Abstract
To characterise mercury (Hg) exposure in Daubenton’s bat (Myotis daubentonii, Kuhl 1817) in southern Sweden, 17 specimens were captured in 2013 and back fur samples were taken for analysis to determine Hg concentrations. The fur Hg levels determined [1.15 ± 0.27 (mean ± standard deviation, n = 17) µg Hg g−1 fresh weight (fw)] represent a baseline for comparison in future assessments of Hg exposure in bat populations in northern Europe. Mercury concentrations were close to those reported in fur from other bat species, but were lower than proposed toxicological thresholds in bats (> 30 µg Hg g−1 fw) and mice (5 µg Hg g−1 fw). This is the first study to examine Hg exposure in bats in Scandinavia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mercury (Hg) has severe impacts as an environmental pollutant, with aquatic ecosystems in particular functioning as an important route for Hg exposure in wildlife (Chan et al. 2003; Driscoll et al. 2013). The situation is especially severe in Sweden, since the environmental quality standard (EQS) of 0.02 mg kg−1 wet weight set to protect ecosystem health under the EU Water Framework Directive is exceeded 10- to 100-fold in aquatic biota across all water bodies (Åkerblom et al. 2014). The EQS is mainly intended to protect wildlife feeding on aquatic biota from secondary poisoning, so it is also important to determine the extent to which wildlife is exposed. Bats foraging in aquatic habitats bioaccumulate Hg by eating insects that emerge from the water (Wolfe et al. 1998; Wada et al. 2010; Little et al. 2015a). Compared with terrestrial foraging bats, aquatic foraging bats have adapted by developing higher levels of protective proteins (metallothioneins), which are used to prevent ecotoxicological effects from heavy metal contamination (Pikula et al. 2010). Owing to the high Hg levels in Swedish aquatic ecosystems, there may be severe Hg exposure in bat populations that forage in regions of Sweden with high Hg levels in aquatic biota. Despite the potential for Hg exposure in bats, this issue has not previously been studied in Sweden. This study rectifies this knowledge gap and provide a baseline that can be used for comparison in future studies. Elsewhere, research focusing on Hg exposure of bats is increasing (Hickey et al. 2001; Zukal et al. 2015).
Bats can be used as bio-indicators, and for this reason bat population mapping is an important component of environmental monitoring programmes. One of the aims of monitoring is to detect heavy metal exposure in bat populations and mitigate remediation efforts (Zukal et al. 2015). Collection of fur to analyse contamination level is a good non-lethal method for determination of heavy metal exposure in bats (Flache et al. 2015; Hernout et al. 2016b). The use of fur from the back of the bat provides heavy metal data that have been shown to be correlated with heavy metal data for other organs, e.g., blood and liver (Massa and Grippo 2000; Nam et al. 2012; Zukal et al. 2015; Hernout et al. 2016b). The collection and analysis of fur also provides the potential to estimate possible ecotoxicological effects by comparison against toxic thresholds.
Environmentally relevant Hg exposure impairs the function of the nervous system and behaviour in mammals (Clarkson and Magos 2006). Toxic effects in bats from Hg exposure include impairment of neurological function [toxic threshold in fur = 100 µg g−1 fresh weight (fw)] (Nam et al. 2012), while damage to mitochondrial DNA has also been reported (toxic threshold in fur = 30 µg Hg g−1 fw) (Karouna-Renier et al. 2014). Shifts in ambulatory activity in wild mouse populations and toxicological effects have been found at fur levels > 5 µg Hg g−1 fw (Burton et al. 1977). There is also a risk of reproductive disorders, with reports of oxidative stress in testes of e.g., rats following Hg exposure (Boujbiha et al. 2009; Burton and Meikle 1980). The effects of Hg also include developmental alterations in the foetus that can cause impairment or even death after birth (Scheuhammer et al. 2007).
Daubenton’s bat (Myotis daubentonii, Kuhl 1817) is common in Sweden, with a distribution that extends above the Arctic circle, and is therefore useful for comparison of Hg exposure between regions (Ahlén 2011; Siivonen and Wermundsen 2008). The aim of this study was to determine the concentrations of Hg in fur from Daubenton’s bat, of both sexes and different stages of maturation, at two sites in southern Sweden that are not subject to local sources of Hg pollution or other disturbances. The hypothesis tested was that Hg concentrations in the fur of Daubenton’s bat are below the reported EQS for Hg in bats. We also tested whether variation in Hg concentration in the fur of Daubenton’s bat could be explained by sex and maturation stage (adult/juvenile). The study was intended to provide a general indication of Hg exposure and to estimate the threat posed by Hg exposure in Swedish populations of Daubenton’s bat.
Materials and methods
Bats were trapped in a mist net placed above the water surface in small streams on 22 July and 23 July 2013 at two different sites: Stockamöllan (coordinates: 55°56′N, 13°22′E) and Södra Åsum (coordinates: 55°38′N, 13°42′E). Both sites are located in the province of Skåne, southern Sweden. Fur samples were collected in conjunction with annual surveillance for the prevalence of bat lyssavirus type 2. Bats were classified as either juvenile or adult based on examination of finger bones (Anthony 1988). Fur samples were clipped from the back of the bats using stainless steel surgical scissors. The samples were stored in plastic tubes and kept frozen (− 18°C) during transport to the laboratory and until analysis. Immediately after fur sampling, the bats were released.
In total, 17 individuals were captured and used for fur sampling. These comprised 7 males and 10 females (Table 1). Thirteen of these individuals were captured at the Södra Åsum site, while only four individuals were captured at Stockamöllan. Bat fur specimens from both adults and juveniles were collected at Södra Åsum, but only adult bats were sampled at Stockamöllan.
Fur was analysed for Hg concentration using an SMS-100 Mercury Analyzer (Perkin Elmer) by thermal decomposition (750°C) followed by amalgamation on a gold trap, thermal desorption and analysis of vapour Hg by atomic absorption spectroscopy (AAS) according to EPA method 7473. Fresh weight of the bat fur samples, determined using a balance with a detection limit of 0.05 mg and weight varied between 2 and 30 mg. The total Hg content in the samples ranged between 0.71 and 3.87 ng Hg (the reported detection limit for the method is 0.005 ng Hg). Blank samples (empty sample boats) were analysed regularly (mean Hg content < 0.01 ng Hg). The accuracy of the Hg analysis was tested at regular intervals during the analysis using fish liver (CNRC DOLT4) and lake sediment (IAEA SL-1) reference material (RM) with a certified concentration of 2.58 µg Hg g−1 dry weight and 0.13 µg Hg g−1 dry weight, respectively. Recovery of RM was 105% ± 2% [mean ± standard deviation (SD)]. The amount of sample material collected could only be used for one analysis of each bat specimen. Analysis of hair replicate samples typically has a precision higher than 97% relative SD (Gashaw et al. 2017).
A weighted arithmetic mean for the Hg concentration of fur samples were calculated based on data from each category of bat (adult/juvenile, female/male, Södra Åsum/Stockamöllan). Differences in Hg concentration in fur of Daubenton’s bat were tested by the non-parametric median test using a 1-way test using all groups available (males/females, adult/juvenile, and Stockamöllan/Södra Åsum). All tests were evaluated for significance at α = 0.05. Statistical calculations were executed in JMP 11.0 (SAS Institute Inc., Cary, NC, USA).
Results and Discussion
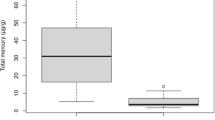
The Hg concentration in fur from the Daubenton’s bat specimens varied between 0.44 and 2.30 µg Hg g−1 fw, with an overall weighted arithmetic mean of 1.15 µg Hg g−1 fw (SD = 0.27 µg Hg g−1 fw) with no significant difference between the sampled groups (median test: χ2 = 10.9, df = 5, p = 0.054) (Table 1; Fig. 1). Fur samples were not washed prior the Hg analysis to remove any external contamination (Flache et al. 2015). By not removing possible exogenous contaminants by washing of the fur samples possible artefacts in the data set can have been introduced, e.g. the high Hg concentration in adult female bat specimen at Södra Åsum (2.31 µg Hg g−1 fw). Data on fur Hg concentration in the present study represented only two populations of bats with a small number of observations (n = 17) and skewness in the number of observations between the studied groups (Table 1). The data still provides an indication of fur Hg concentrations that can be expected in the study region.
Box and whiskers plot of concentrations of Hg in back fur (µg Hg g−1 fw) from adult and juvenile male and female specimens of Daubenton’s bat (Myotis daubentonii) captured at two sites in southern Sweden (Stockamöllan and Södra Åsum). Box plots (Bars = 10 and 90 percentiles; boxes = 25 and 75 percentile; vertical line = median). N number of specimens
Compared with fur Hg concentration reported for other regions and continents (Table 2), bat populations in southern Sweden were exposed to Hg to approximately the same extent as in regions in North America and Asia (Table 2). There are differences between bat species in the published data, with big brown bat (Eptesicus fuscus) having the lowest levels of Hg, while little brown bat (Myotis lucifugus) has the highest reported levels (although those high values were detected at polluted sites) (Nam et al. 2012).
Sex and age represent important sources of variation in Hg bioaccumulation in wild populations of bats (Yates et al. 2013). In Sweden, we have observed sex-biased differentiation in Daubenton’s bat, as small streams and ponds are more commonly used by males while the females use lakes and rivers for foraging, although small streams close to lakes may be used by both sexes (unpublished data). Lower fur Hg concentrations in females bats compared with male bats have been explained by the depuration of Hg during lactation (Lison et al. 2017; Yates et al. 2013) even though it was not possible to detect any difference between sexes in this study. Differentiation between sexes in their foraging and roosting strategies could also add to differences in Hg concentrations between bat populations. Juvenile bats of both sexes forage close to the colony in the same area as the female, especially during lactation, while there are clear differences in foraging strategies between male and female adult Daubenton’s bat (Dietz et al. 2006). Males and females also differ in their strategy of selecting roosting places, with landscape factors (altitude, abundance of ponds, lakes and rivers) playing an important role (Encarnação et al. 2005). New growth fur should be targeted for sampling and analysis of endogenous markers in bats. Moulting cycles in bats are both age and sex-specific and add to the observed variation in endogenous markers (Fraser et al. 2013). Sampling of fur in this study was done in early-mid summer and was earlier then the timing of new-fur growth that generally occur in late summer-fall. Monitoring protocols should acknowledge the above-mentioned factors for sampling of bat populations in the future.
The Hg levels detected in Daubenton’s bat in southern Sweden were 5- to 100-fold lower than the suggested threshold at which genotoxic (30 µg Hg g−1 fw) (Karouna-Renier et al. 2014) or neurological (100 µg Hg g−1 fw) (Nam et al. 2012) effects may occur. However, a much lower threshold (5 µg Hg g−1 fw) has been proposed for behavioural effects in wild mouse populations (Burton et al. 1977). Those toxicity studies were performed at contaminated sites and the Hg exposure in bats at Hg-contaminated sites in Sweden may well reach toxic levels. Bats caught near rivers and lakes with fish that exceed consumption advisory levels (1 mg kg−1 wet weight) in Arkansas (USA) had fur Hg concentration ranging from 1 to 30 µg Hg g−1 fw (Massa and Grippo 2000). Fish Hg levels above these levels have been found in a large proportion of lakes in Sweden (Åkerblom et al. 2014). Despite the uncertainty resulting from the small number of observations, we speculate that a linear relationship between aquatic biota and Hg exposure in Daubenton’s bat across ecosystems could eventually lead to Hg concentrations that are 2- to 20-fold higher in northern Sweden than at southern Swedish sites.
This study on Hg exposure in bats is the first in Scandinavia and provides an estimate for future comparisons on Hg exposure in bat populations in other regions in northern Europe. It also provides an estimate of the environmental stress on bat populations and the potential risk of Nordic bats being affected by the ecotoxicological effects of Hg (Zukal et al. 2015). This type of data is important, since heavy metal exposure is considered to play an important role for the health of bat populations and is thus relevant in monitoring work on bats (Hernout et al. 2016a; Luftl et al. 2003; Walker et al. 2007; Zukal et al. 2015).
The extent to which Hg exposure affects the health of bat populations can only be estimated by comparison of field-collected data with toxic thresholds. The results presented here are important for future establishment of a broader environmental monitoring programme on Hg exposure in bat populations in Sweden. The data also provide a reference for future studies on Hg exposure, risk assessments and decision making to protect the health of bat populations from negative effects of mercury.
References
Ahlén I (2011) Fladdermusfaunan i Sverige. Arternas utbredning och status. Kunskapsläget 2011. Fauna Flora 106:1–19
Åkerblom S, Bignert A, Meili M, Sonesten L, Sundbom M (2014) Half a century of changing mercury levels in Swedish freshwater fish. Ambio 43:91–103
Anthony ELP (1988) Age determination in bats. In: Kunz TH (ed) Ecological and behavioral methods for the study of bats. Smithsonian Institution Press, Washington, DC, pp 47–58
Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, El Feki A (2009) Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol 28:81–89. doi:10.1016/j.reprotox.2009.03.011
Burton GV, Meikle AW (1980) Acute and chronic methyl mercury-poisoning impairs rat adrenal and testicular function. J Toxicol Environ Health Sci 6:597–606
Burton GV, Alley RJ, Rasmussen L, Orton P, Cox V, Jones P, Graff D (1977) Mercury and behavior in wild mouse populations. Environ Res 14:30–34
Chan HM, Scheuhammer AM, Ferran A, Loupelle C, Holloway J, Weech S (2003) Impacts of mercury on freshwater fish-eating wildlife and humans. Hum Ecol Risk Assess 9:867–883. doi:10.1080/713610013
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 34:369–403. doi:10.1080/10408440600845619
Dietz M, Encarnacao JA, Kalko EKV (2006) Small scale distribution patterns of female and male Daubenton’s bats (Myotis daubentonii). Acta Chiropterologica 8:403–415. doi:10.3161/1733-5329(2006)8[403:SSDPOF]2.0.CO;2
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983. doi:10.1021/es305071v
Encarnação JA, Kierdorf U, Holweg D, Jasnoch U, Wolters V (2005) Sex-related differences in roost-site selection by Daubenton’s bats Myotis daubentonii during the nursery period. Mammal Review 35:285–294
Flache L, Becker NI, Kierdorf U, Czarnecki S, Duering R-A, Encarnacao JA (2015) Hair samples as monitoring units for assessing metal exposure of bats: a new tool for risk assessment. Mamm Biol 80(3):178–181. doi:10.1016/j.mambio.2015.01.007
Fraser EE, Longstaffe FJ, Fenton MB (2013) Moulting matters: the importance of understanding moulting cycles in bats when using fur for endogenous marker analysis. Can J Zool 91(8):533–544. doi:10.1139/cjz-2013-0072
Gashaw H, Getahun A, Bravo AG, Deribe E, Åkerblom S, Bishop K (2017) Mercury human exposure in populations living around lake Tana (Ethiopia). Biol Trace Elem Res 175:237–243
Hernout BV, Arnold KE, McClean CJ, Walls M, Baxter M, Boxall ABA (2016a) A national level assessment of metal contamination in bats. Environ Pollut 214:847–858
Hernout BV, McClean CJ, Arnold KE, Walls M, Baxter M, Boxall ABA (2016b) Fur: a non-invasive approach to monitor metal exposure in bats. Chemosphere 147:376–381. doi:10.1016/j.chemosphere.2015.12.104
Hickey MBC, Fenton MB, MacDonald KC, Soulliere C (2001) Trace elements in the fur of bats (Chiroptera: Vespertilionidae) from Ontario and Quebec, Canada. Bull Environ Contam Toxicol 66:699–706
Karouna-Renier NK, White C, Perkins CR, Schmerfeld JJ, Yates D (2014) Assessment of mitochondrial DNA damage in little brown bats (Myotis lucifugus) collected near a mercury-contaminated river. Ecotoxicol 23(8):1419–1429. doi:10.1007/s10646-014-1284-9
Lison F, Espin S, Aroca B, Calvo JF, Garcia-Fernandez AJ (2017) Assessment of mercury exposure and maternal-foetal transfer in Miniopterus schreibersii (Chiroptera: Miniopteridae) from southeastern Iberian Peninsula. Environ Sci Pollut R 24:5497–5508. doi:10.1007/s11356-016-8271-z
Little ME, Burgess NM, Broders HG, Campbell LM (2015a) Mercury in little brown bat (Myotis lucifugus) maternity colonies and its correlation with freshwater acidity in Nova Scotia, Canada. Environ Sci Technol 49(4):2059–2065. doi:10.1021/es5050375
Little ME, Burgess NM, Broders HG, Campbell LM (2015b) Distribution of mercury in archived fur from little brown bats across Atlantic Canada. Environ Pollut 207:52–58. doi:10.1016/j.envpol.2015.07.049
Luftl S, Freitag B, Deutz A, Tataruch F (2003) Concentrations of heavy metals in European bats (Microchiroptera). Fresen Environ Bull 12:353–358
Massa SA, Grippo RS (2000) Mercury levels in Arkansas bats from counties under advisories for fish consumption. Paper presented at the SETAC 21st Annual Meeting, Nashvill, Tennesee, USA
Miura T, Koyama T, Nakamura I (1978) Mercury content in museum and recent specimens of chiroptera in Japan. Bull Environ Contam Toxicol 20:696–701. doi:10.1007/bf01683587
Nam DH, Yates D, Ardapple P, Evers DC, Schmerfeld J, Basu N (2012) Elevated mercury exposure and neurochemical alterations in little brown bats (Myotis lucifugus) from a site with historical mercury contamination. Ecotoxicol 21:1094–1101. doi:10.1007/s10646-012-0864-9
Pikula J et al (2010) Heavy metals and metallothionein in vespertilionid bats foraging over aquatic habitats in the Czech republic. Environ Toxicol Chem 29:501–506. doi:10.1002/etc.80
Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW (2007) Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12–18
Siivonen Y, Wermundsen T (2008) Distribution and foraging habitats of bats in northern Finland: Myotis daubentonii occurs north of the Arctic Circle. Vespertilio 12:41–48
Wada H, Yates DE, Evers DC, Taylor RJ, Hopkins WA (2010) Tissue mercury concentrations and adrenocortical responses of female big brown bats (Eptesicus fuscus) near a contaminated river. Ecotoxicol 19:1277–1284
Walker LA, Simpson VR, Rockett L, Wienburg CL, Shore RF (2007) Heavy metal contamination in bats in Britain. Environ Pollut 148:483–490. doi:10.1016/j.envpol.2006.12.006
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160. doi:10.1002/etc.5620170203
Yates DE et al (2013) Mercury in bats from the northeastern United States. Ecotoxicol 23:45–55
Zukal J, Pikula J, Bandouchova H (2015) Bats as bioindicators of heavy metal pollution: history and prospect. Mamm Biol 80:220–227. doi:10.1016/j.mambio.2015.01.001
Acknowledgements
We thank Anders Lindström, Louise Treiberg-Berntsson and Gunilla Hallgren from the National Veterinary Institute for excellent support during the field work. The work on collection of Daubenton’s bats was financially supported by the Swedish Board of Agriculture.
Funding
This study was funded by the SLU programme for the Environmental Quality Objective of a non-toxic environment within Environmental Monitoring and Assessment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither Staffan Åkerblom nor Johnny De Jong have any conflict of interest to declare.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of bats were followed during this study. Ethical approval was issued on 31 May 2010 by the Swedish Board of Agriculture for capture and sampling of bats (Approval-ID:C192/10).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Åkerblom, S., de Jong, J. Mercury in fur of Daubenton’s bat (Myotis daubentonii) in Southern Sweden and Comparison to Ecotoxicological Thresholds. Bull Environ Contam Toxicol 99, 561–566 (2017). https://doi.org/10.1007/s00128-017-2206-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2206-3