [29]. Results are expressed as a fraction of the total study population, median with inter-quartile range (IQR), mean with standard deviation, 95% confidence interval, or standard error of the mean. Continuous data were analysed using Student’s t test or the Wilcoxon-Mann-Whitney test. Categorical data were analysed using Fisher’s exact test. Correlations were assessed using Spearman’s rank test (two-tailed). Kinetic data were studied with two-way analyses of variance for repeated measurements with Bonferroni’s correction for post hoc analysis. Statistical calculations were performed with the Statistical Program for Social Science release 11.0.0 (Chicago, Ill., USA). A p value less than 0.05 was considered statistically significant. The standardized mortality ratio (SMR) defined as the actual or attributable mortality divided by the PIM-predicted mortality was calculated for each group.
Results
Mortality
Four patients who were positive for S. pneumoniae died, with IPD being the cause of death in three children (13.5%). One patient died on day 5 of treatment on PICU from cerebral oedema due to pneumococcal meningitis complicated by cerebritis. One patient admitted with pneumococcal septicaemia died on day 4 of multiple organ systems failure. Pneumococcal pneumonia was the cause of death due to respiratory failure in a patient with Duchenne’s muscular dystrophy on day 11. A patient with 75% burns died on day 19 from multiple organ system failure, which was not associated with an earlier pneumococcal lower airway infection. A post-mortem examination found pulmonary haemorrhages as part of disseminated intravascular coagulation, but no evidence of lung infection. All eight deaths in the MD group were due the N. meningitidis infection. Four children died from fulminant cardiovascular collapse and one each from cerebral oedema, pulmonary haemorrhage, acute respiratory distress syndrome, multiple organ systems failure.
There were no differences in age, PIM, or even length of PICU stay between the survivors and non-survivors in either the IPD or the MD group (all p values >0.34), but numbers were small with IPD. Death occurred earlier in MD than IPD (p=0.02; Table 1). Death was correlated to inotropic requirement in MD (p=0.03), but not in IPD (p=0.2). There was no association between age and death in those with IPD (p=0.15), with MD (p=0.52), or when both groups were analysed together (p=0.33).
Inflammatory response
CRP levels, white blood cell count (WCC) and platelets count of the children with IPD compared to those with MD over the course of their PICU stay are shown in Figs. 1, 2 and 3, respectively. There were no differences in the inflammatory markers over the course of their PICU stay between survivors and non-survivors in either the IPD or the MD groups (all p values >0.15), but numbers were small with IPD. There was no correlation between admission WCC and death in either IPD (p=0.79) or MD (p=0.61). The prevalence of leucopaenia, defined as WCC lower than 4×109/l on PICU admission, was 19.2% and 12.4% in the IPD and MD populations, respectively, but there was no difference in admission WCC between groups (p=0.72).
Temporal profile of C-reactive protein (CRP) levels of critically ill children with invasive pneumococcal disease compared to those with meningococcal disease (mean, standard error of the mean). The number of patients (n) in each group on admission, at 48 h and at 96 h is shown. *p=0.001 (Bonferroni’s method: p<0.016)
Concurrent and superinfection
All patients were admitted with S. pneumoniae, demonstrating that the infection developed in the community (i.e. imported into PICU). The incidence of concurrent infection was high (27%) in the IPD group. Three children had one other primary endogenous infection, two due to Pseudomonas aeruginosa and one due to Moraxella catarrhalis. Three children had a concurrent viral (RSV) infection. There were no bacterial or fungal superinfections (secondary endogenous or exogenous). One child (4.2%) acquired RSV bronchiolitis while on PICU. In contrast, in the MD group the incidence of concurrent infection was 17.8% (one-half bacterial and one-half viral), of bacterial superinfection 5.9%, and 5.8% acquired a viral infection (RSV or parainfluenza). There was no significant difference in concurrent infection rate between groups (p=0.57).
Abnormal flora on admission
In the IPD group 43% were admitted with abnormal AGNB in throat and/or gut surveillance swabs, compared to 45% in the MD group. Klebsiella, Enterobacter, Citrobacter and P. aeruginosa were the predominant abnormal AGNB. One IPD patient was a carrier of methicillin-resistant Staphylococcus aureus (MRSA).
Antibiotic susceptibility
Twenty-seven isolates were obtained from the 22 patients with IPD, all of which were sensitive to cefotaxime, the first choice of treatment. No isolates were resistant to the macrolides and glycopeptides. Two isolates were resistant to ciprofloxacin and one was intermediate for ciprofloxacin. One isolate (serogroup 9; 3.7%) obtained from lower airway secretions was resistant to penicillin. There was no penicillin resistance in the MD group.
Discussion
Four findings emerge from this prospective comparative cohort study between IPD and MD children requiring paediatric intensive care: (a) The crude mortality was 18.2% and the mortality due to IPD was 13.5%, which was higher than the comparative MD group (4.3%). (b) Inflammation markers in IPD were high during the first week of treatment on PICU but were lower in the first 3–4 days than the children with MD. (c) The concurrent infection rate (27%) was similar to the comparative MD group (17.8%), but there were no superinfections. (d) Penicillin-resistance was not a problem amongst either S. pneumoniae or N. meningitidis isolates.
In this study although there were four deaths, only three children died on the PICU due to IPD, giving an attributable mortality of 13.5%. This is similar to the mortality quoted in the literature [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. However, it was appreciably higher than the comparative group with MD (4.3%). Age may have played a role, as the children with IPD were younger than their counterparts with MD (median 14 vs. 29 months old, p<0.001), but no correlation was observed. Perhaps the IPD group had a more severe and protracted disease course than the MD patients, as suggested by their longer length of stay in the PICU (median 8.5 vs. 3 days, p<0.001) and more required mechanical ventilation (100% vs. 80.1%, p<0.001). On the other hand, more children required inotropic support with MD (63.4% vs. 50%, p=0.03). Additionally, on admission to PICU the risk of death (PIM) was higher in the MD group (p=0.02). The use of SMR should calibrate for disease severity and allow comparison between groups and units [30, 31, 32, 33, 34, 35]. The SMR was different, at 2.0 and 0.52 for IPD and MD, respectively.
The severe generalized inflammation status in both IPD and MD patients often lasted for more than 1 week, but did not explain the basis for IPD patients performing worse than their MD counterparts. On the contrary, the MD patients had a more intense initial inflammatory response and similar ongoing response, and yet still fared better. Figures 1 and 2 show that the CRP and WCC were significantly higher in the MD than the IPD group. The platelets were significantly lower due to endotoxin release by N. meningitidis than in the IPD group (Fig. 3) [36]. Conceivably the MD group had a better outcome because of better initial, early management. Certainly in the United Kingdom over the past 5–10 years there has been heightened public and medical awareness of MD. This has most probably impacted on earlier recognition and therefore earlier appropriate management and treatment of the disease [37, 38].
Although concurrent infection may have adversely affected the IPD group, the incidence was no greater than in the MD group. There were six patients in the IPD group with concurrent infections, three viral and three bacterial, and this is in line with two recent studies on community acquired pneumonia and sepsis in hospitalized children [39, 40]. The intense inflammation response causing a profound immunosuppression during the first week is a likely cause for these concurrent primary endogenous infections with micro-organisms present in the admission flora [26].
Illness severity is a well described independent risk factor for abnormal carriage of AGNB and MRSA [41]. The two groups had similar abnormal carriage rates (IPD 43% and MD 45%), indirectly suggesting similar disease severities on PICU admission. Yet one group fared substantially better. The intense inflammation status associated with invasive infections promotes acquisition and subsequent carriage of hospital flora including AGNB and MRSA [42, 43]. An abnormal carrier state may lead to superinfections and associated mortality in the subset of ventilated patients with ongoing immune suppression [42, 43]. There was a low superinfection rate in our study in both groups. In our unit a policy of restrictive antibiotic usage and SDD using enteral antimicrobials as a technique to eradicate abnormal carriage may have played a role in minimizing superinfections [44]. Previous studies have suggested that mortality in IPD is related to superinfections [17, 42], but this did not play a role in this IPD study group. Only one isolate (3.7%) was resistant to penicillin whilst cefotaxime, the drug of first choice, was invariably active. It is therefore unlikely that penicillin-resistance amongst S. pneumoniae impacted on outcome in our study. A recent prospective study in severely ill adults with pneumococcal bacteraemia showed that combination antibiotic therapy improved survival over monotherapy [45]. Many of our IPD patients had received combination antibiotic therapy (cefotaxime and gentamicin) initially which was later rationalized to monotherapy once cultures and sensitivities were available. Unfortunately the numbers were too small to address this aspect. Similarly the sample size was too small to determine a correlation with regards to corticosteroid therapy and outcome [46].
All the cases of IPD had clinical and laboratory evidence of widespread sepsis and fulfilled criteria for severe sepsis or septic shock [21]. The low number of cases with microbiologically confirmed IPD in this study may be an underestimate. A considerable number of the sepsis cases with an unidentified pathogen (3.9% of the PICU population) may have been caused by S. pneumoniae, as during most of the study period only culture techniques were used to diagnose IPD. Molecular techniques including PCR may improve the diagnosis of IPD and increase the number of IPD cases identified, as commonly used antimicrobials given in the community and the referring hospital render clinical samples of blood, tracheal aspirate and CSF sterile. PCR was routinely used to confirm MD cases. Added to this, MD’s more clearly definable clinical signs (especially the purpuric rash) allowed it to be considered more readily.
It can be argued that the relative paucity of exposure to IPD as opposed to MD within the unit could have influenced the treatment and therefore the mortality. It has been recognized in medical practice that improvement in outcome may reflect a relationship between quantity and quality [32, 33, 34, 35]. However, experience within the PICU with critically ill children with sepsis is substantial (over 200 cases per year), and therefore lack of exposure is unlikely to play a major role.
The sample size may be a limitation in this study. Although the prospective study covered a long period (56 months), only 24 admissions (22 children) with microbiologically confirmed IPD were diagnosed. This utilization of only culture-confirmed pneumococcal disease cases may also have limited the study. The small sample size of 22 children requiring intensive care including mechanical ventilation for IPD compared to 186 children with MD may unfavourably bias this comparison, but the difference in outcome speaks for itself. Serogrouping of S. pneumoniae isolates was not routinely performed, and we were therefore unable to specify which serogroups were common to our study population.
Recent studies have demonstrated substantial declines in the number of cases of IPD following the introduction of a polyvalent pneumococcal vaccine [47, 48]. Pneumococcal vaccination is not routinely used in the United Kingdom. The age of the children with IPD in this study (median 14 months, IQR 3–52) reveals that most would have benefited from vaccination. Early recognition and earlier, more pro-active aggressive treatment of MD at base hospitals/healthcare centres has improved mortality in those presenting to PICU [37, 38]. Perhaps this approach would impact on IPD similarly.
Conclusion
IPD and MD show clinical similarities. Despite the absence of resistance and superinfections developing during intensive care treatment, the mortality and SMR in those with IPD was still much higher than in those MD. We believe that IPD should command the same respect and be treated in an identical way as MD, i.e. prevention in the community, including vaccination and immediate antimicrobials [18, 49, 50].
References
Austrian R (1986) Some aspects of the pneumococcal carrier state. J Antimicrob Chemother 18[Suppl A]:35–45
Rapola S, Salo E, Kiiski P, Leinonen M, Takala AK (1997) Comparison of four different sampling methods for detecting pharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children. J Clin Microbiol 34:1077–1079
Cartwright K (1995) Meningococcal disease. Wiley, Chichester, pp 115–175
Bone RC (1994) Gram-positive organisms and sepsis. Arch Intern Med 154:26–34
Despond O, Proulx F, Carcillo JA, Lacroix J (2001) Pediatric sepsis and multiple organ dysfunction syndrome. Curr Opin Pediatr 13:247–253
Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, Zell ER, Schuchat A, Whitney CG; Active Bacterial Core Surveillance (ABCS)/Emerging Infections Program Network (2001) Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998. JAMA 285:1729–1735
Eskola J, Takala AK, Kela E, Pekkanen E, Kalliokoski R, Leinonen M (1992) Epidemiology of invasive pneumococcal disease in children in Finland. JAMA 268:3323–3327
Davis CWC, McIntyre PB (1995) Invasive pneumococcal infection in children, 1981–1992: a hospital based study. J Paediatr Child Health 31:317–322
Venetz I, Schopfer K, Muhlemann K and the Swiss Pneumococcal Study Group (1998) Paediatric invasive pneumococcal disease in Switzerland, 1985–1994. Int J Epidemiol 27:1101–1104
Shackley F, Knox K, Morris JB, Crook D, Griffiths D, Mayon-White R, George R, Willocks L, Moxon E (2000) Outcome of invasive pneumococcal disease: a UK-based study. Arch Dis Child 83:231–233
Kries R von, Siedler A, Schmitt HJ, Reinert RR (2000) Proportion of invasive pneumococcal infections in German children preventable by pneumococcal conjugate vaccines. Clin Infect Dis 31:482–487
Scheifele D, Halperin S, Pelletier L, Talbot J (2000) Invasive pneumococcal infections in Canadian children, 1991–1998: implications for new vaccination strategies. Clin Infect Dis 31:58–64
Iglesias Sanchez L, Perez-Yarza EG, Garcia-Arenzana JM, Valiente Mendez A, Perez-Trallero E (2002) Epidemiology of invasive pneumococcal disease in children in Gipuzkoa [Spain] from 1981 to 2001. An Esp Pediatr 57:401–407
Espin MI, Sandoval A, Ruiz J, Navarro JA, Garcia J, Perez Flores D (2002) Invasive pneumococcal disease in children in the region of Murcia, Spain. Gac Sanit 16:385–391
Ispahani P, Slack RC, Donald FE, Weston VC, Rutter N (2004) Twenty year surveillance of invasive pneumococcal disease in Nottingham: serogroups responsible and implications for immunization. Arch Dis Child 89:757–762
Goldacre MJ, Roberts SE, Yates D (2003) Case fatality rates for meningococcal disease in an English population, 1963–98: database study. BMJ 327:596–597
Riou B, Richard C, Rimaildo A, Auzépy P (1987) Co-infection or early superinfection of pneumococcal pneumonia. Intensive Care Med 13:352–354
Cartwright K (2002) Pneumococcal disease in Western Europe: burden of disease, antibiotic resistance and management. Eur J Pediatr 161:188–195
Oteo J, Cruchaga S, Campos J, Saez-Nieto JA, Baquero F; Miembros espanoles del Grupo del European Antimicrobial Surveillance System (EARSS) (2003) Antibiotic resistance of 622 Streptococcus pneumoniae isolated from blood and cerebrospinal fluid in 33 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance Study [2000]. Enferm Infecc Microbiol Clin 21:12–19
Klugman KP, Friedland IR, Bradley JS (1995) Bactericidal activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis. Antimicrob Agents Chemother 39:1988–1992
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference. Intensive Care Med 29:530–538
Barlows A, Harsier WJ (1991) Manual of clinical microbiology 5th edn, sect III. American Society of Microbiology, Washington, pp 209–553
National Committee for Clinical Laboratory Standards (1997) Methods for dilution antimicrobial susceptibility test for bacteria that grows aerobically, 4th edn. National Committee for Clinical Laboratory Standards, Wayne, pp M7–A4
Doern GV, Brueggemann AB, Blocker M, Dunne M, Holley HP Jr, Kehl KS, Duval J, Kugler K, Putnam S, Rauch A, Pfaller MA (1998) Clonal relationship among high-level penicillin-resistant Streptococcus pneumoniae in the United States. Clin Infect Dis 27:757–761
Thorburn K, Kerr S, Taylor N, van Saene HKF (2004) RSV outbreak in a paediatric intensive care unit. J Hosp Infect 57:194–201
Saene HKF van, Damjanovic V, Alcock SR (2001) Basics in microbiology for the patient requiring intensive care. Curr Anaesth Crit Care 12:6–17
Sarginson RE, Taylor N, Reilly N, Baines PB, van Saene HKF (2004) Infection in prolonged pediatric critical illness: a prospective four-year study based on knowledge of the carrier state. Crit Care Med 32:839–847
Sarginson RE, Taylor N, van Saene HKF (2001) Glossary of terms and definitions. Curr Anaesth Crit Care 12:2–5
Shann F, Pearson G, Slater A, Wilkinson K (1997) Paediatric Index of Mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med 23:201–207
Leteurtre S, Leclerc F, Martinot A, Cremer R, Fourier C, Sadik A, Grandbastien B (2001) Can generic scores (Pediatric Risk of Mortality and Pediatric Index of Mortality) replace specific scores in predicting outcome of presumed meningococcal septic shock in children? Crit Care Med 29:1239–1246
Festa M, McDermott W, Britto J, Nadel S, Habibi P (1998) Validation and comparison of PRISM and PIM scores in prediction of mortality from meningococcal disease. Abstr. Intensive Care Med 24 [Suppl 1]:S41
Gemke RJ, Bonsel GJ, PICASSO study group (1995) Comparative assessment of pediatric intensive care: a national multi-center study. Crit Care Med 23:238–245
Pollack MM, Patel KM, Ruttimann UE (1995) A look at pediatric intensive care—Dutch style. Crit Care Med 23:221–222
Shann F, Carlin J (1992) The outcome of pediatric intensive care. N Engl J Med 326:1161
Shann F (2000) Where do all the children go? Intensive Care Med 26:6–7
Das J, Schwartz AA, Folkman J (1973) Clearance of endotoxin by platelets: role in increasing the accuracy of the Limulus gelation test and in combating experimental endotoxemia. Surgery 74:235–240
Thorburn K, Baines P, Thomson A, Hart CA (2001) Mortality in severe meningococcal disease. Arch Dis Child 85:382–385
Booy R, Habibi P, Nadel S, de Munter C, Britto J, Morrison A, Levin M; Meningococcal Research Group (2001) Reduction in case fatality from meningococcal disease associated with improved healthcare delivery. Arch Dis Child 85:386–390
Juven T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, Eskola J, Saikku P, Ruuskanen O (2000) Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J 19:293–298
Yagupsky P, Sofer S, Dagan R (2001) Early onset pneumococcal sepsis in children hospitalized for non-infectious life-threatening events. Pediatr Infect Dis J 20:1092–1094
Jarstrand C, Tuneval G (1976) Colonization and clinical superinfection with Gram-negative bacilli in influenza. Scand J Infect Dis 8:229–235
Finland M (1973) Superinfections in the antibiotic era. Postgrad Med 54:175–183
Petros AJ, O’Connell M, Roberts C, Wade P, van Saene HK (2003) Systemic antibiotics fail to clear multi-drug-resistant Klebsiella from a pediatric ICU. Chest 119:862–866
Saene HKF van, Petros AJ, Ramsay G, Baxby D (2003) All great truths are iconoclastic: selective decontamination of the digestive tract moves from heresy to level 1 truth. Intensive Care Med 29:677–690
Baddour LM, Yu VL, Klugman KP, Feldman C, Ortqvist A, Rello J, Morris AJ, Luna CM, Snydman DR, Ko WC, Chedid MB, Hui DS, Andremont A, Chiou CC; International Pneumococcal Study Group (2004) Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med 170:440–444
Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y (2004) Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ 1:47–56
Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A; Active Bacterial Core Surveillance of the Emerging Infections Program Network (2003) Decline in invasive pneumococcal disease after the introduction of protein-conjugate polysaccharide conjugate vaccine. N Engl J Med 348:1737–1746
Kaplan SL, Mason EO Jr, Wald ER, Schutze GE, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Yogev R, Barson WJ (2004) Decrease of invasive infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal vaccine. Pediatrics 113:443–449
Austrian R (1997) The enduring pneumococcus: unfinished business and opportunities for the future. Microb Drug Resist 3:111–115
Peters M, Petros A, Baines P, Loan P, Cullen P, Ralston C, Yates R, Marsh M, Weir P (2002) Genuine reduction in meningococcal deaths results from teamwork. Arch Dis Child 87:560–561
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thorburn, K., Taylor, N., Lopez-Rodriguez, L. et al. High mortality of invasive pneumococcal disease compared with meningococcal disease in critically ill children. Intensive Care Med 31, 1550–1557 (2005). https://doi.org/10.1007/s00134-005-2803-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2803-1