Abstract
Objective
To evaluate and compare the efficacy, infusion rate and recovery profile of vecuronium and cisatracurium continuous infusion in critically ill children requiring mechanical ventilation.
Design and setting
Prospective, randomised, double-blind, single-centre study in critically ill children in a paediatric intensive care unit in a tertiary children’s hospital.
Methods
Thirty-seven children from 3 months to 16 years old (median 4.1 year) were randomised to receive either drug; those already receiving more than 6 h of neuromuscular blocking drugs were excluded. The Train-of-Four (TOF) Watch maintained neuromuscular blockade to at least one twitch in the TOF response. Recovery time was measured from cessation of infusion until spontaneous TOF ratio recovery of 70%.
Results
The cisatracurium infusion rate in nineteen children averaged 3.9±1.3 µg kg−1 min−1 with a median duration of 63 h (IQR 23–88). The vecuronium infusion rate in 18 children averaged mean 2.6±1.3 µg kg−1 min−1 with a median duration of 40 h (IQR 27–72). Median time to recovery was significantly shorter with cisatracurium (52 min, 35–73) than with vecuronium (123 min, 80–480). Prolonged recovery of neuromuscular function (>24 h) occurred in one child (6%) on vecuronium.
Conclusions
Recovery of neuromuscular function after discontinuation of neuromuscular blocking drug infusion in children is significantly faster with cisatracurium than vecuronium. Neuromuscular monitoring was not sufficient to eliminate prolonged recovery in children on vecuronium infusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular blocking (NMB) drugs are used in critically ill children to facilitate mechanical ventilation, reduce oxygen consumption and barotrauma, stabilise intracranial pressure and provide haemodynamic control. Blockade is caused by competing with acetylcholine at the receptor site at the neuromuscular junction. Vecuronium and atracurium infusions have been used in paediatric intensive care units (PICUs), the former because of its fewer cardiovascular side effects and the latter because of its non-enzymatic elimination. Use of the intermediate acting NMB drug vecuronium may be complicated by prolonged weakness [1] and prolonged NMB [2], especially in renal failure due to accumulation of the metabolite 3-desacetylvecuronium (which has 50–70% activity of the parent compound). Administration of atracurium can result in histamine release [3], and its metabolite laudanosine, which is cleared by the liver, has been shown to cause convulsions in animals [4]. However, patients with fulminant hepatic failure have not experienced measurable central neurological effects [5], and there has only been one report of a surgical patient who had a seizure while receiving atracurium [6].
Cisatracurium was introduced into clinical practice in 1996 and is one of one stereoisomers of atracurium, the R-cis, R′-cis isomer. Dose-response studies in adults have established that cisatracurium has a similar NMB profile to atracurium, with no vagolytic or sympatholytic properties. Onset of action is slower [7], and cisatracurium is approx. three times more potent than atracurium [4, 7]. Cisatracurium therefore has less propensity to release histamine [7, 8, 9], providing increased cardiovascular stability and less production of laudanosine, making it safer for use in hepatic disease. Cisatracurium is non-cumulative, undergoing degradation predominantly by non-enzymatic Hofmann elimination [8], and has been shown to have a lower incidence of prolonged paralysis than vecuronium [10]. Recent clinical practice guidelines for sustained NMB in adults recommend cisatracurium or atracurium for patients with significant renal or hepatic disease [11]. Cisatracurium has been used safely in children undergoing general anaesthesia [12, 13, 14, 15] and in PICUs [16, 17] and may be the NMB drug of choice in the PICU.
This study was designed to establish infusion rates and to compare efficacy and recovery profiles of vecuronium and cisatracurium infusions in critically ill children undergoing mechanical ventilation.
Methods
The study was conducted on a 15-bed PICU (no cardiac or neonatal patients) of a tertiary referral centre from February 1999 to December 2000. Children between 3 months and 16 years old requiring NMB as part of their management were eligible. The attending intensivist decided on the indication for NMB which included children who had undergone gastric transposition or tracheal surgery repair and failure to achieve synchronous ventilation with adequate sedation. Children were excluded if there was a history of malignant hyperthermia or allergy to non-depolarising drugs, or if they had received more than 6 h of NMB drug. Forty-six children were originally enrolled and randomised to study drug; nine were subsequently excluded from analysis (Fig. 1). A random allocation sequence was set up in blocks of four by the principle investigator. Children were randomised to receive either cisatracurium or vecuronium by cards inside serially numbered, opaque, sealed envelopes. There was no significant difference in weight, age or gender between the cisatracurium group (n=19) and the vecuronium group (n=18) children, and PICU admission diagnoses were similar in the two groups (Table 1). Two members of PICU nursing staff not involved in the child’s management reconstituted drug infusion according to card instructions in a side room. Both investigators and bedside nurse were kept blinded to drug identity until study completion. Demographic data and reason for admission were recorded. Vital signs, daily liver and renal function tests and current medications were available from the computerised database.
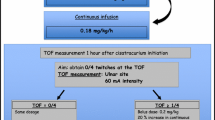
Children received continuous intravenous infusion of NMB drug by syringe pump at an initial rate of 4 µg kg−1 min−1. If clinically indicated patients received an initial bolus dose of 0.1 mg kg−1. Patients already receiving NMB drugs (duration <6 h) were allowed partial recovery (>one twitch) in Train-of-Four (TOF) response prior to initiation of study drug. All children were sedated and analgesed throughout the study using intravenous infusions of midazolam (2–4 µg kg−1 min−1) and morphine (10–60 µg kg−1 h−1).
All children had a peripheral nerve stimulator (TOF-Watch, Organon Teknika) placed using self-adhering cutaneous electrodes on the forearm over the distal ulnar nerve and a miniature acceleration transducer taped to the volar surface of the thumb. Stimulation pulses up to 60 mA are delivered four times at 1 Hz. Titration of infusion dose helps prevent under [18] or over dosage [19] and minimises prolonged paralysis. Standard optimal neuromuscular paralysis for ICU is synonymous with maintaining a TOF at one twitch [12, 18]. In this study infusion was titrated as such provided the child did not exhibit clinical signs indicating the necessity for deeper NMB (e.g. coughing, interbreathing, movement). Dosage alterations were made by increments of 1 µg kg−1 min−1; increases were accompanied by a bolus equivalent to current hourly rate. Additional boluses equivalent to current hourly rate were permitted as clinically indicated at the discretion of the attending intensivist.
Peripheral nerve response to TOF stimulation was monitored and recorded each minute for the first 10 min of the infusion and thereafter every 30 min until a TOF at one twitch was maintained. Once this was established these measurements were repeated at a minimum of 4-h intervals during continuous study drug infusion. Additional measurements were recorded at 30 min after any boluses or alterations in infusion rate.
Recovery time was measured from drug cessation when monitoring was carried out at 5-min intervals until spontaneous TOF ratio recovery of more than 70%. Prolonged recovery time was defined as longer than 24 h to reach a TOF of 70% (or clinical criteria such as head movement) [2], associated risk factors were defined as renal insufficiency (peak creatinine ≥ 60 µmol/l), renal failure (requiring haemofiltration) and steroid therapy. Any child who did not receive more than 6 h of NMB drug infusion, or in whom it was not possible to monitor the degree of NMB was excluded from analysis. The study protocol was approved by the hospital ethics committee and informed, written consent obtained from parents of all children studied.
A sample size of 20 in each group was estimated to give 90% power in order to detect a 10% reduction from reported incidence of prolonged recovery of more than 6 h from 43% to 4% at the 5% significance level [2]. Continuous variables are summarised as median and interquartile range (IQR) where normality cannot be assumed and mean ±standard deviation if normally distributed. Infusion rates were defined as average infusion rate referring to total dose (including any bolus doses) received by infusion for an individual child divided by infusion duration; mean infusion rate refers to the mean of average infusion rates from all children [20]. Data were compared using the Mann-Whitney U test (demographics, Table 1; pharmacodynamics, Table 2), linear regression analysis (average infusion rates and recovery times) and two-tailed Fisher’s exact test with exact confidence intervals for the difference in percentages (prolonged recovery times). In order to assess imprecision arising from the relatively small sample size 95% confidence intervals (CI) around the difference in medians or means were expressed for pharmacodynamic data. Statistical analysis was performed with StatXact (version 4.0.1) and Statistica (version 5.5). The significance level for all statistical hypothesis tests was set at 5%.
Results
Median duration of cisatracurium infusion (63 h, IQR 23–88) did not differ significantly from that of vecuronium (40 h, 27–72), p=0.65 (Table 2). Mean infusion rates for vecuronium (n=18) and cisatracurium (n=19) were 2.6±1.3 and 3.9±1.3 µg kg−1 min−1. All children experienced at least four changes in infusion rate. Linear regression analysis showed no relationship of age with average infusion rate for children receiving cisatracurium (r2=0.0008, p=0.91) or vecuronium (r2=0.10, p=0.19).
Maximum infusion rates reached 10 µg kg−1 min−1 in both groups; one child on vecuronium received increased doses in a mistaken effort to reduce hypertension, one child receiving cisatracurium required successive increases in dose to maintain a TOF at one. Linear regression analysis was performed on infusion rates of 30 children completing 24 h of infusion time to examine the likelihood of an increase or decrease in infusion requirement over time. Of 16 children receiving vecuronium 12 (75%) showed no significant change in infusion rate; three (19%) showed a statistically significant decrease and one (6%) a statistically significant increase. Of children receiving cisatracurium 14 (71%) showed no significant change in infusion rate, and four (29%) had a statistically significant increase.
All increases in dose infusion were accompanied by a bolus dose as per protocol. Additional bolus doses administered at the discretion of the clinician in charge occurred in eight children receiving cisatracurium and three receiving vecuronium; the greatest rate of 0.1 bolus/h occurred in one child in each group; there was no statistical difference between groups (p=0.18). Mean TOF response throughout the period of drug infusion was 0.5 (0.3–0.84) in children receiving cisatracurium and 0.2 (0–0.83) in children receiving vecuronium (p=0.13; Table 2).
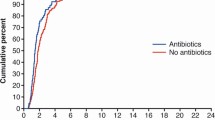
Recovery time to TOF ratio of more than 70% was available for all children included in the analysis. TOF counts at discontinuation of study drug did not differ significantly between groups; 27 children had a TOF of 0, all of whom had TOF counts of 1 or more at other points in the study (Table 2). Median recovery time with vecuronium was 123 min (IQR 80–480) and that with cisatracurium 52 min (35–73; p=0.001, 95% CI 24.1–305.1). There was no relationship between recovery time and NMB drug infusion duration (Fig. 2), age, gender, mean infusion rate, or total dose administered. Six children receiving vecuronium had recovery times longer than 6 h (p=0.0498, 95% CI 1.1–64.3%), one of whom required 27 h to recover (p=0.73). This was a boy who had suffered a head injury and had no renal impairment. No child received intravenous magnesium, and all received antibiotics (aminoglycoside choice at this institution is amikacin).
Haemodynamic variables and vital signs were measured continuously during the study, and no clinically important changes were attributed to either drug. No child showed signs of myopathy following recovery of NMB, and no deaths occurred as a result of the study.
Costs over the 22-month period in 37 studied patients were estimated. The cost of 25 mg cisatracurium was £10 and that of 25 mg vecuronium £10.40. Mean cisatracurium infusion rate was 1.5 times that of vecuronium, using median infusion time (48 h) and median weight (17 kg) an estimation of additional cost of cisatracurium was calculated as £1,054.87. The prolonged recovery time of 27 h for the child receiving vecuronium equates to an extra £1237.50 (cost of 24 h on PICU at the time of the study was £1,100). Therefore additional costs are fully recovered from one single vecuronium patient with prolonged recovery.
Discussion
Current information to guide administration of NMB drugs in the PICU is limited. Factors such as cardiovascular and autonomic interactions, drug toxicity, metabolism and excretion, cost and predictability of onset and offset may be considered. In this study recovery of neuromuscular function (from approximately equivalent depth of NMB) was significantly longer after vecuronium than cisatracurium, and was not correlated with age, renal function, infusion rate or duration of infusion. Hodges et al. [21] found that children less than 1 year old had significantly reduced vecuronium requirements, at 0.9 µg kg−1 min−1 as opposed to 1.64 µg kg−1 min−1 in children over 1 year old. In this study mean infusion rates of vecuronium were higher at 2.6±1.3 µg kg−1 min−1. There was no age association with either drug, although only three children were less than 1 year of age. This was partly due to study protocol excluding infants under 3 months old, and exclusion of three infants in whom it was not possible to monitor neuromuscular transmission. Therefore the higher dose of vecuronium in this study may reflect the higher median age of the children studied. Of those children who received more than 24 h vecuronium infusion one child had a significant increase in average infusion rate and three (19%) a significant reduction, suggesting cumulation which may reflect the change in clearance of vecuronium during prolonged administration [22]. Median recovery time after discontinuation of vecuronium of 123 min (IQR 80–480) was longer than that reported in children by Hodges [21] (65 min, 55–103) and shorter than that reported in adults by Prielipp et al. [10] (median 178 min), who also reported 13 of 58 adult patients (22%) with prolonged recovery (defined as longer than 2 h).
Segredo et al. [2] have reported prolonged recovery (defined as longer than 6 h) in 7 of 16 adult patients (43%) receiving vecuronium. In this study despite neuromuscular monitoring similar significant prolonged recovery occurred in 6 of 18 children (33%). Assessment of prolonged recovery longer than 24 h (occurring in one vecuronium child, 6%) would have more accurately emphasised the effect of prolongation of mechanical support. However, this study was not designed to assess time to weaning from the ventilator and was not appropriately powered to evaluate this, as a sample 25 times greater would have been required. There was no evidence of a relationship between prolonged recovery of 6 or 24 h and previously described associations (metabolic acidosis, elevated magnesium concentrations, female sex and renal failure) [2].
Clinical practice guidelines for adult patients recommend TOF monitoring with a goal of adjusting degree of NMB to achieve one or two twitches [11], which is not standard practice on PICUs. TOF monitoring over the ulnar nerve in infants and small children was difficult, required careful attention for correct functioning, and was impossible in three of six children under 1 year old. This study could not assess whether neuromuscular monitoring reduced recovery times, but this did result in a reduction in vecuronium dosing.
Cisatracurium dose in this study averaged 3.9±1.3 µg kg−1 min−1 which is within the range quoted by others for children on PICU (1.4–22.7 µg kg−1 min−1 [18] and 5.4±3 µg kg−1 min−1 [17]). Cisatracurium doses in children undergoing anaesthesia have been 1.9±0.4 µg kg−1 min−1 in those aged under 1 year and 2±0.5 µg kg−1 min−1 in those aged over 3 years [15], possibly reflecting shorter duration of infusion. In this study 29% of children receiving cisatracurium longer than 24 h required significant increases in dose, suggesting tachyphylaxis. This has been reported in adults [23] and children [18]. Median recovery times were comparable to those found in adult ICU patients [20].
Certain limitations are evident in this study. Sample size was relatively small, and the significance of increased recovery times for children receiving vecuronium needs to be considered together with wide confidence intervals. Although the goal of the protocol was to maintain a TOF at one twitch, average TOF response did not reach this value in either group, and more than half the children had a TOF count of 0 at discontinuation of NMB drug infusion. Despite frequent monitoring it was difficult to maintain a fixed level of NMB, perhaps due to changing body temperature and alterations in muscle blood flow and electrolytes [24]. In some children a TOF ratio of 1 was not correlated with a level of NMB sufficient adequately to manage clinical endpoints such as elimination of coughing during suctioning. Discrepancy between muscle movement and peripheral nerve stimulation has been described in critically ill children [25] and adults [10]. There is probably a mismatch between depth of NMB in the adductor pollicis response and the diaphragm [24], and therefore a TOF count of 0 does not necessarily represent a failure of monitoring or drug titration.
Adverse effects other than prolonged recovery from NMB for vecuronium were not observed. Prolonged weakness in adults [26] and anaphylaxis in adults [27, 28] and children [29, 30] have been reported with cisatracurium. Direct immunogenicity and acrylate-mediated immune activation has been suggested as a mechanism for cisatracurium-related anaphylaxis [15], and the incidence of anaphylactoid reaction has been estimated to be in the order of 1:10,000 [31]. The numbers in this study are not large enough to assess adverse effects, and it was not the aim of the study to assess the safety profiles of either drug. Clinical practice guidelines for sustained NMB in adults recommend economic analysis when choosing NMB drugs. TOF monitoring has been associated with reduced overall costs [32], and prolonged weakness has been associated with substantially higher hospital costs [1]. Cisatracurium infusion has been found to be the least expensive intermediate-acting NMB drug in children undergoing anaesthesia [33] which is consistent with the findings in this study. It could be argued that intermittent NMB governed solely by individual need is far more effective than continuous infusion. There were some children in whom the risks of missed movement outweighed the benefits of intermittent blockade, for example, those who had undergone tracheal surgery or gastric transposition. However, children undergoing NMB for ARDS or head injury could have been managed with intermittent boluses rather then infusion. In this study NMB drug infusion avoided fluctuations in drug levels and resulted in a more precise determination of drug requirement. Further studies comparing different methods of administration of NMB drugs need to be carried out to assess safety and efficacy.
Conclusion
Cisatracurium infusion in the PICU was characterised by a significantly more rapid recovery than vecuronium and was not associated with prolonged NMB. Neuromuscular twitch monitoring assists in titration of NMB drugs but may be unreliable particularly in children aged less than 18 months and was not sufficient to eliminate prolonged recovery in patients receiving vecuronium.
Reference
Rudis MI, Guslits BJ, Peterson EL, Hathaway SJ, Angus E, Beis S, Zarowitz BJ (1996) Economic impact of prolonged motor weakness complicating neuromuscular blockade in the intensive care unit. Crit Care Med 24:1749–1756
Segredo V, Caldwell JE, Matthay MA, Sharma ML, Gruenke LD, Miller RD (1992) Persistent paralysis in critically ill patients after long-term administration of vecuronium. N Engl J Med 327:524–528
Barnes PK, Renzy-Martin N, Thomas VJ, Watkins J (1986) Plasma histamine levels following atracurium. Anaesthesia 41:821–824
Chapple DJ, Miller AA, Ward JB, Wheatley PL (1987) Cardiovascular and neurological effects of laudanosine. Studies in mice and rats, and in conscious and anaesthetized dogs. Br J Anaesth 59:218–225
Bion JF, Bowden MI, Chow B, Honisberger L, Weatherley BC (1993) Atracurium infusions in patients with fulminant hepatic failure awaiting liver transplantation. Intensive Care Med 19 [Suppl 8]
Manthous CA, Chatila W (1995) Atracurium and status epilepticus? Crit Care Med 23:1440–1442
Lepage JY, Malinovsky JM, Malinge M, Lechevalier T, Dupuch C, Cozian A, Pinaud M, Souron R (1996) Pharmacodynamic dose-response and safety study of cisatracurium (51W89) in adult surgical patients during N2O-O2-opioid anesthesia. Anesth Analg 83:823–829
Kisor DF, Schmith VD, Wargin WA, Lien CA, Ornstein E, Cook DR (1996) Importance of the organ-independent elimination of cisatracurium. Anesth Analg 83:1065–1071
Soukup J, Doenicke A, Hoernecke R, Qass J (1997) Cisatracurium-is the stereoisomer an “ideal” relaxant? Histamine liberation and tryptase determination after bolus administration of cistracurium: a comparison with vecuronium. Anaesthesist 46:486–491
Prielipp RC, Coursin DB, Scuderi PE, Bowton DL, Ford SR, Cardenas VJ Jr, Vender J, Howard D, Casale EJ, Murray MJ (1995) Comparison of the infusion requirements and recovery profiles of vecuronium and cisatracurium 51W89 in intensive care unit patients. Anesth Analg 81:3–12
Murray MJ, Cowen J, DeBlock H, Erstad B, Gray AW Jr, Tescher AN, McGee WT, Prielipp RC, Susla G, Jacobi J, Nasraway SA Jr, Lumb PD, Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM) (2002) Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med 30:142–156
Taivainen T, Meakin GH, Meretoja OA, Wirtavuori K, Perkins RJ, Murphy AK, Fisher GR, Waiter MR (2000) The safety and efficacy of cisatracurium 0.15 mg.kg(-1) during nitrous oxide-opioid anaesthesia in infants and children. Anaesthesia 55:1047–1051
Brandom BW, Woelfel SK, Ference A, Dayal B, Cook DR, Kerls S (1998) Effects of cisatracurium in children during halothane-nitrous oxide anesthesia. J Clin Anesth 10:195–199
Meretoja OA, Taivainen T, Wirtavuori K (1996) Cisatracurium during halothane and balanced anaesthesia in children. Paediatr Anaesth 6:373–378
Ruiter J de, Crawford MW (2001) Dose-response relationship and infusion requirement of cisatracurium besylate in infants and children during nitrous oxide-narcotic anesthesia. Anesthesiology 94:790–792
Tobias JD (1997) A prospective evaluation of the continuous infusion of cis-atracurium for neuromuscular blockade in the pediatric intensive care unit patient: efficacy and dosage requirements. Am J Ther 4:287–290
Odetola FO, Bhatt-Mehta V, Zahraa J, Moler FW, Custer JR (2002) Cisatracurium infusion for neuromuscular blockade in the pediatric intensive care unit: a dose-finding study. Pediatr Crit Care Med 3:250–254
Tobias JD (1997) Increased cis-atracurium requirements during prolonged administration to a child. Can J Anaesth 44:82–84
Rudis MI, Sikora CA, Angus E, Peterson E, Popovich J Jr, Hyzy R, Zarowitz BJ (1997) A prospective, randomized, controlled evaluation of peripheral nerve stimulation versus standard clinical dosing of neuromuscular blocking agents in critically ill patients. Crit Care Med 25:575–583
Newman PJ, Quinn AC, Grounds RM, Hunter JM, Boyd AH, Eastwood NB, Pollard BJ, Pearson AJ, Harper NJ, Beale RJ, Sutjarittam M, Elliot JM, Bion JF (1997) A comparison of cisatracurium (51W89) and atracurium by infusion in critically ill patients. Crit Care Med 25:1139–1142
Hodges UM (1996) Vecuronium infusion requirements in paediatric patients in intensive care units: the use of acceleromyography. Br J Anaesth 76:23–28
Segredo V, Caldwell JE, Wright PMC, Sharma ML, Gruenke LD, Miller RD (1998) Do the pharmacokinetics of vecuronium change during prolonged administration in critically ill patients? Br J Anaesth 80:715–719
Kanji S, Barletta JF, Janisse JJ, Kruse JA, Devlin JW (2002) Tachyphylaxis associated with continuous cisatracurium versus pancuronium therapy. Pharmacotherapy 22:823–830
Harper NJ (1993) Neuromuscular blocking drugs: practical aspects of research in the intensive care unit. Intensive Care Med 19 [Suppl 5]
Pena O, Prestjohn S, Guzzetta CE (2000) Agreement between muscle movement and peripheral nerve stimulation in critically ill pediatric patients receiving neuromuscular blocking agents. Heart Lung 29:309–318
Davis NA, Rodgers JE, Gonzalez ER, Fowler AA, III (1998) Prolonged weakness after cisatracurium infusion: a case report. Crit Care Med 26:1290–1292
Toh KW, Deacock SJ, Fawcett WJ (1999) Severe anaphylactic reaction to cisatracurium. Anesth Analg 88:462–464
Clendenen SR, Harper JV, Wharen RE Jr, Guarderas JC (1997) Anaphylactic reaction after cisatracurium. Anesthesiology 87:690–692
Briassoulis G, Hatzis T, Mammi P, Alikatora A (2000) Persistent anaphylactic reaction after induction with thiopentone and cisatracurium. Paediatr Anaesth 10:429–434
Legros CB, Orliaguet GA, Mayer MN, Labbez F, Carli PA (2001) Severe anaphylactic reaction to cisatracurium in a child. Anesth Analg 92:648–649
Krombach J, Hunzelmann N, Koster F, Bischoff A, Hoffmann-Menzel H, Buzello W (2001) Anaphylactoid reactions after cisatracurium administration in six patients. Anesth Analg 93:1257–1259
Zarowitz BJ, Rudis MI, Lai K, Petitta A, Lulek M (1997) Retrospective pharmacoeconomic evaluation of dosing vecuronium by peripheral nerve stimulation versus standard clinical assessment in critically ill patients. Pharmacotherapy 17:327–332
Splinter WM, Isaac LA (2001) The pharmacoeconomics of neuromuscular blocking drugs: a perioperative cost-minimization strategy in children. Anesth Analg 93:339–344
Acknowledgements
We thank Dr. Angie Wade for statistical help and the PICU nurses for their help in conducting the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burmester, M., Mok, Q. Randomised controlled trial comparing cisatracurium and vecuronium infusions in a paediatric intensive care unit. Intensive Care Med 31, 686–692 (2005). https://doi.org/10.1007/s00134-005-2615-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2615-3