Abstract
The objectives of this study were (1) to determine cadmium (Cd) accumulation in the midgut gland of a land snail Helix pomatia L. inhabiting residential areas of the 14 largest cities in Poland, and (2) to examine whether the accumulated Cd exerted any toxic effects. The average accumulation of Cd in the midgut gland of snails, weighing 16–18 g, ranged from 7.00 to 87.3 µg/g dry weight (0.06–0.77 µmol/g) and differed significantly among animals from the various urban areas. This difference in Cd accumulation was not related to city population, but was associated with the topsoil Cd (R2 = 0.868, p < 0.0001). The tissue Cd was not found to produce toxicity (histopathology, programmed cell death, lipofuscin formation or lipid peroxidation), probably due to the induction of sufficiently high quantities of metallothionein and glutathione, well-known protective molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cadmium (Cd) is an environmental pollutant ranked seventh in the priority list of hazardous substances (ATSDR 2007). In humans and animals, Cd can damage various organs and tissues, including kidney, liver, lung, pancreas, testis and bone (Wittman and Hu 2002; Satarug et al. 2003; Zalups and Ahmed 2003; Järup and Akesson 2009). The metal is also classified as a category I human carcinogen (Weisberg et al. 2003). Unfortunately, its amount in the environment still increases mainly due to human activities such as the combustion of coal and oil to produce heat and electricity, non-ferrous metal manufacturing, the production of plastics, alloys, iron and cement, and waste incineration (Wittman and Hu 2002; Pacyna et al. 2007). These activities are generally located in urban areas which, therefore, become more contaminated with Cd than other areas (Sawicka-Kapusta et al. 2003). This is also evidenced by a substantial increase of Cd concentration in the urban soils (Ajmone-Marsan and Biasoli 2010; Luo et al. 2012). However, the impact of soil contamination on human health and soil organisms, especially in residential and recreational areas, is not well studied and remains to be elucidated.
Land snails, including Helix pomatia L., have been shown to exhibit a high capacity to accumulate Cd in the midgut gland (hepatopancreas) (Dallinger and Wieser 1984; Rabitsch 1996; Menta and Parisi 2001; Dallinger et al. 2004), which makes them useful tools for the assessment of the metal pollution in soil (Dallinger 1994). The high capacity of Cd accumulation is related directly to efficient induction of a specific Cd-binding metallothionein (MT) isoform which is responsible for retention and detoxification of the metal in the midgut gland of H. pomatia and other species (Dallinger et al. 1997, 2004; Chabicovsky et al. 2004; Hödl et al. 2010). It has been demonstrated further that pathological alterations such as increased programmed cell death and cell proliferation, the formation of lipofuscin granules, and the disruption of mitochondrial membranes in this organ can occur at Cd concentrations above a threshold of 0.8 µmol/g dry weight, when all binding sites on the MT isoform are saturated with Cd and the resulting non-MT-bound Cd ions can exert their toxic effects (Hödl et al. 2010). One mechanism by which these ions can produce pathology in vertebrates and invertebrates is thought to be through generation of reactive oxygen species and oxidation of membrane lipids and proteins (Roesijadi et al. 1997; Liu et al. 2009; Amachree et al. 2013). So far, however, little is known about the accumulation and toxicity of Cd in the midgut gland of H. pomatia snails ranging in an urban area.
Therefore, the purposes of this study were (1) to determine Cd accumulation in the midgut gland of H. pomatia inhabiting residential areas of the 14 largest cities in Poland, and determine whether this accumulation was related to city population size and soil Cd, and (2) to examine whether the accumulated Cd affected tissue structure, programmed cell death, the formation of lipofuscin granules and lipid peroxidation. In addition, the concentrations of MT and glutathione (GSH) that are linked to a protective effect against Cd toxicity (Chan and Cherian 1992; Nzengue et al. 2008) were determined.
Materials and Methods
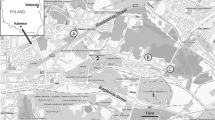
Ten individuals of H. pomatia, weighing 16–18 g, were collected from each of the 14 cities in Poland (Fig. 1) in the period of 15 June–15 July 2011. The snails were sampled from three different sites located in a residential area (gardens, parks) of each city. In addition, from each site the topsoil (0–5 cm) as composite sample (500 g) was taken for determination of Cd and pH. In the laboratory, the soil samples were dried at 60°C, and then crushed and passed through a 1-mm sieve. Soil pH was determined in soil suspensions (1:2.5 soil to water ratio) using a pH meter. The snails were decapitated, and the midgut gland was removed and divided into three portions. One portion (about 200 mg wet weight) was dried at 60°C for 48 h and used for determination of Cd and water (to calculate a dry weight conversion factor). Another one was fixed in 4% formaldehyde for histological examination. The third portion was frozen and kept at −80°C until analysis of MT, GSH and lipid peroxidation. After thawing, a portion (about 250 mg) of this organ was transferred to 1.0 mL chilled 0.25 M sucrose and homogenized with a Teflon pestle in a glass homogenizer. An aliquot (0.2 mL) was taken for determination of lipid peroxidation. The remaining homogenate was centrifuged at 20,000×g for 20 min at 4°C, and the resulting supernatant was removed for MT and GSH assays. The data were expressed on a dry weight basis, using dry weight conversion factors.
Cadmium content in the midgut gland of H. pomatia snails was determined as described in Włostowski et al. (2010). Briefly, a portion of the organ (60–70 mg dry weight) was placed in a glass tube and 2.0 mL of redistilled nitric acid (70%) (Sigma-Aldrich) was added. After 20 h of sample digestion at room temperature, 72% perchloric acid (0.5 mL) was added and the mixture was heated at 100°C for 3 h. Finally, the temperature was raised to 150–180°C and digestion continued for another 3 h. Deionized water was added to the residue after digestion to a volume of 3.0 mL (first solution). A portion of the first solution (200 µL) was evaporated to dryness in a quartz crucible at 130°C, and the residue was redissolved in an appropriate amount of deionized water (second solution). Cd analyses of these solutions were carried out by electrothermal atomic absorption spectrometry (AAS) using a Thermo Sollar M6 instrument with Zeeman correction (ThermoFisher Scientific, Waltham, MA, USA). A standard solution (Sigma-Aldrich, Poznan, Poland) was used to prepare the standard curve. Quality assurance procedures included the analysis of reagent blanks and standard reference material (Bovine liver 1577c—National Institute of Standards and Technology, Gaithersburg, MD). The limit of detection in the acid digest (3 × standard deviation of 10 reagent blanks plus the mean) was 2.00 µg/L. For a 60 mg of tissue the detection limit was 0.10 µg/kg dry weight. The precision expressed as relative standard deviation (RSD) of 10 measurements of the same sample was 5% and the recovery of Cd was 91%–96%.
In the case of soil Cd, 0.5 g of the soil was extracted with 2.0 mL of concentrated nitric acid in a closed polypropylene tube for 5 days, and then heated at 70°C for 24 h (Dallinger et al. 2004). During extraction, the tubes were repeatedly shaken by hand. After this treatment the soil suspensions were centrifuged at 30,000×g for 30 min, and the supernatants were transferred to a quartz crucible and evaporated to dryness. The residue was redissolved in an appropriate amount of deionized water and analyzed for Cd by electrothermal AAS. The analysis of each soil sample was done in triplicate.
MT in the midgut gland was determined by a Cd-saturation method and expressed in µg Cd bound to MT/g dry weight (Włostowski et al. 2010). The total GSH (reduced + oxidized) was measured according to the method of Tietze (1969) by using a glutathione assay kit (NWLSS, Vancouver, WA, USA). Lipid peroxidation was assessed by measuring malondialdehyde (MDA) formation, using the thiobarbituric acid (TBA) assay (Ohkawa et al. 1979).
The fixed portions of the midgut gland were dehydrated in ethanol and xylene, embedded in paraffin, cut into 5 µm sections (Leica microtome), and stained with hematoxylin and eosin for microscopic examination. The number of cells with lipofuscin granules (Fig. 2) were determined in ten random microscopic fields for each snail, using a 40 × objective, and the results were expressed as the mean number of cells with lipofuscin granules per microscopic field. Also condensed bodies (programmed cell death) (Fig. 2) (Chabicovsky et al. 2004) were counted in the same sections.
Representative photomicrograph of a midgut gland section from the Helix pomatia snail under study. Note the residual bodies (lipofuscin granules) within large vacuoles of excretory cells (arrows) and the condensed body (programmed cell death) situated in a basophilic cell (arrow head). Scale bar 20 µm
Data were expressed as mean ± SD. They were analyzed by one-way analysis of variance (ANOVA) followed by the Duncan’s multiple range test (when required, the variables were normalized using log10 transformation). Differences at p < 0.05 were considered statistically significant. The simple regression analysis was used to examine the relationship between Cd accumulation in the midgut gland and the number of inhabitants and soil Cd, as well as between the midgut gland Cd and the MT, GSH, lipid peroxidation and the number of cells with lipofuscin granules. All the statistical analyses were performed using IBM SPSS Statistics 21 program (IBM Corporation, Somers, NY, USA).
Results and Discussion
The concentrations of Cd in the midgut gland of H. pomatia snails and in the soil from a residential area of 14 cities are presented in Table 1. The levels of Cd in the midgut gland differed significantly (p < 0.0001) among snails living in the different cities. The lowest levels of the metal (means below 10 µg/g dry wt) were found in 4 cities (Bialystok, Olsztyn, Gdansk and Warsaw), the highest (87 µg/g dry wt) was noted in Katowice, and medium concentrations (16–35 µg/g dry wt) were observed in animals from the remaining 9 cities (Fig. 1; Table 1). This difference in Cd accumulation was not related to the weight (age) of animals, which was similar (16–18 g) in the snails from all cities. Also, the size of a city (number of inhabitants) (Table 1) did not affect the accumulation of Cd in the midgut gland (R2 = 0.029, p > 0.5). The simple regression analysis revealed, however, that the accumulation of Cd in this organ correlated significantly with the topsoil Cd (R2 = 0.868, p < 0.0001) (Fig. 3), but the soil Cd was not related to the size of a city (R2 = 0.01). Although soil pH has been demonstrated to affect Cd accumulation in snails (Pauget et al. 2012), this factor could only have a negligible effect in the H. pomatia snails because the soil pH (6.95–7.35) was almost identical in all cities.
The midgut gland was also analyzed for MT and GSH contents (Table 1). The Cd-binding capacity of MT in this organ followed a pattern similar to that of Cd concentration, and the capacity exceeded the total concentration of this metal in the snails from all urban areas (Tables 1, 2). Furthermore, the concentration of GSH in the midgut gland correlated positively with Cd concentration (Table 2), suggesting that this compound could also be induced by the metal.
In this work, the toxicity of Cd was evaluated by assessing histopathology, programmed cell death (condensed bodies) and lipofuscin formation (residual bodies) (Fig. 2), as well as lipid peroxidation (Table 1). No histopathological change (necrosis) was observed, and evidence of programmed cell death was very rarely observed in the midgut gland of all snails from the 14 cities. These data were not suitable for statistical analysis. Residual bodies were observed exclusively in the excretory cells (Fig. 2), and their means differed significantly (p < 0.05) amongst the cities (Table 1). However, there was no significant relationship of the tissue Cd to the lipofuscin formation (Table 2). Likewise, lipid peroxidation (an index of oxidative stress) in the midgut gland differed significantly (p < 0.05) among snails from the various cities (Table 1), but did not correlate significantly with tissue Cd (Table 2).
The present work demonstrated that Cd accumulation in the midgut gland of H. pomatia from an urban area was strongly related to topsoil Cd. Thus, these data confirm the notion that H. pomatia snails are suitable animals for the assessment of Cd contamination in soil (Dallinger 1994). The current data indicate further that the accumulation of Cd in the midgut gland is not associated with the population of a city, which suggests that the inhabitants do not affect the soil and tissue concentrations of Cd. Indeed, the levels of Cd in the soil and midgut gland of H. pomatia from the largest city (Warsaw) were similar to those from the smaller ones (Bialystok, Gdansk, Olsztyn), and significantly lower than those from other cities (Table 1). Thus, it may be concluded that the differences in Cd concentration among the urban areas are linked to other factors; for instance, a different natural concentration of Cd in the bedrock and/or industrial emission of Cd to the atmosphere may play an important role (Luo et al. 2012). The first possibility may be confirmed by the fact that the concentrations of Cd in the soil and midgut gland of H. pomatia from Kielce (204,000 inhabitants) are 2–3-fold higher than those from Warsaw (1,720,000 inhabitants) although the emission of Cd to the atmosphere from the territory of the two districts is similar (about 100 g/km2/year) (Hławiczka 2008). On the other hand, the highest concentrations of Cd observed in Katowice (Fig. 1; Table 1) are probably due to long-term emission of Cd to the atmosphere, which is the highest in Poland and now amounts to 600 g/km2/year (Hławiczka 2008). This pollution is probably caused by heavy industry situated in this area which includes, among others, coal-based power station and heating units, hard coal mines, an iron factory and a non-ferrous metal smelter (Kennedy et al. 1999). However, irrespective of Cd origin (natural or anthropogenic) the lowest environmental levels of this metal are characteristic of Olsztyn, Bialystok, Gdansk and Warsaw (north-eastern part of Poland), slightly higher for Lublin and Torun, 3–5-fold higher for Cracow, Kielce, Lodz, Poznan, Rzeszow, Szczecin and Wroclaw, and highest for Katowice (Fig. 1). In this regard it is worth noting that the renal Cd levels in non-smoking inhabitants from Katowice, Cracow and Lodz have been shown to be significantly higher than those found in people from Bialystok (Piotrowski et al. 1996). These data suggest that environmental Cd in the urban areas affects the tissue Cd not only in H. pomatia snails but also in humans. In the case of humans, both house dust and the consumption of home-grown crops are considered to be important routes of exposure to Cd (Järup and Akesson 2009; Ajmone-Marsan and Biasoli 2010).
The average accumulation of Cd in the midgut gland of H. pomatia from the 14 cities ranged from 7.00 to 87.3 µg/g dry weight (0.06–0.77 µmol/g) (Table 1). It has been recently demonstrated that pathological changes in the midgut gland of H. pomatia such as the increasing formation of lipofuscin granules occurs only at Cd concentrations above a threshold of 0.8 µmol/g dry weight (90 µg/g) (Hödl et al. 2010). Thus, it can be concluded that observations of pathological effects in the midgut gland of free-ranging snails in these urban areas would be unlikely. Indeed, there was no histopathology and only single cells undergoing programmed cell death were observed; in addition, no correlation was found between the midgut gland Cd and the number of cells with lipofuscin granules (Table 2), suggesting the lack of toxic effects of the metal accumulated in this organ. No toxic effects of Cd in the midgut gland could have resulted from the Cd induction of appropriate amounts of MT (Tables 1, 2). It is well known that the Cd-MT isoform of H. pomatia is involved in Cd detoxification (Dallinger et al. 1997; Hödl et al. 2010) and its Cd-binding capacity in the midgut gland of snails from all cities exceeded the total concentration of Cd, which indicated that the non-MT-bound Cd (if any) was too low to exert toxic effects. In addition, GSH that is also known to provide a protection against Cd toxicity (Chan and Cherian 1992; Nzengue et al. 2008) increased in relation to Cd concentration in the midgut gland, thereby preventing Cd-induced oxidative stress; no correlation between lipid peroxidation and Cd concentration in the gland suggested that this may have been the case. It cannot be ruled out, however, that the induction of lipid peroxidation by Cd in the snails may occur at much higher concentrations of this metal than those required for GSH or MT induction. Nevertheless, no Cd toxicity observed in the midgut gland of H. pomatia may be linked to sufficiently high concentrations of the two protective molecules.
In conclusion, the results of this study showed that the accumulation of Cd in the midgut gland of H. pomatia snails from urban areas was directly related to topsoil Cd concentration, which was not related to city population size. Relatively high levels of Cd in the midgut gland of snails from some urban areas appeared not to produce toxic responses, most probably due to the induction of appropriate quantities of MT and GSH.
References
Ajmone-Marsan F, Biasoli M (2010) Trace elements in soil of urban areas. Water Air Soil Pollut 213:121–143
Amachree D, Moody AJ, Handy RD (2013) Comparison of intermittent and continuous exposures to cadmium in the blue mussel Mytilus edulis: accumulation and sub-lethal physiological effects. Ecotoxicol Environ Saf 95:19–26
ATSDR (2007) The priority list of hazardous substances. http://www.atsdr.cdc.gov/toxicsubstances
Chabicovsky M, Klepal W, Dallinger R (2004) Mechanisms of cadmium toxicity in terrestrial pulmonates: programmed cell death and metallothionein overload. Environ Toxicol Chem 23:648–655
Chan HM, Cherian MG (1992) Protective roles of metallothionein and glutathione in hepatotoxicity of cadmium. Toxicology 72:281–290
Dallinger R (1994) Invertebrate organisms as biological indicators of heavy metal pollution. Appl Biochem Biotechol 48:27–31
Dallinger R, Wieser W (1984) Patterns of accumulation, distribution and liberation of Zn, Cu, Cd and Pb in different organs of the land snail Helix pomatia L. Comp Biochem Physiol C 79:117–124
Dallinger R, Berger B, Hunziker P, Kägi JHR (1997) Metallothionein in snail Cd and Cu metabolism. Nature 388:237–238
Dallinger R, Lagg B, Egg M, Schipflinger R, Chabikovsky M (2004) Cd accumulation and Cd-metallothionein as a biomarker in Cepaea hortensis (Helicidae, Pulmonata) from laboratory exposure and metal-polluted habitats. Ecotoxicology 13:757–772
GUS (2011) Demographic yearbook of Poland. Central Statistical Office, Warsaw
Hławiczka S (2008) Emission of cadmium, lead, arsenic, nickel, chromium, copper and zinc to the atmosphere in Poland in the years 1980–2005. Ochr Pow Probl Odpad 42:137–147 (in Polish)
Hödl E, Felder E, Chabicovsky M, Dallinger R (2010) Cadmium stress stimulates tissue turnover in Helix pomatia: increasing cell proliferation from metal tolerance to exhaustion in molluscan midgut gland. Cell Tissue Res 341:159–171
Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Kennedy SW, Godzik S, Dmowski K, Handy R, Kedziorski A, Kramarz P et al (1999) Report of the working group on the Katowice Administrative District, Poland; a review of research done to date, and recommendations for future research. In: Peakall DB, Walker CH, Migula P (eds) Biomarkers: a pragmatic basis for remediation of severe pollution in eastern Europe. Kluwer Academic Publishers, Utrecht, pp 191–210
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238:209–214
Luo XS, Yu S, Zhu YG, Li XD (2012) Trace metal contamination in urban soils of China. Sci Total Environ 421–422:17–30
Menta C, Parisi V (2001) Metal concentrations in Helix pomatia, Helix aspersa and Arion rufus: a comparative study. Environ Pollut 115:205–208
Nzengue Y, Steiman R, Garrel C, Lefebvre E, Guirand P (2008) Oxidative stress and DNA damage induced by cadmium in the human keratinocyte HaCaT cell line: role of glutathione in the resistance to cadmium. Toxicology 243:193–206
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pacyna EG, Pacyna JM, Fudala J, Strzelecka-Jastrząb E, Hlawiczka S, Panasiuk D et al (2007) Current and future emissions of selected heavy metals to the atmosphere from anthropogenic sources in Europe. Atmos Environ 41:8557–8566
Pauget B, Gimbert F, Scheifler R, Coeurdassier M, de Vaufleury A (2012) Soil parameters are key factors to predict metal bioavailability to snails based on chemical extractant data. Sci Total Environ 431:413–425
Piotrowski JK, Orłowski C, Bem EM, Bryś M, Baran E (1996) The monitoring of cadmium, zinc and copper in the kidneys and liver of humans deceased in the region of Cracow (Poland). Environ Monit Assess 43:227–236
Rabitsch WB (1996) Metal accumulation in terrestrial pulmonates at a lead/zinc smelter site in Arnoldstein, Austria. Bull Environ Contam Toxicol 56:734–741
Roesijadi G, Brubacher LI, Unger ME, Anderson RS (1997) Metallothionein mRNA induction and generation of reactive species in molluskan hemocytes exposed to cadmium in vitro. Comp Biochem Physiol C 118:171–176
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PEB, Williams DJ et al (2003) A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83
Sawicka-Kapusta K, Zakrzewska M, Bajorek K, Gdula-Argasińska J (2003) Input of heavy metals to the forest floor as a result of Cracow urban pollution. Environ Int 28:691–698
Tietze F (1969) Enzymatic method for the quantitative determination of nanogram amounts of total and oxidized glutathione: application to mammalian blood and other tissues. Anal Biochem 27:502–522
Weisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192:95–117
Wittman R, Hu H (2002) Cadmium exposure and nephropathy in a 28-year-old female metals worker. Environ Health Persp 110:1261–1266
Włostowski T, Dmowski K, Bonda-Ostaszewska E (2010) Cadmium accumulaton, metallothionein and glutathione levels, and histopathological changes in the kidneys and liver of magpie (Pica pica) from a zinc smelter area. Ecotoxicology 19:1066–1073
Zalups RK, Ahmed S (2003) Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol 186:163–188
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Włostowski, T., Kozłowski, P., Łaszkiewicz-Tiszczenko, B. et al. Accumulation of Cadmium in and Its Effect on the Midgut Gland of Terrestrial Snail Helix pomatia L. from Urban Areas in Poland. Bull Environ Contam Toxicol 93, 526–531 (2014). https://doi.org/10.1007/s00128-014-1346-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1346-y