Abstract
Objective
Endotracheal suctioning can cause alveolar collapse and impede ventilation. One reason is the gas flow through a single-lumen endotracheal tube (ETT) provoking a gradient between airway opening and tracheal (Ptr) pressures. Separately extending the patient tubing limbs of a suitable ventilator into the trachea via a double-lumen ETT should maintain Ptr. Can this technique reduce the side effects?
Design and setting
Bench and animal studies in a university hospital laboratory.
Interventions
A lung model was ventilated via single and double-lumen ETTs. Closed-system suctioning was applied with catheters introduced into the single-lumen ETT or the expiratory lumen of the double-lumen ETT via swivel adapter. Seven anesthetized pigs (lungs lavaged) underwent three runs of ventilation and suctioning through (a, b) an 8.0-mm ID single-lumen ETT, (c) a double-lumen ETT (41Ch outer diameter, OD). In (a) the single-lumen ETT was disconnected for suctioning, in (b) and (c) ventilator mode was set to continuous positive airway pressure mode, and the ETTs remained connected.
Measurements and results
Bench: Suction through single-lumen ETTs impaired ventilation and led to strongly negative Ptr (common: −10 to −20 mbar); the double-lumen ETT technique maintained ventilation and pressures. Animals: Lung gas content (computed tomography, n=4) and arterial oxygen partial pressure, initially 1462±65 ml/532±76 mmHg, were significantly reduced by suctioning through single-lumen ETT: to 302±79 ml/62±6 mmHg with disconnection and to 851±211 ml/158±107 mmHg with closed suction. With double-lumen ETT they remained at 1377±95 ml/521±56 mmHg.
Conclusions
The double-lumen ETT technique minimizes side effects of suctioning by maintaining Ptr.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endotracheal suctioning after disconnecting the airway from the ventilator (“open” suctioning) can provoke severe hypoxemia and cardiocirculatory problems [1, 2]. One reason is the interruption of both continuous and cycling elevation in airway opening pressure (Pao) by the ventilator. In addition, a gradient (ΔP) from Pao to tracheal pressure (Ptr) develops because the flow of air drawn down the endotracheal tube (ETT) in compensation for the suction flow meets an ETT resistance elevated by the suction catheter [2, 3]. Alveolar collapse ensues, the degree depending on disease state [1, 2, 4, 5]. Pao can be maintained by leaving the ETT connected to the ventilator (“closed-system” suctioning). However, ΔP is not eliminated and Ptr cannot be controlled; thus the potential for alveolar collapse is reduced but not abolished [3, 6, 7].
Ventilators in pressure preset modes operate as demand flow generators during inspiration as during suctioning. Several models measure the target pressure through their expiratory limb, which is flowless in this phase and reliably transmits the pressure at the junction of the limbs—normally the Y-piece. A double-lumen ETT (DL-ETT) allows extending both limbs into the trachea separately [8, 9, 10], moving the point of pressure regulation there as well [10]. Accordingly, Ptr should be reliably maintained at the set level even during suctioning; −ΔP should be automatically compensated for by the ventilator.
Related questions were to be addressed concerning suctioning and the reduction in its side effects by using a DL-ETT: The pressure-flow-relationship of ETTs partially obstructed by suction catheters were defined. In a mechanical model the hypothesis was tested that Ptr and ventilation are better preserved if closed-system suctioning is applied through a DL-ETT. The short-term effects of suctioning with and without preservation of Ptr were also studied in animals with lungs prone to alveolar collapse. We hypothesized that negative side effects of suctioning can be minimized by maintaining Ptr at the set level. The results were presented in part at the annual meetings of the European Society of Intensive Care Medicine in 2001 and 2003 [11, 12, 13].
Methods
Pressure-flow relationship of endotracheal tubes partially obstructed by suction catheters
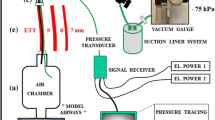
Single-lumen ETTs (SL-ETT; internal diameter, ID: 5.0, 6.0, 7.0, 8.0, and 8.5 mm, lo contour, Mallinckrodt, Athlone, Ireland) and one lumen of a DL-ETT (Mallinckrodt Bronchocath, outside diameter, OD: 13.6 mm, 41 Ch, the bronchial lumen shortened to the length of the tracheal one, 36 cm) were subjected to constant flows up to 1.5 l/s in both directions by a ventilator in volume control mode (Evita 1, Dräger Medical, Lübeck, Germany) with suction catheters (length, 60 cm, OD: 3.3–6.0 mm, 10–18 Ch; Braun, Melsungen, Germany) in place. Signals from a pneumotachygraph (PT; Hamilton Medical, Bonaduz, Switzerland) and a pressure tap between ventilator and ETT were picked up by solid-state transducers (range, ±5 mbar or −30 to +80 mbar; SensorTechnics, Rugby, UK), digitized and analyzed off-line.
Impact of suction on ventilation in a model lung
A VentAid Training/Test Lung (Michigan Instruments, Grand Rapids, Mich., USA; compliance 0.05 l/mbar) was modified to simulate spontaneous ventilation by coupling the two bellows and regularly pressurizing one (the “muscle”) by a ventilator (Evita 1) in biphasic positive airway pressure (BIPAP) mode (high pressure 10 or 20 mbar; respiratory rate, RR, 20 breaths/min; ratio of inspiratory to expiratory time, I:E, 1:2). Ventilation to the “lung” bellows was provided by another ventilator (Evita 2) in spontaneous breathing mode without or with pressure support (10 mbar above continuous positive airway pressure, CPAP, in conjunction with “muscle” pressure of 10 mbar) or in BIPAP mode (high pressure 10 or 20 mbar above positive end-expiratory pressure, PEEP; RR 20 breaths/min, I:E 1:2) with passive model, i.e., the “muscle” ventilator disconnected. The “lung” was connected to the ventilator via PT, a model “trachea” (plexiglass tube, ID 23 mm), and SL-ETT (7.0 or 8.5 mm ID) or DL-ETT (as described above). The two lumina of the DL-ETT were connected separately to the two limbs of the ventilator tubing. Suction (pump model B1102, Weimann Medical Devices, Dänischenhagen, Germany) was applied through catheters (OD, 4.7 mm, 14 Ch, or 6.0 mm, 18 Ch; Braun) introduced into the SL-ETT or the expiratory lumen of the DL-ETT via swivel adapter (Fig. 1, Table 1). The test lung does not have an expiratory reserve volume; mechanical stops prevent excursions below the elastic equilibrium lung volume (EELV) at atmospheric pressure. Since suction can lower end-expiratory volume below EELV, EELV was elevated by PEEP or CPAP up to 25 mbar, which guaranteed free excursions. Adaequate combinations pressures ensured excursions of the test lung to remain within the linear part of its pressure-volume relationship. Signals from PT and pressure taps were processed as described above. Pressure values reported for this part of the study are to be understood as relative to PEEP/CPAP.
Schematic representation of connections for single-lumen endotracheal tube (SL-ETT, left) and double-lumen endotracheal tube (DL-ETT, right) in both parts of the study. The suction catheter shown was only introduced for suctioning and removed during ventilation. For open suctioning through the SL-ETT (animal study) the Y-piece was disconnected from the signal tap in the “Y” position; the tap stayed connected to the ETT and picked up the flow of air the suction drew into the ETT. Letters denote points of measurement of various signals: P pressure; V′ flow; CO2 partial pressure of CO2 in respiratory gas. Further taps for P in the model lung not shown in the drawing: “lung” and “muscle.” For explanation of abbreviations see Table 1
Impact of suction on animals with lungs prone to alveolar collapse
With approval of the Committee on Animal Studies of Uppsala University and in accordance with the NIH “Principles of Laboratory Animal Care” [14], seven pigs weighing 27.1 kg (range 23–32) were premedicated (intramuscularly: 6 mg/kg zolazepam, 6 mg/kg tiletamine, 2.2 mg/kg xylazine, 0.04 mg/kg atropine), anesthetized (induction, intravenously: 2.5 µg/kg fentanyl; maintenance, per hour: 25–50 mg/kg ketamine, 90–180 µg/kg midazolam, 3–6 µg/kg fentanyl, and 0.25–0.50 mg/kg pancuronium), and tracheotomized (7.0 mm ID tracheostomy tube, Mallinckrodt).
Volume-controlled ventilation (VCV) was provided by a Servo 300 ventilator (Siemens-Elema, Solna, Sweden; RR 30 breaths/minute, inspiratory time 25% + 10% end-inspiratory pause (plateau), tidal volume chosen for maintaining PaCO2 between 35 and 45 mmHg, i.e., 8–12 ml/kg, PEEP 5 mbar, FIO2 1.0). Alveolar surfactant was depleted by repeated lavages with 1-l aliquots of body warm isotonic saline solution until PaO2/FIO2 stayed below 100 mmHg. A short piece of plastic tubing (22 mm ID) connected to the tracheostomy tube served as an externalized “trachea” for the ETTs under study (Fig. 1).
A single study course comprised three runs in random order. In one, ventilation and suctioning took place through a DL-ETT (as described above), otherwise through an SL-ETT (Mallinckrodt Lo Contour, 8 mm ID; Fig. 1). The tracheostomy tube was clamped during change of ETTs to avoid pressure loss.
Each run consisted of four phases: (a) 2 min of pressure-controlled ventilation (PCV; RR 15/min, I:E 1:1, PEEP 30 mbar, inspiratory pressure 60 mbar) for complete alveolar recruitment; (b) VCV as described above, but PEEP 16 mbar, for 5 min; (c) suctioning, (d) VCV as in phase 2. Suctioning took place in apnea. In one of the SL-ETT runs the ventilator was disconnected from the ETT; in the other one as well as in the DL-ETT run it stayed connected and was set to CPAP 16 mbar (pressure trigger, minimum threshold). The ventilator was modified to regulate demand flow based on the pressure at the Y-piece (SL-ETT) or the “trachea” (DL-ETT) by deviating the line from its inspiratory pressure transducer to the expiratory limb of its circuit (for details see the Electronic Supplemental Material). The compressed air injector pump (MS-33, AGA, Espoo, Finland, with 2-l receptal) was switched on with suction blocked by a clamp on the connection tube. The suction catheter (5.3 mm, 16 Ch OD; Maersk Medical, Lynge, Denmark) was inserted into the SL-ETT or the expiratory lumen of the DL-ETT via a swivel adapter until it extended 1 cm beyond the tip of the ETT; then suction was started by slowly (2–4 s) releasing the clamp and continued for 20 s. The catheter was withdrawn. Apnea was maintained (5–10 s) for the computed tomography (CT) examination mentioned below before proceeding to phase 4. The negative pressure generated by the pump, −650 mbar during occlusion, fell to −65 mbar (approximate mean) with the steady state flow of 22.0±1.5 l/min after an initial transient of −205 mbar effecting a peak flow of 41.0±8.2 l/min
Signals for flow, pressure, and CO2 were picked up at appropriate locations (Fig. 1; CO2SMO+, Novametrix Medical Systems, Wallingford, Conn., USA) and recorded together with breathwise ventilator data, including dynamic respiratory system compliance [=tidal volume/(inspiratory plateau pressure-PEEP)]. Vascular pressures, electrocardiography, SpO2, and intra-arterial blood gases were monitored (7.5-Ch pulmonary artery catheter, Edwards Lifesciences LLC, Irvine, Calif., USA, in an internal jugular vein; Paratrend 7FL catheter, model S7004S in a carotid artery, with Trend-Care TCM7000 monitor, Diametrics, Roseville, Minn., USA; SC9000XL monitor, Siemens, Danvers, Minn., USA). Arterial blood was sampled for gas analysis before and immediately after suctioning and at the end of the run. In four animals CT images of the thorax (Sensation 16, Siemens, Erlangen, Germany) were taken during apnea immediately after suctioning and during endexpiratory occlusions at the end of phases 2 and 4. These were analyzed for gas content in the lungs by calculating the product of voxel volume times the individual voxels’ fractional gas content, which was assumed to be 0 for voxels with a density of 0 HU or greater, 100% for those with −1000 HU, and proportional to the absolute value of HU between them (Maluna, the Mannheim Lung Analyzing Tool, version 2.02). Two animals were subjected to two study courses, one with and one without CT, thus providing data from nine courses for analysis.
Results are presented as mean ±standard deviation with addition of range, if deemed appropriate. Differences between conditions were tested for significance by analysis of variance and t test for paired samples with Bonferroni’s correction for multiple testing unless stated otherwise.
Results
Bench studies
An increase in ETT resistance by suction catheters was a function of dimensions; catheters of recommended [15] sizes—OD equal to one-half ID of the ETT—increased it approx. 2.5-fold. Regardless of ventilation mode without suction the catheters impeded mainly inspiration with SL-ETTs and expiration with the DL-ETT. Suction through the SL-ETT brought about a further reduction in ventilation and a fall in Ptr, despite the ventilator maintaining set pressures at the Y-piece. In contrast, suction through the DL-ETT restored ventilation and lowered neither Ptr nor pressure in the lung. Suction in PSV rendered the ventilator unable to detect the end of the inspiratory “effort.” It maintained the support pressure level for a few seconds, then went on to deliver gas at CPAP level, resuming its response to trigger signals only after suction had been stopped. Thus breath by breath support was not available during suction. Table 2 summarizes the main results for controlled ventilation and unsupported “spontaneous breathing.” Suction through an SL-ETT forces a ventilator or a patient to bring up higher driving pressures for a given inspiratory flow (Table 3). More detailed data on the ETT pressure-flow relationship and on ventilation during suctioning, including representative tracings of spontaneous breathing under various conditions, are provided in the Electronic Supplemental Material accompanying this publication.
Animal study
The recruitment maneuver and the subsequent VCV created identical starting conditions for the interventions in all runs. End-expiratory occlusion pressure during VCV always equaled set PEEP, i.e., dynamic hyperinflation did not occur. Lower PaCO2 throughout the DL-ETT runs reflected smaller serial dead space. Suction through the SL-ETT effected a ΔP from airway opening to trachea, 7.9±1.2 mbar (6–10) during steady state flow of 22.0±1.5 l/min (19–25) after an initial transient of 22.2±9.6 mbar (12–36) with 41±8 l/min (28–54). With the DL-ETT tracheal pressure was held at the set level throughout the procedure, the pressure at the inspiratory limb connector being higher. Figure 2 shows representative samples of pressures and flow during the intervention. The decrease in Ptr by suctioning paralleled a reduction in lung volume and the appearance of atelectases, which were partially resolved by the subsequent VCV. Figure 3 shows CT images and Fig. 4 traces of ventilated volume immediately after the intervention from a representative study. Suction through the SL-ETT reduced respiratory system compliance and PaO2, most markedly with disconnection (Fig. 5). It also led to a small but significant decrease in central venous pressure followed by a transient increase in systemic and pulmonary arterial pressures 0.5–1 min later. Table 4 summarizes the results.
Traces of flow and pressures during suction in a representative animal. Time points highlighted by arrows: A stop of ventilation, disconnection, or switch to CPAP mode and insertion of suction catheter without suction; B start of suction by unclamping the connection tube; C suction stopped, removal of catheter, continued apnea (5–10 s) for computed tomography; subsequent resumption of ventilation not shown. Time scale divisions denote 1-s intervals. The flow seen while suction was in effect was the gas flowing into the trachea as a compensation for suction flow. The flow tracings for closed suction show some triggering and regulation artifacts superimposed to an otherwise stable demand flow. With the double-lumen endotracheal tube the continuous positive airway pressure was maintained in the “trachea,” with the single-lumen endotracheal tube at the airway opening, i.e., the Y-piece
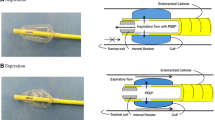
Transversal section images from chest computed tomography in a representative animal. Immediately after the intervention (row 2) atelectases appeared in previously fully recruited lungs (row 1), most prominently after disconnection (column a) and practically absent after suction through the double-lumen endotracheal tube (column c). Breath by breath recruitment after resumption of VCV was incomplete in the single-lumen endotracheal tube runs (row 3, columns a and b)
Recruitment by resumption of ventilation after suction in a representative study. The traces stem from the recorded flow signals. Bars gas volumes measured from computed tomography images; ventilation volumes are referenced to respective computed tomography volumes at end of run. Recruited volume was largest after suctioning with disconnection and smallest after closed suction through the double-lumen endotracheal tube
Discussion
The study shows that the DL-ETT technique allows maintenance of set pressures in the trachea as well as ventilation—both spontaneous and pressure controlled—during suctioning. It minimizes alveolar collapse and the subsequent impairments of gas exchange and respiratory system mechanical properties.
Suctioning side effects and techniques to reduce them
Open suctioning poses well known hazards [1, 9, 16]. The loss of pressure from the ventilator plus the pressure drop along the ETT due to the suction flow cause alveolar collapse [4, 5, 17], the degree of the ensuing hypoxia depending on disease severity [18]. Increasing FIO2 can mitigate hypoxia [1, 19] but may aggravate atelectasis by oxygen absorption [20, 21]. Alveoli are recruited again by ongoing ventilation [5, 9, 20], but maintenance of elevated airway pressure during suctioning is desirable because recruitment by tidal ventilation is incomplete [5]. Ventilating a collapsed lung without a prior dedicated recruitment maneuver can damage it [22], while such maneuvers also bear other risks such as barotrauma, and cycles of collapse and reopening can injure the lungs [23, 24, 25, 26]. Tracheal gas insufflation can elevate airway pressure during suctioning [4] but may cause barotrauma in the case of outflow obstruction [9, 27, 28] because Ptr is not monitored
Closed-system suctioning can maintain elevated airway pressures and ventilation if the ventilator is set properly to deliver no more and no less gas than the suction removes. Otherwise intrapulmonary pressures may fall or rise to potentially dangerous levels [3, 7]. Suction flow usually exceeds minute ventilation, and a sufficiently sensitive trigger must allow for additional breaths [5, 17], i.e., an increase in respiratory rate. This should not be equated with autotriggering, which to many clinicians implies a malfunctioning ventilator. On the other hand, a catheter in the ETT without suction flow impedes expiration and may provoke hyperinflation [3, 7]. Pressure preset modes are considered safer [3, 5] because pressures at the site of measurement stay within the bounds of the settings.
With standard equipment the most distal location for pressure measurement is the Y-piece. Suction-related ΔP from Y-piece to trachea is small under ideal conditions, i.e., catheters as thin and vacuum levels as low as recommended in guidelines [15] plus an ETT free of kinks or secretions that might elevate resistance [3]. The already low risk of alveolar collapse is further reduced by the ongoing ventilation repeatedly expanding the lungs. However, ΔP can be higher for several reasons. A clinician wanting better clearance of thick secretions may use wider suction catheters and a more powerful vacuum than recommended, and kinks or secretions may compromise ETT patency [3]. No clinical study has yet specifically addressed the effective ΔP in clinical practice, but there is indirect evidence from recent patient studies in which the reduction in end-expiratory lung volume during closed-system suctioning was quantified by respiratory inductive plethysmography [5, 17]. This has been measured at −133.2±129.9 [17] and −284±317 ml (–−9 to −841) [5] despite proper trigger setting and catheters of recommended or merely slightly wider sizes. The compensation for ΔP—ongoing VCV [17] or PSV with elevated inspiratory pressure [5]—was by principle unable to adapt to individual patients’ condition. Our study shows a reliable way to minimize these shortcomings: ventilation in a pressure preset mode with regulation of gas delivery based on continuous measurement of Ptr. The ventilator automatically compensates for the momentary individual ΔP even in the absence of cycling ventilation; there is no need for a minimum tidal volume or a recruitment maneuver.
Clinical implications, limitations of the study, and specific features of the setup
The short-term study on a small group of animals and the bench study were intended to show principle features of the DL-ETT technique and to highlight the differences versus suctioning through SL-ETTs. In the animal study we did not include settings ideal for closed-system suctioning through SL-ETTs because, as noted above, previous studies have shown that it can perform well under favorable conditions. However, the negative side effects seem to be almost as pronounced as with open suctioning if conditions are less than ideal. Rather than deviating from the optimum by poorly reproducible ETT obstructions or kinks we chose a catheter elevating ΔP to about 2.5 times the value to be expected with the recommended size (4.0 mm, 12 Ch). And we stopped ventilation, which would have counteracted the alveolar collapse.
The potential reduction in suctioning side effects by maintaining Ptr and thus lung volume may not justify intubating a patient with a special ETT. However, the DL-ETT technique offers additional benefits: it allows better monitoring of respiratory system mechanical properties [8, 29] and minimizes both apparatus dead space and imposed work of breathing [8, 9, 10, 30, 31, 32, 33]. It thus supports strategies to foster spontaneous breathing in a wider range of circumstances as well as a “lung protective ventilation strategy” [34] based on permanent maintenance of sufficient PEEP for collapse prevention, recruitment maneuvers only when necessary, and small tidal volumes for keeping peak distending pressures as low as possible.
Related techniques offer only some of the advantages. Calculation-based automatic tube compensation [35, 36] can compensate for ΔP during ventilation, but during suctioning the underlying assumptions about ETT resistance are invalid. Also, as with measuring Ptr via a separate catheter in an SL-ETT [30, 31], it has no effect on dead space. The coaxial DL-ETT created by introducing an inner tube into an SL-ETT [8, 9] is not meant to allow suctioning while the inner tube is in place, but removing it for the procedure precludes maintenance of pressure.
The DL-ETT technique confines the choice of ventilators to suitable models. The same is true for other techniques, such as the “recruitment” during suction by PSV suggested in [5]. This is feasible only with ventilators continuing cycling support during the procedure, which, for example, is not the case with the Dräger machines used in our bench study. Generally the individual reaction of a ventilator model to closed-system suctioning, which may include malfunction [3], must be considered by the clinician. In the animal study logistical reasons forced us to use a ventilator requiring a modification for performance as needed. The positive results despite less than optimal equipment appear to support the proof of principle.
The DL-ETT uses only part of its cross-section for gas flow at any time. This disadvantage must be compensated by adequate design. Expiration is driven by the elastic recoil of the patient’s respiratory system whereas the ventilator provides the force for inspiration. Hence the expiratory lumen should be as wide as possible while the inspiratory side merely needs to allow the necessary flows with driving pressures less than 100 mbar, the range presently available ventilators can provide without modification. An expiratory lumen such as that of the modified Bronchocath and an inspiratory lumen resembling a 4.5- to 5.0-mm ID SL-ETT would result in a tube with OD equivalent to that of an 8.5 mm ID SL-ETT. Such a device is not available presently. For the present studies the Bronchocath was a suitable surrogate despite its unnecessarily wide inspiratory lumen; previous work has shown that ventilators can cope with a narrow inspiratory lumen in a DL-ETT setup [8, 9].
In the in vivo study we extended the animals’ airway to the outside so we could measure pressure at the tip of the ETTs and change the ETTs between runs without loss of PEEP. This arrangement increased serial dead space by approximately 30 ml, comparable to filters or similar common equipment. The suction catheters did not reach the animal; thus stimulation of the tracheal mucosa was impossible, and the hemodynamic changes seen with suctioning had to be associated with changes in intrathoracic pressures. Reflexes arising from tracheal stimulation as well as those from atelectasis and hypoxia can increase small airway resistance during suctioning [20]. The latter stimulus should be reliably avoidable by the DL-ETT technique.
The impact of the DL-ETT technique on clearance of secretions must be addressed in a separate study. Suction removes only material transported to the central airways; thus both mobilization of secretions in the periphery and the access of the catheter to material in trachea or bronchi are an issue. Closed-system suctioning with any type of ETT may impede access to secretions by the intermittent inspiratory flows from ongoing ventilation [37]. On the other hand, the DL-ETT technique helps to preserve spontaneous breathing during the procedure and thus may enhance patient contribution to mobilization of secretions. As demonstrated in our study, it also allows the use of wider catheters and higher suction flows than presently recommended.
Conclusion
A DL-ETT in conjunction with a suitable ventilator allows reliable preservation of lung volume, oxygenation, spontaneous ventilation, and hemodynamic stability during closed-system suctioning.
References
Barnes CA, Kirchhoff KT (1986) Minimizing hypoxemia due to endotracheal suctioning: a review of the literature. Heart Lung 15:164–178
Johnson KL, Kearney PA, Johnson SB, Niblett JB, MacMillan NL, McClain RE (1994) Closed versus open endotracheal suctioning: cost and physiologic consequences. Crit Care Med 22:658–666
Stenquist O, Lindgren S, Karason S, Søndergaard S, Lundin S (2001) Warning! Suctioning. A lung model evaluation of closed suctioning systems. Acta Anaesthesiol Scand 45:167–772
Brochard L, Mion G, Isabey D, Bertrand C, Messadi AA, Mancebo J, Boussignac G, Vasile N, Lemaire F, Harf A (1991) Constant-flow insufflation prevents arterial oxygen desaturation during endotracheal suctioning. Am Rev Respir Dis 144:395–400
Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, Richard JC, Mancebo J, Lemaire F, Brochard L (2003) Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med 167:1215–1224
Monaco FJ, Meredith KS (1992) A bench test evaluation of a neonatal closed tracheal suction system. Pediatr Pulmonol 13:121–123
Taggart JA, Dorinsky NL, Sheahan JS (1988) Airway pressures during closed system suctioning. Heart Lung 17:536–542
Lethvall S, Sondergaard S, Karason S, Lundin S, Stenqvist O (2002) Dead-space reduction and tracheal pressure measurements using a coaxial inner tube in an endotracheal tube. Intensive Care Med 28:1042
Lethvall S, Lindgren S, Lundin S, Stenqvist O (2004) Tracheal double-lumen ventilation attenuates hypercapnia and respiratory acidosis in lung injured pigs. Intensive Care Med 30:686–692
Pluemers C, Marien T, Reissmann H, Pothmann W, Schulte am Esch J (2002) Double lumen tube abolishes the additional loads on ventilation posed by conventional airway adjuncts. Intensive Care Med 28: Suppl1. S52
Reissmann H, Maisch S, Plümers C, Böhm S, Pothmann W (2001) Ventilation through a double lumen endotracheal tube allows suction without sub-PEEP tracheal pressure. Intensive Care Med 27: Suppl2 S211
Maisch S, Reissmann H, Böhm S, Plümers C, Nierhaus A (2001) Increase of endotracheal tube resistance and interference with ventilation by suction catheters. Intensive Care Med 27: Suppl2 S211
Reissmann H, Böhm SH, Suarez-Sipmann F, Tusman G, Buschmann C, Pesch T, Thamm O, Hedenstierna G (2003) Suctioning through a double lumen ET tube can prevent alveolar collapse and worsening of oxygenation. Intensive Care Med 29: Suppl 1 S149
National Research Council (United States), Commission on Life Sciences, Institute of Laboratory Animal Resources (1996) Guide for the care and use of laboratory animals. National Academy Press
American Association for Respiratory Care (1993) AARC clinical practice guideline. Endotracheal suctioning of mechanically ventilated adults and children with artificial airways. Respir Care 38:500–504
De Campo T, Civetta JM (1979) The effect of short-term discontinuation of high-level PEEP in patients with acute respiratory failure. Crit Care Med 7:47–49
Cereda M, Villa F, Colombo E, Greco G, Nacoti M, Pesenti A (2001) Closed system endotracheal suctioning maintains lung volume during volume-controlled mechanical ventilation. Intensive Care Med 27:648–654
Carlon GC, Fox SJ, Ackerman NJ (1987) Evaluation of a closed-tracheal suction system. Crit Care Med 15:522–525
Craig KC, Benson MS, Pierson DJ (1984) Prevention of arterial oxygen desaturation during closed-airway endotracheal suction: effect of ventilator mode. Respir Care 29:1013–1018
Lu Q, Capderou A, Cluzel P, Mourgeon E, Abdennour L, Law-Koune JD, Straus C, Grenier P, Zelter M, Rouby JJ (2000) A computed tomographic scan assessment of endotracheal suctioning-induced bronchoconstriction in ventilated sheep. Am J Respir Crit Care Med 162:1898–1904
Neumann P, Rothen HU, Berglund JE, Valtysson J, Magnusson A, Hedenstierna G (1999) Positive end-expiratory pressure prevents atelectasis during general anaesthesia even in the presence of a high inspired oxygen concentration. Acta Anaesthesiol Scand 43:295–301
Rimensberger PC, Pristine G, Mullen JBM, Cox PN, Slutsky AS (1999) Lung recruitment during small tidal ventilation allows minimal positive end-expiratory pressure without augmenting lung injury. Crit Care Med 27:1940–1945
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. JAMA 282:54–61
Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF (2003) Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med 167:1620–1626
Taskar V, John J, Evander E, Robertson B, Jonson B (1997) Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med 155:313–320
Suh GY, Koh Y, Chung MP, An CH, Kim H, Jang WY, Han J, Kwon OJ (2002) Repeated derecruitments accentuate lung injury during mechanical ventilation. Crit Care Med 30:1848–1853
Kacmarek RM (2002) A workable alternative to the problems with tracheal gas insufflation? Intensive Care Med 28:1009–1011
Nahum A, Marini JJ (1994) Tracheal gas insufflation as an adjunct to conventional ventilation. Yearbook of intensive care and emergency Medicine (Baltimore) 17:511–523
Karason S, Søndergaard S, Lundin S, Wiklund J, Stenquist O (2001) Direct tracheal airway pressure measurements are essential for safe and accurate dynamic monitoring of respiratory mechanics. A laboratory study. Acta Anaesthesiol Scand 45:173–179
Messinger G, Banner MJ, Blanch PB, Layon AJ (1995) Using tracheal pressure to trigger the ventilator and control airway pressure during continuous positive airway pressure decreases work of breathing. Chest 108:509–514
Banner MJ, Blanch PB, Gabrielli A (2002) Tracheal pressure control provides automatic and variable inspiratory pressure assist to decrease the imposed resistive work of breathing. Crit Care Med 30:1106–1011
Larsson A (1992) Elimination of apparatus dead space—a simple method for improving CO2 removal without increasing airway pressure. Acta Anaesthesiol Scand 36:796–799
Liebenberg CS, Raw R, Lipman J, Moyes DG, Cleaton-Jones PE (1999) Small tidal volume ventilation using a zero deadspace tracheal tube. Br J Anaesth 82:213–216
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino G, Filho GL, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, de Carvalho CR (1998) Effects of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Guttmann J, Eberhard L, Fabry B, Bertschmann W, Wolff G (1993) Continuous calculation of intratracheal pressure in tracheally intubated patients. Anesthesiology 79:503–513
Fabry B, Haberthür C, Zappe D, Guttmann J, Kuhlen R, Stocker R (1997) Breathing pattern and additional work of breathing in spontaneously breathing patients with different ventilatory demands during inspiratory pressure support and automatic tube compensation. Intensive Care Med 23:545–552
Lindgren S, Almgren B, Högman M, Lethvall S, Houltz E, Lundin S, Stenqvist O (2004) Effectiveness and side effects of closed and open suctioning: an experimental evaluation. Intensive Care Med 30:1630–1637
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Reissmann, H., Böhm, S.H., Suárez-Sipmann, F. et al. Suctioning through a double-lumen endotracheal tube helps to prevent alveolar collapse and to preserve ventilation. Intensive Care Med 31, 431–440 (2005). https://doi.org/10.1007/s00134-004-2537-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2537-5