Abstract
Objective
To evaluate, in a model of cerebral air embolism (CAE), the effects of ventilation-induced hypocapnia and hyperoxemia on intracranial pressure (ICP), cerebral perfusion pressure (CPP), brain oxygen (PbrO2), brain carbon dioxide (PbrCO2), brain pH (brpH) and levels of brain glucose and lactate.
Design and setting
Prospective animal study in a university medical center.
Subjects
Fifteen Landrace/Yorkshire pigs.
Interventions
In 15 anesthetized pigs ICP, PbrO2, PbrCO2 and brpH were measured with multi-parameter sensors, and brain glucose and lactate by microdialysis. All these parameters were recorded for 2 h after injection of air into the internal carotid artery. Nine animals were hyperventilated (PaCO2 ±25 mmHg) and hyperoxygenated (FiO2 1.0) and six animals were normoventilated (PaCO2 ±40 mmHg with an FiO2 0.4) and served as controls.
Results
In the treatment group the ICP rose from 8±1 to 52±6 mmHg, which was similar to that in the control group (12±1 to 57±8 mmHg). At the end of the 2-h study period, there were no significant differences in PbrO2, PbrCO2 and brpH between the two groups. The decreased brain glucose and increased brain lactate reached severe pathological values in both groups by the end of the 2-h study period.
Conclusions
Hypocapnia and hyperoxemia in acute CAE did not improve pathological functional brain parameters compared with normoventilated controls. Similarly, the pathological changes in brain glucose/lactate could also not be improved by hypocapnia and hyperoxemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral air embolism (CAE) is a potential life-threatening result of many medical and surgical procedures [1, 2]. It may also occur as a result of various diagnostic and surgical procedures when air is accidentally infused into the systemic circulation [3]. Air bubbles which occlude the brain vasculature may cause acute neurological events, such as coma, disorientation, focal sensory deficits or motor deficits [4]. Another problem faced by clinicians is CAE occurring in divers due to rapid ascent [5]. In addition, arterial air embolism can lead to various cardiovascular symptoms, regardless of the underlying cause [1, 6].
The acute therapy for CAE consists of administration of 100% oxygen in combination with hyperventilation [7, 8]. The rationale for this combined intervention is twofold: first, the increase in FiO2 will improve the ‘off-gassing’ of the bubbles and also improve oxygenation in occluded brain tissue by diffusion and, second, the hyperventilation-induced hypocapnia should induce a reduction in intracranial pressure (ICP), improving cerebral perfusion [8].
However, there is no proof as to whether this treatment does lead to functional improvement of brain parameters. In the current study, therefore, we wanted to investigate whether it would be rational to use hyperventilation in combination with hyperoxemia to improve the functional state of the brain after air embolization. For this purpose, ICP, cerebral perfusion pressure (CPP), brain oxygen (PbrO2), brain carbon dioxide (PbrCO2) and brain pH (brpH) were measured using the microsensor technology [9, 10], and the brain metabolism of glucose and lactate were measured using microdialysis [11, 12]. We hypothesized that, after air embolization, hypocapnia combined with hyperoxemia will improve the functional state of the brain.
Methods
Animal care
This study was approved by the Animal Committee of the Erasmus Medical Center Rotterdam. Care and handling were in accordance with the European Community guidelines.
Instrumentation and microdialysis analyses
In 15 crossbred Landrace/Yorkshire pigs (30–35 kg) of either sex anesthesia was induced with 10 mg/kg i.m. ketamine (Ketalin 100 mg/ml, Apharmo, Arnhem, The Netherlands) and 0.1 mg/kg i.m. midazolam (Dormicum, Roche Ned., Mijdrecht, The Netherlands). Muscle relaxation was induced by 0.2 mg/kg i.v. pancuronium bromide (Pavulon, Organon Teknika, Boxtel, The Netherlands).
After intubation, the animals were connected to a ventilator (Servo Ventilator 300, Siemens-Elema, Sweden) and ventilated in a pressure-controlled mode according to the ‘open lung concept’ [13]. After the initial recruitment maneuver (2–3 breaths up to a pressure of 40 cmH2O) all the animals were ventilated with a PEEP of 6 cmH2O, peak pressure of 15±3 cmH2O, an I/E ratio 1:2 and an inspired oxygen fraction (FiO2) of 0.4. The applied pressure differences resulted in tidal volumes of 10–12 ml/kg and the frequency was set to maintain normocapnia (PaCO2 of 35–40 mmHg). Arterial blood gases were measured by the ABL 505 (Radiometer, Copenhagen, Denmark).
Anesthesia was maintained with i.v. ketamine (10 mg/kg per h) and midazolam (1 mg/kg per h). Although ketamine is known as a cerebro-vasodilator, we do not believe it adversely affected the overall study because ketamine combined with midazolam was reported to have no harmful side effects on cerebral hemodynamics in a study on head-injured patients [14]. Muscle relaxation was maintained with i.v. pancuronium bromide (starting with 0.2 mg/kg per h and adapted as required) while body temperature (measured rectally) was kept within the normal range (37–38°C) by means of a heating mattress.
Subsequently, arterial catheters were inserted in both femoral arteries. A multi-parameter sensor (Paratrend/Trendcare, Philips Medical, Böblingen, Germany) was inserted through one of the catheters for continuous measurements of PaO2, PaCO2, pH and temperature, and calibrated with conventional blood gas analyses (ABL 505). The arterial blood pressure and heart rate were measured using a transducer (Statham P23XL, Spectramed, Oxnard, CA, USA) and continuously recorded on a Hewlett Packard monitor (Merli 68S, Agilent, Böblingen, Germany). The CPP is calculated as the difference between the mean arterial pressure (MAP) and ICP. Through the other catheter, a 5 Fr sheath was inserted in the femoral artery and the angio-catheter (Biocompatibles, Farnham, Surrey, UK) was introduced by means of a guidewire into the internal carotid artery (left or right side). After introduction, the position of the catheter was controlled by arteriography; the diameter of the catheter was small enough to allow blood flow through the internal carotid artery.
After surgical exposure of the skull, four 6 mm burr holes were made: two at 1.5 cm left and right of the sagittal suture, 4 cm caudal of the upper margin of the orbita, the other two burr holes 1 cm frontal of the coronalis suture both, also left and right of the sagittal suture. Through a cut in the dura mater a calibrated ICP sensor (Codman, Neuro monitor, Johnson & Johnson, Berkshire, UK) was inserted in the frontal left burr hole to a depth of 20 mm into the brain parenchyma and two multi-parameter sensors (Paratrend/Trendcare, Philips Medical, Böblingen, Germany) were inserted in both caudal burr holes to a depth of 25 mm into the white brain tissue. Two microdialysis probes (CMA/20, Carnegie Medicine, Solna, Sweden) were inserted into the same caudal burr holes by means of a steel guiding needle. The membrane of the microdialysis probe has a cut-off value of 20 kDa. Probes were perfused with an artificial cerebrospinal fluid (manufacturers guide; Carnegie Medicine, Solna, Sweden) at a rate of 2 μl/min with a micro-injection pump (CMA100, Carnegie Medicine, Solna, Sweden). Dialysate volumes of 20 μl (sampling time 10 min) were collected in micro-vials and stored at –800C until analysis. Analysis of the microdialysis samples for glucose and lactate levels was performed with a CMA600 analyzer (CMA, Carnegie Medicine, Solna, Sweden).
Experimental protocol
After completion of all surgical procedures, there was a 1-h stabilization period at an FiO2 of 0.4 before baseline measurements were made. Subsequently, an “oxygen test” [15]was conducted to ensure correct placement of the multi-parameter sensor in the brain tissue. In short, FiO2 was increased from 0.4 to 1.0 for 15 min and the PbrO2 had to increase sharply; if the PbrO2 did not increase by at least 15 mmHg, the sensor was advanced a further 3–5 mm into the brain. PbrO2 had to return to baseline values after the FiO2 was returned to 0.4.
Thirty minutes after PbrO2 had returned to baseline, the baseline values were recorded for all brain parameters and microdialysis parameters. Then, 0.5 ml/kg of air was injected through the catheter in the internal carotid artery at about 1 ml/sec, followed by 3 ml saline at about 1 ml/sec. For the 2 h following embolization, 20 μl dialysates was collected at 10-min intervals. Similarly, at the same time intervals, multi-parameter sensor data of the brain and ICP were recorded. Data on heart rate, MAP, rectal/brain temperature and blood gases were recorded continuously and are presented at baseline and at 60 min and 120 min after embolization for both groups of animals.
Treatment group
In nine animals, 3 min after embolization, hyperventilation was started by increasing the frequency and increasing the FiO2 from 0.4 to 1.0. The frequency was set to maintain hypocapnia (PaCO2 ±25 mmHg). Despite an increased respiratory rate, the end expiratory flow was always zero; thus, no intrinsic PEEP may have led to changes in mean airway pressure. The ventilator setting and the FiO2 level of 1.0 were maintained for the 2-h measuring period.
Control group
In six animals, 3 min after embolization, normoventilation (PaCO2 ±40 mmHg with an FiO2 of 0.4) was maintained; these animals served as the control group.
At 2 h after embolization, measurements were stopped and the sensors were again calibrated to assess any drift in the measured values. All animals were killed by an overdose of pentobarbital (Euthesate 200 mg/ml, Apharmo, Arnhem, The Netherlands).
Statistical analysis
The data were analyzed using the Instat 2.0 Biostatistics Package (GraphPad Software 93–98, San Diego, USA). Inter- and intra-group comparisons were analyzed with repeated measures ANOVA, with Dunnet’s multiple comparison test as post-hoc test. Statistical significance was accepted at a p value less than 0.05. Results are expressed as means ± SD.
Results
All animals were in stable cardiocirculatory conditions at baseline and survived the 2-h study period. Table 1 gives data on heart rate, MAP, rectal/brain temperature and blood gases at baseline and at 60 min and 120 min after air embolization for both groups of animals.
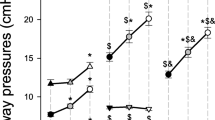
Compared to baseline, the ICP increased significantly over time in both groups during the 2-h study period (Fig. 1a), but there were no significant differences between the two groups. In the treatment group the ICP increased significantly over time from 8±1 (baseline) to 52±6 mmHg; in the control group ICP increased significantly over time from 12±1 (baseline) to 57±8 mmHg. There was a significant difference in CPP between the two groups at 20–70 min after embolization (Fig. 1b).
a Intracranial pressure (ICP) over 2 h. Values are means ± SD. *p<0.05 intra-group versus baseline, open dots intracranial pressure (control), closed dots intracranial pressure (treatment) b Cerebral perfusion pressure (CPP) over 2 h. Values are means ± SD. # p<0.05 inter-group, open triangles cerebral perfusion pressure (control), closed triangles cerebral perfusion pressure (treatment)
Compared with baseline, after air embolization in both groups there was an immediate and significant increase in PbrCO2, which decreased to almost baseline values after 40 min and remained stable for the rest of the study (Fig. 2). In the treatment group PbrO2 rose immediately when switching the FiO2 from 0.4 to 1.0, then decreased again and finally reached the same levels as the normocapnic group ventilated with an FiO2 of 0.4 (Fig. 2). In both groups, brpH decreased immediately after air embolization, then slowly improved, but did not return to baseline values during the 2-h study period (Fig. 3).
Data on brain oxygen (PbrO 2 ) and brain carbon dioxide (PbrCO 2 ) in cerebral air embolism over 2 h. Values are means ± SD. *p<0.05 intra-group versus baseline, # p<0.05 inter-group. open circles PbrO2 (control), closed circles PbrO2 (treatment), open triangles PbrCO2 (control), closed triangles PbrCO2 (treatment)
Figure 4 presents data on brain glucose and lactate during the 2-h study period. Ten minutes after air embolization, in the treatment group there was a decrease in brain glucose, which continued to decrease until the end of the study period. In the control group, after an initial decrease there was a slight increase during the subsequent 60 min followed by another slight decrease at the end of the study. After embolization, lactate sharply increased in both groups and remained high in the treatment group and slightly decreased in the normoventilated group (Fig. 4).
Discussion
The hypothesis of this study was that hypocapnia combined with hyperoxemia would improve the functional state of the brain in CAE. However, the results clearly show that there was no improvement in the brain parameters measured and, therefore, the hypothesis can not be supported.
It is well known that cerebral oxygenation is a crucial factor in maintaining the normal physiology of brain metabolic function. The development of microsensor and microdialysis technology permits the measuring of PbrO2 together with PbrCO2 and brpH, thus enabling an estimation of acidosis and glucose/lactate levels in the brain. However, one has to be aware that a limitation of this technology is that values derived from the sensor/probe represent data from a very local area only and do not represent the entire brain. The experimental event described in the present study is more or less a global insult that should have affected almost all regions of the brain (even the contralateral side by shunting), but predominantly the areas monitored by the sensors/probes. In addition, for the microdialysis, one has to consider that the total amount of glucose/lactate recovered depends on the membrane length of the probe and the flow rate used. Applying our experimental set-up, in vitro we could recover 22% and 39%, respectively, from known concentrations of glucose and lactate. These in vitro percentages were then used to correct the values of glucose and lactate measured in the brain, as recommended by Hillered et al. [16].
Cerebral air embolism is an iatrogenic complication of numerous invasive medical procedures in anesthesia and intensive care [2, 4]. Intensive care physicians are confronted with this severe complication during high-risk interventions, e.g. in cardiovascular surgery using extracorporeal circulation, in neurosurgery and in routine procedures involving placement of central venous catheters [3, 4]. Air bubbles in the cerebral arteries cause an abrupt occlusion of blood flow in the areas supplied by these vessels, leading to ischemic damage if arterial obstruction continues [17]. In fact, bubbles have a natural tendency to dissolve [18] and several interventions can be used to accelerate this process [8]. Otherwise they can remain in the intravascular space for more than 24 h [19]; for bubbles with a diameter of 4 mm it can take up to 560 min before they disappear [20]. Moreover, the pathophysiology of CAE is complex and involves more than vessel occlusion alone; CAE can damage cerebral endothelium, initiating a complex inflammatory response that causes secondary injury and cerebral edema resulting in an increase in ICP [17, 21, 22].
A high FiO2 increases oxygen content in the blood and leads to blood denitrogenation; this increases the pressure gradient of nitrogen between the air bubbles and blood, and thus improves the diffusion of nitrogen from the bubble into the blood, leading to disappearance of the bubble [23]. Rat studies have shown that with increasing oxygen concentration bubbles resolve more rapidly than with normal air breathing [24, 25]. In mathematical models it was calculated that the time needed for a bubble to disappear is 12 h with an FiO2 of 1.0 and up to 40 h with an FiO2 of 0.4 [26, 27]. When comparing these theoretical data with in vivo data (using computer tomography to measure the bubble clearance) the absorption time was also three times faster when switching FiO2 from 0.4 to 1.0 [28].
In the present study, the baseline values for ICP were comparable with data from other animal studies [10, 14]. After air embolization in our animals, ICP rose progressively over the study period to very high pathological values independent of hypocapnia and hyperoxemia.
In the current study, all the detrimental changes in all the other measured parameters can be explained by the following two mechanisms. On the one hand by occlusion (leading to hypoxia) and, on the other, by the increase in ICP. There was a significant decrease in MAP in the hyperventilated group resulting in a significant decrease in CPP in the hypocapnic group (at 20–70 min), which probably caused the deterioration of the PbrO2 after the initial increase. In both groups, the end values of brain glucose and lactate were at the levels of compromised brain tissue in severe head injuries [29, 30].
In our study, compared with the normoventilated group, hyperventilation resulted in a smaller increase in PbrCO2 within 10 min after air embolization; surprisingly, after 30 min PbrCO2 values in the treatment group were almost the same as controls, despite continuous hyperventilation. It has been discussed whether or not hyperventilation has a detrimental effect on the brain [9]: reducing the PaCO2 to 25 mmHg decreases the global cerebral blood flow by 30–40%, which is a level associated with mild cerebral ischemia [31] and, in some pathological conditions, hyperventilation can even be detrimental e.g. in stroke [32], permanent focal cerebral ischemia [32], during neonatal extracorporeal-membrane oxygenation [33] and in infants with perinatal anoxia [34]. Similarly, in a study in humans, reduced EEG activity induced by hyperventilation could be reversed by hyperbaric oxygen, which indicates that hyperventilation reduces oxygen delivery and limits cerebral metabolism [35]. Finally, brain lactate concentrations increased during severe hypocapnia and were inversely proportional to PaCO2, which suggests insufficient oxygen to maintain oxidative metabolism [31].
In conclusion, in the present study hypocapnia combined with hyperoxemia (FiO2 of 1.0) in CAE did not improve the functional state of the brain as compared with normocapnia (FiO2 of 0.4). Further studies should investigate whether even hypercapnia may have beneficial effects on the functional state of the brain.
References
Murphy BP, Harford FJ, Cramer FS (1985) Cerebral air embolism resulting from invasive medical procedures. Ann Surg 201:242–245
Muth CM, Shank ES (2000) Gas embolism. New Engl J Med 342:476–482
Heckmann JG, Lang CJG, Kindler K, Huk W, Erbguth FJ, Neundörfer B (2000) Neurologic manifestations of cerebral air embolism as a complication of central venous catheterization. Crit Care Med 28:1621–1625
Blanc P, Boussuges A, Henriette K, Sainty JM, Deleflie M (2002) Iatrogenic cerebral air embolism: importance of an early hyperbaric oxygenation. Intensive Care Med 28:559–563
Gorman DF (1984) Arterial gas embolism as a consequence of pulmonary barotrauma. In: Desola A et al. (eds) Diving and hyperbaric medicine. Barcelona, European Underwater Biomedical Society pp 348–368
Evans DE, Kobrine AI, Weathersby PK, Bradley ME (1981) Cardiovascular effects of cerebral air embolism. Stroke 12:338–344
Schmidt GA (1992) Pulmonary embolic disorders: thrombus, air and fat. In: Hall JB, Schmidt GA, Wood LDH (eds) Principles of critical care. McGraw-Hill, New York, pp 1476–1492
Tovar EA, Del Campo C, Borsari A, Webb RP, Dell JR, Weinstein PB (1995) Post-operative management of cerebral air embolism: gas physiology for surgeons. Ann Thorac Surg 60:1138–1142
Zauner A, Doppenberg E, Soukup J, Menzel M, Young HF, Bullock R (1998) Extended neuromonitoring: new therapeutic opportunities. Neurol Res 20 (Suppl):S85–90
Menzel M, Rieger A, Roth S, Soukop J, Furka I, Miko I, Molnar P, Peuse C, Hennig C, Radke J (1998) Comparison between continuous brain tissue PO2, PCO2, pH and temperature and simultaneous cerebrovenous measurement using a multisensor probe in a porcine intracranial pressure model. J Neurotrauma 15:265–276
Hamberger A, Jacobson I, Nystrom B, Sandberg M (1991) Microdialysis sampling of the neuronal environment in basic and clinical research. J Intern Med 230:375–380
Hamani C, Luer MS, Dujovny M (1997) Microdialysis in the human brain: review of its applications. Neurol Res 19:281–288
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
Kolenda H, Gremmelt A, Rading S, Braun U, Markakis E (1996) Ketamine for analgosedative therapy in intensive care treatment of head-injured patients. Acta Neurochir 138:1193–1199
McKinley BA, Morris WP, Parmley L, Butler BD (1996) Brain parenchyma PO2, PCO2 and pH during and after hypoxic, ischemic brain insult in dogs. Crit Care Med 24:1858–1868
Hillered L, Persson L (1999) Neurochemical monitoring of the acutely injured human brain. Scand J Clin Lab Invest 59 (Suppl 229):9-18
Hossmann KA (1998) Experimental models for the investigation of brain ischemia, review. Cardiovasc Res 39:106–120
Butler BD, Luehr S, Katz J (1989) Venous gas embolism: time course of residual pulmonary intravascular bubbles. Undersea Biomed Res 16:21–29
Fries CC, Levowitz B, Adler S, Cook AW, Karlson KE, Dennis C (1957) Experimental cerebral gas embolism. Ann Surg 145:461–470
Hlastala MP, Farhi LE (1973) Absorption of gas bubbles in flowing blood. J Appl Physiol 35:311–316
Francis TJR, Gorman DF (1993) Pathogenesis of the decompression disorders. In: PB Bennett, DH Elliott (eds) The physiology and medicine of diving, 4th edn. Saunders, Philadelphia, pp 454–480
Hulst RA van, Lameris TW, Hasan D, Klein J, Lachmann (2003) Effects of cerebral air embolism on brain metabolism in pigs. Acta Neurol Scand 108:118–124
Kyttä J, Tanskanen P, Randell T (1996) Comparison of the effects of controlled ventilation with 100% oxygen, 50% oxygen in nitrogen and 50% oxygen in nitrous oxide on responses to venous air embolism in pigs. Br J Anaesth 77:658–661
Hyldegaard O, Madsen J (1989) Influence of heliox, oxygen and N2-O2 breathing on N2 bubbles in adipose tissue. Undersea Biomed Res 16:185–193
Hyldegaard O, Madsen J (1994) Effect of air, heliox and oxygen breathing on air bubbles in rat tissues. Undersea Hyperbaric Med 21:413–424
Himm JF, Homer LD (1999) A model of extravascular bubble evolution: effect of changes in breathing gas composition. J Appl Physiol 87:1521–1531
Dexter F, Hindman BJ (1997) Recommendations for hyperbaric oxygen therapy of cerebral air embolism based on a mathematical model of bubble absorption. Anesth Analg 84:1203–1207
Annane D, Troché G, Delisle F, Devauchelle P, Paraire F, Raphaël JC, Gajdos P (1994) Effects of mechanical ventilation with normobaric oxygen therapy on the rate of air removal from cerebral arteries. Crit Care Med 22:851–857
Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS (1999) Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med 27:1965–1973
Stahl N, Ungerstedt U, Nordstrom CH (2001) Brain energy metabolism during controlled reduction of cerebral perfusion pressure in severe head injuries. Intensive Care Med 27:1215–1223
Brian JE (1998) Carbon dioxide and the cerebral circulation. Anesthesiology 88:1365–1386
Dexter F (1997) Research synthesis of controlled studies evaluating the effect of hypocapnia and airway protection on cerebral outcome. J Neurosurg Anesth 9:217–222
Laffey JG, Kavangh BP (1999) Carbon dioxide and the critically ill—too little of a good thing. Lancet 354:1283–1286
Hashimoto K, Takewuchi Y, Takahina S (1991) Hypocarbia as a pathogenic factor in pontosubicular necrosis. Brain Dev 13:155–157
Reivich M, Cohen PJ, Greenbaum L (1966) Alterations in the electroencephalogram of awake men produced by hyperventilation: effects of 100% oxygen at 3 atmospheres pressure. Neurology 16:303–307
Acknowledgments
We thank Stefan Krabbendam, Gonda Schoonhoven, Debbie Heppener and Rob van Bremen (Department of Experimental Cardiology) for technical assistance, and Laraine Visser-Isles for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was performed at the Department of Anesthesiology, Erasmus MC-Faculty, University Medical Center Rotterdam, the Netherlands, and was financially supported by the Royal Netherlands Navy, Ministry of Defense.
An editorial regarding this article can be found in the same issue (http://dx.doi.org/10.1007/s00134-004-2209-5)
Rights and permissions
About this article
Cite this article
van Hulst, R.A., Haitsma, J.J., Lameris, T.W. et al. Hyperventilation impairs brain function in acute cerebral air embolism in pigs. Intensive Care Med 30, 944–950 (2004). https://doi.org/10.1007/s00134-003-2119-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2119-y