Abstract
Microplastics (MPs) have emerged as a global environmental concern due to their persistent nature. In South Africa, microplastic research has primarily focused on marine systems. However, recent years have seen a shift in focus to studying MPs in South African freshwaters. In this study, MPs with a minimum size of 0.055 mm in surface water of the Vaal River, South Africa, were reported. MPs were 100% prevalent, with a mean numerical abundance of 0.68 ± 0.64 particles/m3. Small-sized MPs of < 1 mm accounted for the largest proportion. MPs were chemically identified as high-density polyethylene, low-density polyethylene, and polypropylene according to their Raman spectra. The prevalence of fragments (41.6%) and fibers (38.5%) over pellets (8.1%) indicates that microplastics are from secondary sources. The prevalence of polyethylene and polypropylene is consistent with microplastics being from secondary sources. These polymers are commonly used in single-use plastics, packing bags, textiles, and containers. These characteristics are of great concern due to their implications on the bioavailability and toxicological impacts of MPs. Consequently, these properties may pose more hazards to aquatic biota inhabiting the Vaal River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Microplastics (MPs) result from the breakdown of larger pieces of plastics as well as manufactured MPs (Park and Park 2021). Microplastics enter the aquatic environment from different sources, including effluents from wastewater treatment plants (WWTPs), sewage sludge, shipping activities, atmospheric fallout, direct disposal from the public, beach littering, and runoff from agricultural, recreational, industrial, and urban areas (Nikiema et al. 2020a, 2000b ; Bellasi et al. 2020). They are divided into two categories, namely primary (manufactured plastic products produced at a microscopic size) and secondary (small plastic particles that are formed by the breakdown of larger plastic items) (Sun et al. 2019; Onoja et al. 2022).
Environmental contamination by MPs is of great concern. In addition to their possible health risks, the potential hazard associated with MPs includes exposure to their constituent additives such as plasticizers, flame retardants, pigments, and stabilizers (Li et al. 2023). Further, MPs have the potential to act as micro-vectors for toxic elements and organic contaminants, adsorbing and desorbing them depending on the prevailing environmental conditions (Onoja et al. 2022). Plastics are also known to be good adsorbents of some trace elements e.g. mercury, lead, and cadmium among others (Liu et al. 2023). This capability varies depending on the functional groups pendent on the backbone structure of the plastic (Saad 2022a). MPs are reported to interact with aquatic organisms including plants. Alterations in tissue morphologies, gene expression, enzyme activity, swimming patterns, and kidney functions are some of the toxicological effects reported in several aquatic biota (Saad 2023).
MP research has primarily focused on marine systems, however, the past few years have seen a shift in focus to studying MPs in South African freshwaters (Dahms et al. 2020; Saad et al. 2022b; Weideman et al. 2019). South Africa has a well-established plastics manufacturing sector, however, recycling is insufficient. It is one of the top twenty countries that produce large masses of plastic waste, which ends up in aquatic environments. Yet, MP pollution in the freshwater of South Africa has not been thoroughly investigated (Saad et al. 2022b). This study aimed to investigate the presence, abundance, and sources of MPs in one of the most essential freshwater systems in South Africa, the Vaal River. The main objective was to report the concentration levels of MPs in surface water, using a volume-reduced sampling method, for different areas of the Vaal River system.
Materials and Methods
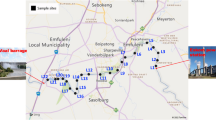
Sampling was conducted in June 2021 in Vereeniging, a city located in the south of Gauteng province, bordering the Free province. Samples were collected from the middle part of the Vaal River over 50 km, between the Lethabo weir and the Vaal River barrage (Fig S1). This region of the Vaal is known for different anthropogenic activities including industrial, agricultural, and recreational activities. Eskom–Lethabo power station and Rand Water Lethabo pump station are also located in this area. This part of the river receives water flowing from five tributaries and discharges from WWTPs. Sampling was conducted using a plankton net with 0.25 m, 50 cm, and 0.055 mm diameter, length, and mesh size, respectively. Twenty surface water samples were collected, and the sampled material was concentrated inside a removable net bucket with a sieve gauze-covered side window and stop-cock, through which it was transferred into glass jars. GPS and flowmeter readings at the beginning and the end of each tow were recorded and the volume filtered through the net was calculated using a flowmeter (General Oceanics model 2030R) attached to the net (Ramaremisa et al. 2022). Samples were stored in glass jars, transferred to the laboratory, and preserved at 4 °C.
Samples were digested by wet peroxide oxidation process (WPO) using Fenton’s reagent (0.07 M FeSO4·7H2O catalyst and 30% (v/v) H2O2). 60 mL of Fenton’s reagent was added to each sample in a 1:1 volume ratio. The catalyst solution was added first and the H2O2 solution was slowly added, until no further reaction. The samples were subsequently swirled to allow the solution to soak into the sample. Samples were left for 24–48 h at room temperature to achieve complete digestion before separation (Ramaremisa et al. 2022).
Upon digestion, MPs were separated based on their density using both NaCl and NaI, in a two-step density separation protocol (Ramaremisa et al. 2022). Firstly, 45 mL of saturated NaCl (1.2 g/mL) was mixed with each sample, left for 24 h, and filtered. Subsequently, 45 mL of NaI (1.8 g/mL) was added and left for another 24 h before filtration. The two-step protocol ensures high extraction of high-density MPs while avoiding excessive use of NaI. Samples were then vacuum filtered through GF/A filter papers (Whatman® : 47 mm diameter, pore size: 1.6 μm), air-dried in a fume hood, and stored in covered glass Petri dishes until analysis.
Following the extraction, the physical properties of potential MP particles were identified using a Nikon stereomicroscope (Nikon MET SMZ745T, Japan) at 50x magnification. Particles suspected to be MPs were photographed with an imaging source camera (TIS) USB 3.0 and processed with NIS Elements-D imaging software Version 5.30 (Nikon Cooperation, Japan). In addition to identifying their shape and colour, the software was also used to count particles as well as measure particle size. MPs were grouped into six size classes: <0.5 mm, 0.5–1 mm, 1–2 mm, 2–3 mm, 3–4 mm, and 4–5 mm. MPs were identified based on the criteria proposed in the literature, namely, a maximum size of 5 mm, absence of biological structures, and homogeneous colours (Ramaremisa et al. 2022).
The polymer type of MPs in this study was identified using Raman spectroscopy. Frequently observed MP particles were analysed by a Horiba LabRAM HR Raman spectrometer with an Olympus BX41 microscope attachment, using a 514.5 nm (green) line of a Lexel Model 95-SHG argon ion laser. LabSpec v5 software is used for acquisition and analysis of spectra, the spectra ranged from 0 to 3500 cm1 and the wavelength of the incident laser was set to 1–2 μm. The chemical composition of polymers was identified by comparison with reference spectra in the SLOPP Library of Microplastics and polymer databases of KnowItAll software (Bio-Rad Laboratories, Inc.).
Extreme caution was taken during sample collection, sample processing, and analysis to prevent cross-contamination. Except for the plankton net, no plastic apparatus or containers were used during sampling and sample preparation, and experimental equipment and containers were washed with filtered Milli-Q® Type 1 Ultrapure water and rinsed with filtered ethanol. All laboratory experiments were conducted in a laminar flow cabin in a lab dedicated to MPs, and cotton lab coats were worn during all stages to prevent contamination risk from clothing fibers. To mitigate against the potential contamination of samples by MPs in chemicals, all solutions were filtered through a Whatman® GF/F glass microfiber filter (Cytiva Danaher Group, Buckinghamshire, United Kingdom) before use. In addition, blank experiments were conducted with filtered Milli-Q® Type 1 Ultrapure water to count for possible procedural contamination. All blanks were processed along with the samples. No MPs were detected in the blanks.
Results and Discussion
All samples showed the presence of MPs with a mean abundance of 0.68 ± 0.64 particles/m3. Abundance per sample location is given in Fig. 1.
Higher abundance was detected at locations L5, L6, L7, L8, L9 and L10 with over 0.4 particles/m3 each. It is noted that MP pollution is attributed to several factors, most significantly, the proximity of the water body to highly populated urban areas, industrial hubs, (WWTPs), and other anthropogenic activities such as tourism, fishing, and agriculture (Saad et al. 2022c). These sampling spots are located between Vereeniging and Vanderbijlpark (Fig S1), where potential sources of MPs exist. Potential sources in the area include industries (Arcelor Mittal Vaal works and Vaal Rubberising Centre, which are located near L6); Water and wastewater treatment works (L6 is 1.5 km from Rand water Vereeniging pump station); urban settlements (L 8 and L 9 are near the North–West University Vaal Triangle campus and Vaal University of Technology in Vanderbijlpark); recreational activities (L6 is less than 1 Km from Riviera aquatics club, L9 is near Emerald Resort animal world, and the Aquadome and Eligwa boat club are located close to L10); informal settlements (L 7 is near to informal human settlements under the R59 road). Informal settlements are profoundly marked by environmental degradation and pollution due to illegal dumping, poor waste management, and logistical challenges (Verster and Bouwman 2020; Grangxabe et al. 2023; Kekana et al. 2023). This explains the high abundance of MPs in L7 .
In addition, L9 and L10 are approximately 1.4 km and 1.176 km away from the confluence of the Vaal River and Taaibosspruit River. The increase of MPs in main rivers at confluences of tributaries is well documented (Dahms et al. 2020). Further downstream a sharp increase is observed at L16, which is about 1 km away from the confluence of the Vaal River with Leeuspruit River. However similar observations were not made at the confluence of the Vaal River and Rietspruit, this could be due to the Vaal-Rietspruit River confluence being stretched out into a Lake (Loch Vaal) as shown in Fig S2. Subsequently, MPs may be more abundant further away from the area sampled.
Morphological characteristics (shape, color, and size) of MP particles were analysed. Microscope images of the most frequently detected shapes are shown in Fig. 2.
Fragments (41.6%) and fibers (38.5%) dominated other shapes. Films and pellets were less-existent, representing only 11.8% and 8.1%, respectively (Fig. 3). The high prevalence of fragments and fibers over pellets indicates that MPs are from secondary sources Ramaremisa et al. 2022).
Domestic discharge from households around the Vaal River may contribute immensely to the prevalence of fibrous MPs, mainly, from machine washing (Lahiri et al. 2023). Sun et al. (2019) investigated the pervasiveness of fibers in the treatment stages and reported the persistence of fibers during wastewater treatment processes. Notably, fibers and fragments are frequently reported as the most abundant shapes of MPs in several freshwater bodies, examples include the Karnafully River in Bangladesh, the Chengdu River in China, and several freshwater lakes and Rivers in Russia (Chen et al. 2022; Frank et al. 2022; Hossain et al. 2022). Weideman et al. (2019) reported a high abundance of fibers (98%) in bulk water samples.
In terms of size, it is observed that smaller sizes less than 0.5 mm were the most abundant, accounting for 38.8% of the total particles, followed by 28.3% of MPs in the range 0.5–1 mm, and 22.1% in the range 1–2 mm. While larger particles (2–5 mm) are less abundant and represent 10.7% of the total particles detected in all samples. Figure 4 shows the size distribution of MPs in the surface water samples. In riverine systems, MPs are susceptible to environmental weathering such as phyto, thermal, microbial, and chemical degradation. These degradation processes result in a continuous decrease in MP size over time (Bellasi et al. 2020; Onoja et al. 2022; Ramaremisa et al. 2022), which explains the dominance of small-sized MPs in this study. The dominance of small-sized MPs has been reported widely (Govender et al. 2020; Ramaremis et al. 2022; Saad et al. 2022a, 2022b; Saad and Alamin 2024).
The relationship between MP abundance and their size was examined by the Spearman correlation test (Fig. 5). A p-value of 0.6 indicates an insignificant correlation. This could be explained by the fact that MP size is affected by the environmental degradation processes mentioned above and not directly linked to the abundance.
Coloured MPs were found to be more abundant (82.4%) compared to transparent MPs (17.6%) as shown in Fig S3. The dominance of coloured MPs has been reported in several freshwater studies around the world (Zhao et al. 2015). This is obvious as plastics are commonly coloured during manufacturing to enhance their appearance for different applications.
Post-visual identification, the chemical composition of MPs was further examined by Raman spectroscopy. The most frequent MP particles were identified as high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP), according to their Raman spectra (Fig S4). Differences between sample spectra and library spectra may reflect one or a combination of the following factors, the presence of chemical additives in the MPs, partial polymer degradation, and the presence of residues of biofilms that had colonised the plastic surfaces over time (Frère et al. 2016; Käppler et al. 2016). PE and PP were frequently reported in MP monitoring studies in surface water, which could be attributed to their extensive use in manufacturing different products. Additionally, the low density of these polymers makes them buoyant, thus abundant in the surface water (Govender et al. 2020). The polymer types, therefore, influence their distribution and transport in aquatic environments based on their density. While high-density polymers are known to be bioavailable for benthic organisms, low-density polymers such as PE and PP are consumed by pelagic organisms such as plankton. However, due to biofouling, low-density polymers can sink and become bioavailable for benthic biota. Benthic organisms can also consume low-density MPs via trophic transfer (Lagarde et al. 2016; Long et al. 2015).
Several adverse effects, including physical damage and changes, oxidative stress, and immune and gene destructions, are observed in organisms that consumed PE MPs (Hamed et al. 2022; Rochman et al. 2014).
The abundance of MPs in this study is moderate in comparison with other freshwater studies. Worldwide freshwater studies were selected to compare with the current study (Table 1).
The values show that the level of MP abundance in the Vaal River was higher than those reported in two Chinese freshwater systems. Higher levels of MP contamination were reported in three Italian freshwater bodies and another two Chinese Rivers. However, the comparison of MP abundance among different studies must be performed with some caution, due to significant methodological differences in sampling, sample preparation, and visual identification. The Vaal River is exposed to discharges from industrial, mining, power generation, commercial agriculture, nature conservation, as well as urban and rural human settlements (Saad et al. 2022b). Fishing activities, domestic sewage, poor plastic waste management, population densities, and different anthropogenic activities are considered to be the major contributors to MPs abundance most of these cases.
Conclusions
Surface water of the Vaal River were studied to document the presence, abundance, and the sources of MPs in the Vaal River. The abundance of MPs is comparable to the findings of some freshwater studies from other parts of the world. Fragments and fibers represented over 80% of the detected MPs, the majority of which were coloured particles of sizes below 0.5 mm. Polymer types were identified as HDPE, LDPE, and PP. Different anthropogenic activities were identified as potential sources of MPs in the Vaal River including, industries, tourism, fishing, and agriculture.
This report on the status of MP abundance in surface water of the Vaal River provides a reference for further monitoring studies, which may assist in extending our knowledge of MP pollution in South African freshwater systems.
Data Availability
Data are available upon request.
References
Bellasi A, Binda G, Pozzi A et al (2020) Microplastic contamination in freshwater environments: a review, focusing on interactions with sediments and benthic organisms. Environments 7(4):30
Campanale C, Stock F, Massarelli C et al (2020) Microplastics and their possible sources: the example of Ofanto river in southeast Italy. Environ Pollut 258:113284. https://doi.org/10.1016/j.envpol.2019.113284
Chen J, Rao C, Yuan R et al (2022) Long-term exposure to polyethylene microplastics and glyphosate interferes with the behavior, intestinal microbial homeostasis, and metabolites of the common carp (Cyprinus carpio L.). Sci Total Environ 814:152681. https://doi.org/10.1016/J.SCITOTENV.2021.152681
Dahms HTJ, van Rensburg GJ, Greenfield R (2020) The microplastic profile of an urban African stream. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.138893
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments - a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657. https://doi.org/10.1016/j.envpol.2016.03.012
Frank Y, Ershova A, Batasheva S et al (2022) Microplastics in freshwater: a focus on the Russian Inland waters. Water 14:3909
Frère L, Paul-Pont I, Moreau J et al (2016) A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter. Mar Pollut Bull 113:461. https://doi.org/10.1016/j.marpolbul.2016.10.051
Govender J, Naidoo T, Rajkaran A et al (2020) Towards characterising microplastic abundance, typology and retention in mangrove-dominated estuaries. Water. https://doi.org/10.3390/w12102802
Grangxabe XS, Maphanga T, Madonsela BS et al (2023) The escalation of Informal Settlement and the high levels of illegal dumping post-apartheid: systematic review. Challenges. https://doi.org/10.3390/challe14030038
Hamed M, Monteiro CE, Sayed AEDH (2022) Investigation of the impact caused by different sizes of polyethylene plastics (nano, micro, and macro) in common carp juveniles, Cyprinus carpio L., using multi-biomarkers. Sci Total Environ 803:149921. https://doi.org/10.1016/J.SCITOTENV.2021.149921
Hossain MJ, AftabUddin S, Akhter F et al (2022) Surface water, sediment, and biota: the first multi-compartment analysis of microplastics in the Karnafully river, Bangladesh. Mar Pollut Bull 180:113820. https://doi.org/10.1016/j.marpolbul.2022.113820
Käppler A, Fischer D, Oberbeckmann S et al (2016) Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal Bioanal Chem 408:8377. https://doi.org/10.1007/s00216-016-9956-3
Kekana HN, Ruhiiga TM, Ndou NN, Palamuleni LG (2023) Environmental justice in South Africa: the dilemma of informal settlement residents. GeoJournal. https://doi.org/10.1007/s10708-022-10808-z
Lagarde F, Olivier O, Zanella M et al (2016) Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ Pollut 215:331–339. https://doi.org/10.1016/J.ENVPOL.2016.05.006
Lahiri SK, Azimi Dijvejin Z, Golovin K (2023) Polydimethylsiloxane-coated textiles with minimized microplastic pollution. Nat Sustain. https://doi.org/10.1038/s41893-022-01059-4
Lam TWL, Fok L, Lin L et al (2020) Spatial variation of floatable plastic debris and microplastics in the Pearl River Estuary, South China. Mar Pollut Bull 158:111383. https://doi.org/10.1016/j.marpolbul.2020.111383
Li W, Wang S, Wufuer R et al (2023) Distinct soil microplastic distributions under various farmland-use types around Urumqi. China. Sci Total Environ 857:159573. https://doi.org/10.1016/J.SCITOTENV.2022.159573
Liu Y, Cui W, Li W et al (2023) Effects of microplastics on cadmium accumulation by rice and arbuscular mycorrhizal fungal communities in cadmium-contaminated soil. J Hazard Mater 442:130102. https://doi.org/10.1016/J.JHAZMAT.2022.130102
Long M, Moriceau B, Gallinari M et al (2015) Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar Chem. https://doi.org/10.1016/j.marchem.2015.04.003
Nikiema J, Asiedu Z, Mateo-Sagasta J et al. (2020a) Catalogue of technologies to address the risks of contamination of water bodies with plastics and microplastics. United Nations Environment Programme.
Nikiema J, Mateo-Sagasta, Asiedu Z et al. (2020b) Water pollution by plastics and microplastics- A review of technical solutions from source to sea. United Nations Environment Programme.
Onoja S, Nel HA, Abdallah MAE, Harrad S (2022) Microplastics in freshwater sediments: Analytical methods, temporal trends, and risk of associated organophosphate esters as exemplar plastics additives. Environ Res 203:111830
Park H, Park BS (2021) Review of microplastic distribution, toxicity, analysis methods, and removal technologies. Water 13(19):2736
Ramaremisa G, Ndlovu M, Saad D (2022) Comparative Assessment of Microplastics in Surface Waters and sediments of the Vaal River, South Africa: abundance, composition, and sources. Environ Toxicol Chem 41:3029–3040. https://doi.org/10.1002/ETC.5482
Rochman CM, Kurobe T, Flores I, Teh SJ (2014) Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci Total Environ 493:656–661. https://doi.org/10.1016/J.SCITOTENV.2014.06.051
Saad D (2022) Selective removal of cr(III) from aqueous solution using cross-linked polyethylenimine: experimental optimization and modeling. Polym Bull. https://doi.org/10.1007/s00289-021-03578-0
Saad D (2023) Why Microplastics are exceptional contaminants? https://doi.org/10.5772/INTECHOPEN.109173
Saad D, Alamin H (2024) The first evidence of microplastic presence in the River Nile in Khartoum, Sudan: using Nile Tilapia fish as a bio-indicator. Heliyon 10:e23393. https://doi.org/10.1016/J.HELIYON.2023.E23393
Saad D, Chauke P, Cukrowska E et al (2022) First biomonitoring of microplastic pollution in the Vaal river using carp fish (Cyprinus carpio) as a bio-indicator. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.155623
Saad D, Ndlovu M, Ramaremisa G, Tutu H (2022) Microplastics in freshwater environment: the first evaluation in sediment of the Vaal River. South Africa. Heliyon 8:e11118. https://doi.org/10.1016/J.HELIYON.2022.E11118
Saad D, NdlovuM RamaremisaG et al (2023) Characteristics of microplastics in sediment of the Vaal River, South Africa: implications on bioavailability and toxicity. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-023-05168-1
Sun J, Dai X, Wangc Q et al (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37
Tan X, Yu X, Cai L et al (2019) Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 221:834–840. https://doi.org/10.1016/j.chemosphere.2019.01.022
Verster C, Bouwman H (2020) Land-based sources and pathways of marine plastics in a South African context. S Afr J Sci 116(5–6):1–9
Weideman EA, Perold V, Ryan PG (2019) Little evidence that dams in the Orange-Vaal River system trap floating microplastics or microfibres. Mar Pollut Bull 149:110664. https://doi.org/10.1016/j.marpolbul.2019.110664
Zhang L, Xie Y, Zhong S, Liu J, Qin Y, Gao P (2021) Microplastics in freshwater and wild fishes from Lijiang River in Guangxi, Southwest China. Sci Total Environ 755:142428. https://doi.org/10.1016/j.scitotenv.2020.142428
Zhao S, Zhu L, Li D (2015) Microplastic in three urban estuaries, China. Environ Pollut 206:597–604. https://doi.org/10.1016/j.envpol.2015.08.027
Acknowledgements
The authors would like to thank Prof Rudolph Erasmus for the Raman Spectroscopy analysis.
Funding
Open access funding provided by University of the Witwatersrand. This project was supported by the Royal Society, UK, and funded by Government’s Grand Challenges Research Fund (GCRF), [Grant Number: FLR\R1\201062].
Author information
Authors and Affiliations
Contributions
Study conception and design, funding acquisition, and resources [DSd]. Methodology, data collection, and analysis were performed by [GR]. The first draft of the manuscript was written by [DS] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saad, D., Ramaremisa, G., Ndlovu, M. et al. Microplastic Abundance and Sources in Surface Water Samples of the Vaal River, South Africa. Bull Environ Contam Toxicol 112, 23 (2024). https://doi.org/10.1007/s00128-023-03845-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03845-y