Abstract
Objective
To test the effects of low perfusion caused by emerging sepsis on the performance of two new pulse oximetry techniques: Masimo SET in comparison with Nellcor Oxismart XL.
Design
Cohort study with random allocation of two pulse oximetry devices to two sensor sites.
Setting
University animal research facility.
Subjects
Twenty-five adult, anesthetized, ventilated rabbits.
Interventions
Pneumonia/sepsis was induced by tracheal instillation of E. coli.
Measurements and results
Oxygen saturation was measured by pulse oximetry (SpO2) and recorded continuously until death. Arterial oxygen saturation (SaO2) was measured hourly by CO oximetry and whenever a difference of >5% between the devices occurred. SpO2 sensors were positioned at both forelegs and switched hourly. There was no difference in total signal dropout time [median 3.8 min (range 0.4–66.6 min) vs 3.3 min (range 0–94.5 min), Masimo SET vs Oxismart XL]. There were fewer episodes with a false SpO2 reading [1 (range 0–7) vs 2 (range 0–17)] using the Masimo SET vs the Oxismart XL as verified by CO oximetry; p<0.05. Average bias (SpO2–SaO2) was significantly different between the two devices, and variability of bias values increased across time with both devices.
Conclusions
Both devices were capable to measure SpO2 during most of the experimental time in this model of low perfusion and therefore appear to be highly sensitive to pick up a signal; however, low perfusion caused by emerging sepsis may result in inaccurate measurements with both devices. These episodes were less common with the Masimo SET vs the Oxismart XL.
Similar content being viewed by others
Introduction
Pulse oximetry is widely used in anesthesia as well as emergency, and critical care settings to monitor arterial oxygenation and to guide adjustments of FiO2. Different filter techniques and software algorithms to separate noise from the true signal are used in pulse oximetry devices [1], which may result in a different performance when challenged by artifacts, such as motion or low perfusion [2, 3, 4]. The effect of low perfusion on pulse oximetry has been studied in healthy subjects using exposure to hypothermia and tourniquet-occlusion techniques [4, 5, 6], or during hemorrhagic hypotension [7]; however, there are very few studies during low perfusion in subjects with sepsis, and these studies are limited by the fact that arterial oxygen saturation as measured by pulse oximetry (SpO2) was compared with arterial oxygen saturation as measured by CO oximetry (SaO2) at only very few different time points [8, 9]. As sepsis is a common event in ICU patients [10], it is important to know the performance characteristics of more recently available pulse oximetry techniques in this setting. We have previously shown that low perfusion caused by experimental sepsis may result in significant signal dropout when using an older standard pulse oximeter (Nellcor N-200), and that SpO2 measurements may be underestimated considerably [11].
The objective of this study was to test the hypothesis that a more recently available pulse oximetry technique (Masimo SET) would be less prone to signal loss, and therefore the total signal dropout time would be at least 50% shorter in comparison with a Nellcor Oxismart XL pulse oximeter in an animal model of emerging sepsis.
Materials and methods
All animals were cared for according to the current version of the German law on the protection of animals and to NIH guidelines for the care and use of laboratory animals. The protocol was approved by the appropriate government agencies. Twenty-five adult anesthetized, ventilated New Zealand White Rabbits received a tracheal instillation of E. coli to induce pneumonia/sepsis and were supported as described in detail previously [11].
Arterial hemoglobin saturation (SpO2) was simultaneously measured with an IVY Model 405T (IVY Medical Systems, Branford, Conn.), equipped with a LNOP-Neo Sensor and Masimo SET (Masimo, Irvine, Calif.), and a Nellcor N-395 pulse oximeter equipped with a Sensor Type D-YS (Nellcor Puritan Bennett, Pleasonton, Calif.) and Nellcor Oxismart XL technique. To separate the arterial signal from noise signals, the Masimo SET uses an adaptive filter that identifies and removes frequency components in common with both signals and plots the remaining signal on a power curve where the arterial signal is identified. We expected that this very sophisticated technique would allow signal detection during conditions with a low-signal-to-high-noise ratio. In the Nellcor Oxismart XL a different adaptive filter technique is used, which takes into account the rate of SpO2 change analyzed and assumes that rapid changes along with a high noise level may be caused by noise as well. After closely shaving both forelegs, the pulse oximeter sensors were randomly assigned to one foreleg each and switched hourly. Sensor sites were shielded against ambient light using an opaque cover throughout the study. SpO2 readings and plethysmography curves of both pulse oximeters were recorded continuously.
Arterial blood gases and arterial hemoglobin O2 saturation (SaO2), i.e., functional saturation, as measured by CO oximetry (Omni 3, Roche Diagnostics, Graz, Austria), were drawn and analyzed within 2 min at hourly intervals, or whenever the absolute value of the difference between both SpO2 readings was >5% for more than 60 s.
The primary outcome measure was the total signal dropout time. It was calculated as the sum of all episodes without a signal from instillation of E. coli until death for each of the two devices. Secondary outcome measures were the number and duration of episodes with signal dropout, duration of final signal dropout, and the number and duration of episodes with a false SpO2 reading, defined as a bias (difference between SpO2 and SaO2) of ≥5% or ≤−5%, and the maximal bias during these episodes. Bias was further calculated for both pulse oximeters at hourly intervals in each animal. Modified Bland-Altman plots of these hourly measured bias values were used to compare time-dependent changes of the accuracy of pulse oximetry measurements across experimental time [12]. Mean±2SD values of the differences (SpO2–SaO2) measured at each time point were calculated using an ANOVA model for repeated measurements. Moving averages derived from three consecutive measurements were used for smoothing of mean±2SD intervals. Paired t tests, Wilcoxon signed-rank tests, chi-square tests (including Yates correction), and repeated measures ANOVA on ranks, where appropriate, were used to compare differences between devices.
Results
Signal dropout
The animals survived until 10 h (range 4–31 h) after tracheal instillation of E.coli. Our hypothesis was not confirmed, as there was no significant difference in total signal dropout time comparing the two devices (Table 1). Although there were more episodes of signal dropout with the Masimo SET, the median duration of these episodes was shorter compared with the Nellcor Oxismart XL. There was no difference in the final dropout time, i.e., the time interval between final device failure and death.
Episodes of a false SpO2 reading
We observed 108 episodes of a false SpO2 reading in 21 of 25 animals (Masimo SET: 33 episodes; Nellcor Oxismart XL: 75 episodes). Fourteen animals had episodes with both devices, two had episodes with only the Masimo SET, 5 animals had episodes with only the Nellcor Oxismart XL, and 4 animals had no episode with either device. The Masimo SET pulse oximeter had fewer episodes of a false pulse oximetry reading than the Oxismart XL (p<0.05; Table 2), but there was no significant difference in the maximal bias or the median duration of these episodes (Table 2).
Bias and precision
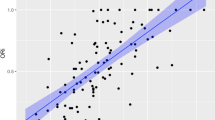
Figure 1 shows the changes of mean bias and variability of bias values as a measure of precision across experimental time. Mean bias was very small initially with both devices (Fig. 1, time=0 h); however, whereas the Masimo SET overestimated oxygen saturation, the Oxismart XL tended to underestimate oxygen saturation, resulting in a significant difference in bias values comparing the two devices (p<0.05, RM ANOVA on ranks). Variability increased across time with both devices, as can be seen from the mean±2SD intervals. Individual bias values were beyond the ±2% error limit given by the manufacturers of most pulse oximetry devices designed for clinical use in 61 of 264 (23%; Masimo SET) vs 81 of 265 (31%; Nellcor Oxismart XL) of all hourly SpO2/SaO2 measurements.
Modified Bland-Altman plots of the measured bias values (SpO2–SaO2) across experimental time. Each dot refers to one measurement in each animal at each time point. Mean bias along with mean±2SD values of these individual bias values. Upper panel Masimo SET; lower panel Nellcor Oxismart XL. Bias across time was significantly different between the devices (p<0.05, RM-ANOVA on ranks): the Masimo SET overestimated SaO2, whereas the Oxismart XL tended to underestimate SaO2. Variability of individual bias values increased with both devices indicating decreasing precision across time. Note that three outliers in the upper panel and four outliers in the lower panel are not shown because they were beyond the y-axis scale
Discussion
In this study we did not find a significant difference in total signal dropout time between devices. Episodes of signal dropout were significantly more common with the Masimo SET compared with the Nellcor Oxismart XL; however, they were shorter in duration as well. We speculate that this observation may be related to differences in data processing. Considering the fact that most of our animals had severely impaired hemodynamics toward the end of the study both systems seem to be very sensitive to pick up a signal. Both devices seem to work properly during normal hemodynamic conditions in this animal species, as suggested by the fact that during the first 30 min of the experiment only one of the animals had one single signal dropout of approximately 40 s with one device, and that there was no bias beyond ±2% at baseline in any animal with both devices.
We observed episodes of false SpO2 readings as compared with CO oximetry with increasing bias toward the end of the experimental time. The fact that one device was overestimating, whereas the other one was underestimating, true arterial oxygen saturation during adverse hemodynamic conditions might be of potential clinical interest. Other investigators found an increased bias in infants with poor peripheral perfusion caused by cardiopulmonary bypass and/or hypothermia [13], and in adults during circulatory failure [8], or with sepsis and low systemic vascular resistance [9]. Other investigators found increased bias of transcutaneously measured pulse oximetry values in comparison with SaO2 in severely sick surgical adult patients with increased body temperature or low mean arterial blood pressure [14]. In the latter study the accuracy of a simultaneously used reflectance pulse oximetry device placed in the esophagus was improved in comparison with sensor placement at fingers [14].
The bias/precision data of our previous study [11] was limited by the fact that an FiO2=1.0 was used throughout that study resulting in an SpO2 close to 100% for most of the experimental time as the pulse oximeter was working mainly on the flat part of the oxygen–hemoglobin dissociation curve, which may have limited errors caused by potential over-reading of the true SaO2. In this study the FiO2 was adjusted to maintain a PaO2 in a target range used in many non-neonatal critical care settings (100–150 mmHg). In contrast to our previous study [11], we were now able to demonstrate in this study that SpO2 might be overestimated considerably, and the bias values calculated from hourly SpO2 and SaO2 measurements now show a nearly symmetrical distribution across time.
As pulse oximetry is widely used to adjust FiO2 in many emergency and critical care settings, it is not reassuring that a pulse oximeter can display a normal looking plethysmography curve along with a heart rate matching the ECG heart rate, but a false SpO2 reading. Whereas with one device (Nellcor) the user may be alerted by the decreasing size of the plethysmography curve, this is not the case with the IVY 405T monitor, as this device expands its plethysmography display automatically to fit the screen.
Currently, it is unknown how often episodes of false SpO2 occur in a specific clinical setting, and how often these episodes would lead caretakers to inappropriate adjustments of FiO2 or ventilator pressures, resulting in hyperoxic tissue injury such as chronic lung disease or retinopathy of prematurity in preterm neonates, or in inappropriate decreases in FiO2 promoting hypoxic tissue damage, such as hypoxic brain injury. The currently available pulse oximeters may perform better during low perfusion, i.e., they pick up a signal when previously available devices would have failed; however, our study results suggest that the price for this increase in sensitivity may be decreased accuracy during adverse hemodynamic conditions. We have not noticed a readily available physiological variable such as blood pressure or heart rate to be of any value to predict device failure; however, perfusion index (=pulsating signal indexed against non-pulsating signal) as a measure of local tissue perfusion is currently under investigation in another study to predict device failure.
Conclusion
In conclusion, we have shown that two more recently available pulse oximetry techniques were capable of measuring SpO2 during most of the experimental time in this model of low perfusion caused by emerging sepsis; however, our data suggest that inaccurate measurements of SpO2 may occur in this setting with both devices. Further studies are necessary to elucidate the exact mechanisms responsible for false SpO2 readings. Meanwhile, pulse oximetry readings suggesting a changing FiO2 requirement should alert the clinician taking care of a patient with impaired hemodynamics to validate this reading using arterial blood gas analyses and/or CO oximetry as there may be a potential for inappropriate interventions.
References
Rusch TL, Sankar R, Scharf JE (1996) Signal processing methods for pulse oximetry. Comput Biol Med 26:143–159
Hay WW Jr, Rodden DJ, Collins SM, Melara DL, Hale KA, Fashaw LM (2002) Reliability of conventional and new pulse oximetry in neonatal patients. J Perinatol 22:360–366
Bohnhorst B, Peter CS, Poets CF (2000) Pulse oximeters’ reliability in detecting hypoxemia and bradycardia: comparison between a conventional and two new generation oximeters. Crit Care Med 28:1565–1568
Trivedi NS, Ghouri AF, Shah NK, Lai E, Barker SJ (1997) Effects of motion, ambient light, and hypoperfusion on pulse oximeter function. J Clin Anesth 9:179–183
Langton JE, Lassey D, Hanning CD (1990) Comparison of four pulse oximeters: effects of venous occlusion and cold-induced peripheral vasoconstriction. Br J Anaesth 65:245–247
Gehring H, Hornberger C, Matz H, Konecny E, Schmucker P (2002) The effects of motion artifact and low perfusion on the performance of a new generation of pulse oximeters in volunteers undergoing hypoxemia. Respir Care 47:48–60
Barrington KJ, Ryan CA, Finer NN (1986) Pulse oximetry during hemorrhagic hypotension and cardiopulmonary resuscitation in the rabbit. J Crit Care 1:241–246
Ibanez J, Velasco J, Raurich JM (1991) The accuracy of the Biox 3700 pulse oximeter in patients receiving vasoactive therapy. Intensive Care Med 17:484–486
Secker C, Spiers P (1997) Accuracy of pulse oximetry in patients with low systemic vascular resistance. Anaesthesia 52:127–130
Brun-Buisson C, Doyon F, Carlet J, French Bacteremia-Sepsis Study Group (1996) Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. Am J Respir Crit Care Med 154:617–624
Hummler HD, Pohlandt F, Franz AR (2002) Pulse oximetry during low perfusion caused by emerging pneumonia and sepsis in rabbits. Crit Care Med 30:2501–2508
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 2:307–310
Villanueva R, Bell C, Kain ZN, Colingo KA (1999) Effect of peripheral perfusion on accuracy of pulse oximetry in children. J Clin Anesth 11:317–322
Vicenzi MN, Gombotz H, Krenn H, Dorn C, Rehak P (2000) Transesophageal versus surface pulse oximetry in intensive care unit patients. Crit Care Med 28:2268–2270
Acknowledgements
We thank N. Claure, University of Miami, for his technical support. We also thank AVL Medizintechnik, Graz, Austria, for kindly providing the AVL Omni 3 blood gas analyzer, and Masimo Corp., Irvine, California, for kindly providing the IVY 405T pulse oximeter. This study was funded by a grant from the German Research Foundation, Bonn, Germany (DFG: FR 1455/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hummler, H.D., Engelmann, A., Pohlandt, F. et al. Accuracy of pulse oximetry readings in an animal model of low perfusion caused by emerging pneumonia and sepsis. Intensive Care Med 30, 709–713 (2004). https://doi.org/10.1007/s00134-003-2116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2116-1