Abstract
Objective
To determine the homeostatic balance of patients with ventilator-associated pneumonia (VAP) with respect to the adequacy of antimicrobial therapy.
Design and setting
Descriptive observational study in a 12-bed medical intensive care unit in a university-affiliated hospital.
Patients
Twenty-nine patients with VAP documented by quantitative culture of bronchoalveolar secretions and a control group of eight mechanically ventilated patients.
Methods
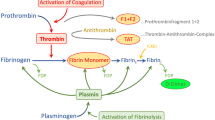
Serial bronchoalveolar lavage fluid (BALF) samples were assayed for prothrombin activation fragment (F1+2), thrombin-antithrombin (TAT) complex, fibrinolytic activity, urokinase-type plasminogen activator (u-PA), and plasminogen activator inhibitor type 1 (PAI-1) on days 1, 4, and 7 after VAP onset.
Results
Pathogens isolated from patients with inadequate empirical antimicrobial coverage included methicillin-resistant Staphylococcus aureus (n=2), Pseudomonas aeruginosa (n=4), and Acinetobacter baumannii (n=1). Compared to those who received adequate antibiotic therapy, TAT, F1+2, and PAI-1 levels increased while u-PA levels remained unchanged. Despite antibiotic adjustment on day 4, TAT levels remained elevated in those who lacked adequate antimicrobial coverage and were significantly correlated with PaO2/FIO2. The procoagulant activity was accompanied by a local depression of fibrinolytic capacity that was attributed mainly to increased BALF PAI-1 levels. Nonsurvivors showed significantly higher levels of TAT and PAI-1 than survivors. No significant correlation between the bacterial burden and the homeostatic derangements was documented.
Conclusions
The lung inflammatory response seems to promulgate a local procoagulant activity associated with hypoxemia in those with inadequate antibiotic therapy. The homeostatic derangement seems to be independent of the lung bacterial burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventilator-associated pneumonia (VAP) is a frequent complication in patients requiring mechanical ventilation with an incidence rate of 10–25% [1, 2] and a crude mortality ranging from 10% to 40% [3]. Mortality rates, however, have been reported as high as 91% in patients receiving inadequate antibiotic therapy [4]. In these patients refractory hypoxemia, increased capillary permeability, and reduced lung compliance often herald the terminal event.
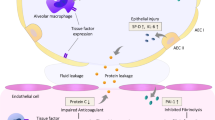
During severe infection pronounced disturbances in intra-alveolar homeostatic response have been described in both experimental models of sepsis and endotoxemia [5] and in mechanically ventilated patients with severe pneumonia [6]. An increase in procoagulant activity and a decrease in pulmonary fibrinolysis are consistently reported. These changes are thought to represent transient abnormalities with complete restitution of the delicate gas exchange barrier. However, in persistent inflammatory condition thrombin and fibrin degradation products may compromise the endothelial monolayer integrity [7] and lead to overwhelming systemic inflammatory response causing multiorgan system failure. As such, differential alveolar hemostatic balance in regard to appropriateness of antimicrobial therapy has not hitherto been performed either clinically or experimentally. We hypothesized that inadequate antimicrobial therapy alters the homeostatic balance in favor of a persistent procoagulant state. The current investigation was undertaken to delineate the temporal impact of empirical antimicrobial therapy on the alveolar coagulation and fibrinolytic pathways of patients with VAP. (Some of the results of this study have been reported previously in the form of an abstract [8].)
Methods
Study population
The study was conducted in a 12-bed medical intensive care unit at a tertiary hospital affiliated with the University at Buffalo over a 16-month period. The protocol was approved by the local institutional review board, and informed consent was obtained from the health care proxy or the next of kin. A total of 53 consecutive patients requiring mechanical ventilation for at least 72 h were considered for inclusion if they developed new pulmonary infiltrates on chest radiography along with at least two of the following criteria: (a) fever 38°C or higher, (c) leukocytes 10,000 mm3 or more, (c) purulent respiratory secretions. Of the 53 patients with suspected VAP 29 completed the study; 7 were extubated prior to completion of the protocol, 4 were excluded because of lack of consent or refusal to participate, 2 received antibiotics prior to performing the bronchoalveolar lavage (BAL) procedure at VAP onset, 2 had positive blood cultures, and 9 did not meet the threshold for the definition of VAP. Of the 29 patients who completed the study 22 (76%) received adequate antimicrobial therapy and 7 had at least one micro-organism resistant to the antibiotic coverage prescribed on suspected VAP onset. The reason for mechanical ventilation was chronic obstructive pulmonary disease (n=4), severe community acquired pneumonia (n=9), septic shock (n=7), cerebrovascular accident (n=6), and postoperative respiratory failure (n=3). The general characteristics of the study population are shown in Table 1. There were no differences with regards to age, gender, use of previous antibiotics, radiological scores, or underlying comorbid diseases between the two groups. The severity of illness and the length of time on mechanical ventilation prior to the onset of VAP were significantly different, however. There was at least one comorbid illness in 76%, and 15% had two or more. In patients with more than one VAP episode during the study period only the first infection was included in the analysis to ensure independence of observations. Exclusion criteria included bacteremia, acute respiratory distress syndrome, initiation of antibiotic therapy within 48 h of clinical suspicion of VAP for alternative reasons, anticoagulation therapy, immunosuppression, organ transplantation, and the presence of hematological or solid malignancies.
Data collection
Data recorded at study enrollment included age, gender, comorbid diseases, reasons for mechanical ventilation, duration of mechanical ventilation before study onset, antibiotics prior to VAP onset, radiological score [9], and Acute Physiology and Chronic Health Evaluation (APACHE) II score [10]. Clinical and laboratory data were collected daily from the onset of VAP up to 7 days. The choice of initial antimicrobial therapy was determined by the treating physicians. Patients suspected of having VAP underwent bronchoscopic BAL prior to initiation or change in antimicrobial coverage. Four aliquots (20 ml each) of sterile saline were instilled and aspirated. The first 20 ml recovered was discarded. The rest of the samples were pooled together. One-half the amount was sent for microbiology processing, and the rest was filtered through two layers of sterile gauze and centrifuged at 250 g for 10 min at 4°C. The cell free supernatant was stored in small aliquots at −70°C for homeostatic assays. Cells were resuspended in phosphate-buffered saline and counted by means of a Neubaeur chamber as described elsewhere [11]. The viability of the cells was assessed by trypan blue. Differential cell counts were performed on cytopsin preparations stained with a modified Giemsa-based Diff-Quick stain (Baxter Scientific Products, McGraw Park, Ill., USA). BAL fluid (BALF) total proteins levels were measured by a modified Lowry assay [12].
Microbiological analysis
Microbiological specimens were processed as described previously [13]. Both Gram and Wright stains were carried out on cytocentrifuge preparations. Patients were considered to have VAP when the following criteria were present: 2% or more of the cells in the cytocentrifuge preparations from BALF contained intracellular bacteria and at least one bacterial species grew at a concentration of104 cfu/ml or more from the BALF sample.
Homeostatic activity
Coagulation activity in BALF was determined by measuring specific markers for thrombin generation: prothrombin fragments F1+2 and thrombin-antithrombin complexes using their respective enzyme-linked immunosorbent assays (ELISAs; Hemex Laboratories, Phoenix, Ariz., USA). The activity of the fibrinolytic system was assayed by an amidolytical assay as previously described [14]. Urokinase-type plasminogen activator (u-PA) and plasminogen activator inhibitor type 1 (PAI-1) levels were measured with their respective ELISAs (Hemex). BALF controls were obtained from eight patients without cardiac or pulmonary disease who were intubated for airway protection. Patients with suspected but unconfirmed pneumonia were not included in the control group. A repeat non-bronchoscopic-directed BAL (Ballard Medical Products, Draper, Utah, USA) was performed on day 4 before adjusting the antibiotics for the susceptibility patterns of recovered pathogens, and on day 7 of VAP onset. Sequential microbiological and coagulation profiles were determined at each of these time points as described previously.
Definitions
Adequate therapy was defined as the use of at least one antibiotic to which all isolates recovered from BALF were susceptible in vitro. Septic shock was defined as a systemic inflammatory response to infection (i.e., the presence of two or more of the following: temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min or PaCO2 <32 torr, and leukocyte count >12,000 cells/mm3 or >10% bands) in the presence of hypotension (systolic blood pressure <90 mmHg or a reduction of >40 mmHg from baseline in the absence of other causes) [15].
Statistical analysis
Descriptive analysis was performed using the NCSS 2000 software (NCSS Statistical Analysis System, Kaysville, Utah, USA). Means were compared using Student’s t test when normally distributed and the Mann-Whitney test otherwise. Proportions were compared using the χ2 test with Yate’s correction or Fisher’s exact test when necessary. A nonlinear regression analysis was performed to look at the association between the thrombin antithrombin levels and the PaO2/FIO2 ratio. Analysis of variance for repeated measures was carried out for sequential measurements and post hoc tests were used for comparison of all pairs of columns. Statistical significance was defined as p<0.05.
Results
Microbiological findings
Overall 35 pathogens were recovered from bronchoalveolar sampling (Table 2). The most frequently isolated organisms were Staphylococcus aureus (n=9) and Pseudomonas aeruginosa (n=11). Seven of the nine S. aureus isolates were methicillin resistant. Gram-negative enteric bacilli were more likely to be isolated from the group of patients receiving adequate antimicrobial therapy than in those who were inadequately treated. Polymicrobial infection was present in six patients. Two patients had S. aureus plus P. aeruginosa; the others were S. aureus plus Escherichia coli, S. aureus plus Streptococcus viridans, Acinetobacter baumannii plus P. aeruginosa, and Stenotrophomona maltophilia plus P. aeruginosa.
The most frequently prescribed antibiotics for the 22 patients with adequate antimicrobial coverage were combination of piperacillin/tazobactam and vancomycin (n=11), imipenem and vancomycin (n=7), piperacillin/tazobactam plus gentamycin plus vancomycin (n=3), and cefipime and amikacin (n=1). In those who received initial inadequate antimicrobial coverage empirical antimicrobial coverage included monotherapy in two cases and consisted of piperacillin/tazobactam in one and cefipime in the other. A combination of ciprofloxacin plus gentamycin plus vancomycin (n=1), piperacillin/tazobactam plus ciprofloxacin (n=3), and cefipime plus flagyl (n=1).
Course of clinical and infectious parameters
Figure S 1 (a–c) shows the time-dependent analysis performed for the clinical indices of the study population. Leukocyte count, temperature, and PaO2/FIO2 ratio were similar between those who had adequate and those had inadequate therapy on the day on which VAP was diagnosed. The mean positive end-expiratory pressure levels were also comparable between the two groups (5.2±0.7 vs. 5.1±0.4 cmH2O, respectively). The trends began to diverge on day 3 and reached statistical significance on day 4 for temperature (99.0±1.8°F vs. 100.7±1.8°F, p=0.001) and leukocyte count (8.8±1.9 vs. 11.8±3.6 cells/mm3, p<0.001). The PaO2/FIO2 ratio lagged behind the other variables in term of improvement until day 5 (296.9±90.0 vs. 139.5±83.1, p<0.001) and remained significantly different between the two groups even after the antimicrobial coverage was adjusted for susceptibilities. The bacterial load remained elevated in those who received inadequate antibiotic coverage but declined following change of antibiotic treatment (Fig. S.2). No significant differences were found when comparing the daily changes in leukocyte counts, temperatures, and PaO2/FIO2 ratio and the predominant pathogens.
Coagulation profiles
The prothrombin activation fragments F1+2 and the thrombin-antithrombin complex levels in BALF were higher at the time of study enrollment for in groups than in controls (Fig. 1a, 1b). While thrombin-antithrombin levels declined in those who received adequate treatment, thrombin generation remained elevated in those who had inadequate coverage as reflected by a persistent production of prothrombin activation fragments (p=0.001) and a 1.4-fold increase in thrombin-antithrombin levels on day 4 after VAP onset (p=0.03). Interestingly, thrombin-antithrombin levels remains elevated on day 7 in those who lacked adequate antimicrobial coverage (p<0.001) even though the prothrombin generation dropped during the same period. The ratio of PaO2/FIO2 to thrombin antithrombin levels was notably lower in those who received inadequate antibiotic treatment (Fig. 2). Although we did not find a correlation between bacterial load and that of coagulation indicators, there was a significant correlation between PaO2/FIO2 and the thrombin antithrombin complex levels (R2=0.61, p<0.001; Fig. S.3).”
Bronchoalveolar lavage fluid procoagulant activity—thrombin-antithrombin (TAT) complexes and F1+2 levels— in those who received adequate antibiotic coverage (closed circles) and those who did not (open squares). *p<0.05, †p<0.001 (Bonferroni’s post hoc analysis) between day 1 and day 4 in those with inadequate antimicrobial coverage
The overall BALF fibrinolytic activity was substantially lower in both groups of patients with VAP than in controls (Fig. 3a). The most prominent suppression was noted in patients who received inadequate antimicrobial therapy. Analysis of this reduction showed that this was caused predominantly by a rise in the concentrations of PAI-1 (Fig. 3b) rather than u-PA levels (Fig. 3c). Even after antibiotic change was implemented on day 4, the levels of PAI-1 remains elevated on day 7 in those who had initial inadequate antimicrobial coverage although not statistically significant (p=0.2).
Bronchoalveolar lavage fluid fibrinolytic activity (a), urokinase-type plasminogen activator (u-PA, b) and plasminogen activator inhibitor type 1 (PAI-1, c) levels in those who received adequate antibiotic coverage (closed circles) and those who did not (open squares). *p<0.05, † p=0.009 (Bonferroni’s post hoc analysis) between day 1 and day 4 in those with inadequate antimicrobial coverage
Outcome
The crude mortality for the study group was 28% (8 of the 29). Five patients succumbed to multiorgan system failure. Two died of septic shock related to Pseudomonas aeruginosa and Candida glabrata, and one had life support measures withdrawn. Four of those who received inadequate antimicrobial therapy (57%) died, compared to four of those who were treated appropriately (18%; p=0.07). Of interest, there was a significant difference in day 7 levels of BALF thrombin-antithrombin complexes between those who survived and those who died (p=0.04; Fig. 4). A similar trend was also observed in BALF PAI-1 levels, but the difference did not reach statistical significance (p=0.1).
Discussion
The most important findings of this study demonstrate: (a) an intra-alveolar shift of the local homeostatic balance to the procoagulant side, (b) a significant correlation between severity of hypoxemia and thrombin-antithrombin complexes, and (c) a lack of normalization of the fibrinolysis pathway despite antibiotic adjustment.
Alveolar homeostasis in severe pneumonia has been investigated in both experimental and clinical studies; however, the temporal patterns of change in the coagulation pathway have not been described previously, particularly in regard to adequacy of antimicrobial therapy. In the current study we observed a rapid resolution of the homeostatic disturbances in patients who had adequate empirical antimicrobial coverage. In comparison, there was a marked increase in the procoagulant activity in the alveolar lining milieu in those with initial inadequate antimicrobial coverage. The rise in thrombin-antithrombin complex and the prothrombin activation peptide fragment F1+2 paralleled the threefold increase in these markers in the BALF in experimental models of low-dose endotoxemia [5]. Similarly, our findings are supported by previous observations in BALF of patients with severe pneumonia requiring mechanical ventilation. Gunther and colleagues [6] described a twofold increase in the magnitude of procoagulant activity of subjects with respiratory failure attributed to severe pneumonia compared to control subjects. This increase was linked exclusively to the activation of the extrinsic coagulation pathway as was evidenced by an increase in tissue factor and factor VII activities [6, 16]. Although we have not elucidated the pathway by which the increased coagulation has occurred, clinical evidence for the role of tissue factor in BALF coagulation is derived from studies in which inhibition of the tissue factor/factor VII pathway inhibited thrombin generation and fibrin deposition [17].
We observed a persistent elevation in thrombin-antithrombin complex levels in those who received inadequate antimicrobial coverage despite antibiotic adjustment. The increased fibrin deposition is thought to be related to either enhanced procoagulant, decreased fibrinolysis, or both. We found a persistent depression of u-PA levels starting with the occurrence of VAP and lasting across the period of the study irrespective of the change in the antibiotic treatment. We attribute the absence of significant variability to the complex interaction of u-PA with its receptor. A recent experimental study in murine model suggested that the net balance of unoccupied u-PA receptors is the one responsible for inflammatory cell recruitment into the lungs during pneumonia [18]. By comparison, PAI-1 concentrations in the BALF of patients who received inadequate antimicrobial treatment reached a peak on the fourth day of VAP and then declined gradually without, however, returning to control levels. A similar trend was also observed in a recent study that documented a suppressed fibrinolytic activity in the alveolar space of patients with VAP [18]. In a similar design, Shultz and colleagues [19] noted that the diagnosis of VAP was preceded by a decrease in fibrinolytic activity in bronchial lavage which appeared to be caused by a marked increase in levels of PAI-1. The significance of these findings is highlighted by the increased mortality in patients with sepsis with elevated PAI-1 activity [20, 21]. Our results suggest this potential association but are not conclusive because the study was not adequately powered to investigate this association.
As a result of the homeostatic imbalance, deposition of intra-alveolar fibrin may well contribute to the poor gas exchange seen in those who received inadequate treatment. Fibrin has been shown to impair surfactant function by incorporating the lipophilic surfactant components into the fibrin clot [22]. The loss of the surfactant properties leads to alveolar instability and increased shunt fraction across the pulmonary vasculature. While this phenomenon could be transient in nature, the accumulation of fibrin may lead to enhanced local inflammation which might, at least in the initial phase of the lung injury, play a positive role in pulmonary host defense. If persistent, however, the inflammatory process could overwhelm the homeostatic balance resulting in spill over to the systemic circulation. This sequence could potentially initiate the sequence of multiorgan system failure. Although these findings may be nonspecific, the elevated thrombin-antithrombin complexes in those who died support this hypothesis. However, more clinical studies are needed to confirm this theory.
Potential limitations worth of consideration include the higher severity of illness in those who received inadequate antimicrobial therapy that might account for the advanced homeostatic disturbances in this group of patients. The absence of significant difference in the coagulation/fibrinolytic parameters on admission argues, however, against this possibility. Second, the sample size was relatively small given the difficulty of enrolling and retaining critically ill patients enrolled for the duration of the study. This limitation should be considered when interpreting the relationship between BALF PAI-1 levels and outcome. Third, we did not measure the changes in plasma levels of corresponding homeostatic indices over the duration of the trial. However, previous studies have demonstrated lung compartmentalization with regard to both to inflammatory cytokines production [23] and thrombin generation [6, 19] with minimal or no changes in plasma. Third, our results cannot rule out a possible relationship between the virulence of the infectious agents and the degree of activation of the coagulation disorders in the alveolar space. Further studies are needed to elucidate the role of bacterial-specific antigens and the coagulation cascade.
Conclusion
Our prospective temporal evaluation of the intra-alveolar homeostatic balance in patients with VAP revealed a procoagulant shift along with depression of fibrinolysis, most notably in patients who received inadequate antimicrobial coverage. The extent of fibrin deposition was correlated with the severity of hypoxemia observed in clinical trials of VAP patients with poor outcome. Further studies are needed to determine whether local control of thrombin generation or enhanced fibrin degradation would translate into improved outcome.
References
Ibrahim EH, Tracy L, Hill C, Fraser V, Kollef M (2001) The occurrence of ventilator associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest 120:555–561
Chastre J, Fagon JY (2002) Ventilator associated pneumonia. Am J Respir Crit Care Med 165:867–903
Craven DE, Steger KA (1996) Nosocomial pneumonia in mechanically ventilated adult patients: epidemiology and prevention in 1996. Semin Respir Infect 11:32–53
Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly E (1997) Impact of BAL data on the therapy and outcome of ventilator associated pneumonia. Chest 111:676–685
Levi M, van der Poll T, ten Cate H, Kuipers B, Biemond BJ, Jansen HM, ten Cate J (1998) Differential effects of anti-cytokine treatment on bronchoalveolar hemostasis in endotoxemic chimpanzees. Am J Respir Crit Care Med 158:92–98
Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wolbruck B, Seeger W (2000) Alveolar fibrin formation caused by enhanced procaogulant and depressed fibrinolytic capacities in severe pneumonia. Am J Respir Crit Care Med 161:454–462
Ge M, Tang G, Ryan TJ, Malik AB. Fibrinogen degradation product fragment D induces endothelial cell detachment by activation of cell-mediated fibrinolysis (1992) J Clin Invest 90:2508–2516
El-Solh A, Okada M, Dhillon R, Aquilina A, Berbary E (2004) Homeostatic activity in inadequately treated ventilator associated pneumonia. Am J Respir Crit Care Med 169:A373
Fagon JY, Chastre J, Hance AJ, Guiguet M, Trouillet J, Domart Y, Pierre J, Gibert C (1988) Detection of nosocomial lung infection in ventilated patients: use of a protected specimen brush hand quantitative culture techniques in 147 patients. Am Rev Respir Dis 138:110–116
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Agusti C, Xaubet A, Luburich P, Ayuso MC, Roca J, Rodriguez-Roisin R (1996) Computed tomography-guided bronchoalveolar lavage in idiopathic pulmonary fibrosis. Thorax 51:841–845
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
El-Solh A, Aquilina A, Dhillon R, Ramadan F, Nowak P, Davies J (2002) Impact of invasive strategy on management of antimicrobial treatment failure in institutionalized older people with severe pneumonia. Am J Respir Crit Care Med 166:1038–1043
Verheijen J, Mullaart E, Chang GT, Kluft C, Wijngaards G (1982) A simple spectrophotometric assay for extrinsic (tissue type) plasminogen activator applicable to measurements in plasma. Thromb Haemost 48:266–269
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) ACCP-SCCM Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101:1644–1655
Idell S, Gonzalez K, Bradford H, MacArthur CK, Fein AM, Maunder RJ, Garcia JG, Griffith DE, Weiland J, Martin TR (1987) Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Contribution of tissue factor associated with factor VII. Am Rev Respir Dis 136:1466–1474
Biemond BJ, Levi M, ten Cate H, Soule HR, Morris LD, Foster DL, Bogowitz CA, van der Poll T, Buller HR, ten Cate JW (1995) Complete inhibition of endotoxin-induced coagulation activation in chimpanzees with a monoclonal Fab fragment against factor VII/VIIa. Thromb Haemost 73:223–230
Rijneveld A, Levi M, Florquin S, Speelman P, Carmeliet P, van Der Poll T (2002) Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J Immunol 168:3507–3511
Schultz M, Millo J, Levi M, Hack C, Weverling G, Garrard C, van der Poll T (2004) Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax 59:130–135
Pralong G, Calandra T, Glauser MP, Schellekens J, Verhoef J, Bachmann F, Kruithof EK (1989) Plasminogen activator inhibitor 1: a new prognostic marker in septic shock. Thromb Haemost 61:459–462
Mesters RM, Florke N, Ostermann H, Kienast J (1996) Increase of plasminogen activator inhibitor levels predicts outcome of leukocytopenic patients with sepsis. Thromb Haemost 75:902–907
Gunther A, Kalinowski M, Elssner A, Seeger W (1994) Clot-embedded neutral surfactant: kinetics of fibrinolysis and surface activity. Am J Physiol 267:L618–L624
Millo JL, Schultz M, Williams C, Weverling GJ, Ringrose T, Mackinlay CI, van der Poll T, Garrard CS (2004) Compartmentalization of cytokines and cytokine inhibitors in ventilator associated pneumonia. Intensive Care Med 30:68–74
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported in part by the American Lung Association of New York
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
El-Solh, A.A., Okada, M., Pietrantoni, C. et al. Procoagulant and fibrinolytic activity in ventilator-associated pneumonia: impact of inadequate antimicrobial therapy. Intensive Care Med 30, 1914–1920 (2004). https://doi.org/10.1007/s00134-004-2391-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2391-5