Abstract
Mercury has been determined in caps, stipes and whole fruiting bodies of Yellow Knights mushrooms and the beneath top layer of soils from ten geographically distant locations in Poland. The Yellow Knights can be considered as an effective accumulator of total Hg. The mean values of bioconcentration factor (BCF) of Hg in caps of Yellow Knights for nine of the locations ranged from 22 ± 9 to 75 ± 13 (total range 9.0–90) and for stipes from 13 ± 7 to 52 ± 9 (total 4.4–93). The top layer (0–10 cm) of soils in these nine sites contained Hg with mean (±SD) concentration ranging from 0.019 ± 0.003 to 0.046 ± 0.007 ng/g dry weight. Mercury was less accumulated (BCF 4.9 ± 2.7 for a whole fruiting bodies) by Yellow Knights that emerged at the most contaminated site, where soil contained 0.059 ± 0.028 ng Hg/g dw. The potential of Yellow Knights communities to bioconcentrate Hg (determined as BCF) in fruiting bodies varied between the localities more than tenfold and decreased highly with increase of Hg content of the top soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury emissions and diffusion in the environment and contamination of food resources remain an actual problem of debate and concern worldwide. Wild grown mushrooms are highly vulnerable to Hg contamination (Falandysz et al. 2001a; Melgar et al. 2009). One of the reasons for elevated Hg content of forest mushrooms can be the degree of forest soil’s pollution with this element (Suchara and Sucharová 2002). Wild grown mushrooms can be very effective accumulators of Hg and some of them highly bioconcentrated methyl mercury (Chudzyński et al. 2009, 2011; Drewnowska et al. 2012a, b; Falandysz 2012; Falandysz et al. 2012a, b; Grgić et al. 1992; Stijve and Roschnik 1974).
Yellow Knight Tricholoma equestre, mushroom, synonymous to “Man on Horseback” is specie popular during the fall and early winter and is widely distributed in Europe and North America (Læssøe et al. 1996). This mushroom is known also as Tricholoma flavovirens that is mycorrhizal with the conifer trees – especially of the Pinus family, and is found on sandy soils. In Poland the Yellow Knight is traditionally regarded as tasty mushroom, which is suitable for drying, pickling, stewing, souring and freezing. In a recipe on mushroom soups, “the Yellow Knights’ soup”, it is noted that the fresh specimens do not need to be blanched before soup cooking – that is the specimens, when “cleaned up, cut and well rinsed in running tap water, are poured directly into boiling bullion and boiled for 40 min”. A common practice is blanching of mushrooms (a short-time boiling) by boiling them for 2, 3, 5 or 10 min in boiling and slightly salty water – depending on the gourmet recipe. The Yellow Knight is officially registered as edible mushroom in Poland. There are some recent controversies on its edibility especially when consumed for several days in large amount. Because of this, in Germany, this mushroom is actually classified in the group of dangerous species.
This study documents the pollution of forest soil by Hg as well as the evaluating the Hg content, bioconcentration and distribution in T. equestre mushrooms collected from ten spatially distant sites in the Central Europe country of Poland. None of these sites surveyed was recognized as being under the influence of local Hg pollution.
Materials and Methods
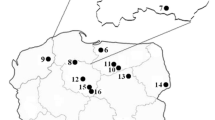
The Yellow Knights, T. equestre (L.) P. Kumm., 1871, mushrooms and soil top layer (0–10 cm; ca. 100 g) beneath to the fruiting bodies were collected from ten spatially distant areas across Poland in 1999–2007 (Table 1).
The fruiting bodies after careful cleanup from the plant material and particles of soil with a plastic knife were rinsed with distilled water and oven dried at 65°C to constant weight. Dried caps and stipes of fruiting bodies were milled in porcelain mortar and further kept in brand new sealed polyethylene bags in clean condition until chemical analysis. Soil substrate samples, after removal of the visible organisms, small stones, sticks and leaves were air dried under clean condition at room temperature for approximately 6 weeks and then sieved through a pore size of 2 mm.
Total Hg content of materials was determined by cold-vapour atomic absorption spectroscopy (CV-AAS). The samples matrix was pyrolised and the released mercury was first amalgamated and then desorbed from gold wool (Mercury analyzer type MA-2000, Nippon Instruments Corporation, Takatsuki, Japan). Analytical control and assurance quality (AC/AQ) were achieved by the analysis of several reference standard materials (Jarzyńska and Falandysz 2011). One of these materials was the CS-M-1 certified reference material (dried fruiting bodies of mushroom Cow Bolete (Suillus bovinus) produced by the Institute of Nuclear Chemistry and Technology in Warsaw, Poland. This material is routinely used in the analysis performance check-up and its’ declared total Hg content is 0.174 ± 0.018 μg/g dw. In this study, Hg content of the CS-M-1 certified reference material was 0.176 ± 0.004 μg/g dw (n = 3). With every set of 10 mushroom or soil samples examined one blank sample was run and no interference was noted. The analytical equipment under the condition used enabled the determination of Hg at linear range spanning from 0.002 to 1,000 ng in a sample of up to 0.5 g mass. Discrepancies between the certified values and concentrations quantified were well below 10 %. Duplicates and blanks followed with every set of ten samples examined. For blank samples, no major interferences were found for the elements quantified. Limit of detection for Hg was 0.005 μg Hg/g dw. Coefficients of variation for these measurements on routine runs were well below 10 %.
All data produced were statistically treated using the computer software Statistica version 8.0.
Results and Discussion
Data on Hg content of top layer of forest soils and fruiting bodies of T. equestre as well as on the Hg distribution between caps and stipes and the Hg bioconcentration factor (BCF) values by species are presented in Table 1.
The unit less BCF value is used as a measure of accumulation potential of elements by mushroom. The BCF is a quotient of chemical element content in fruiting body against its content in soil substratum. The BCF values of Hg in caps and stipes of T. equestre were rather high for most sites and did indicate a strong potential of this mushroom to sequestrate Hg. BCF up to 90 was noted in a single cap, while median values of BCF varied for most of the sites between 18 and 75; the mean values ranged from 22 ± 9 to 75 ± 13 (Table 1). In the Commune Kościelna Wieczfnia of the Mława County, the mean value of BCF was 4.9 ± 2.7 (median was 4), and this is distinctly low compared to other areas in this study. Mercury content of top layer of forest soil in the Commune Kościelna Wieczfnia was higher compared to other sites examined, i.e. 0.059 ± 0.028 μg/g dw, while median for nine other sites varied between 0.013 and 0.045 μg/g dw, and the means ranged from 0.019 ± 0.003 to 0.046 ± 0.007 ng/g (Table 1). Nevertheless, these data on Hg in forest soils do not show any on pollution problem and could be considered as “baseline” levels.
Mercury content was higher in caps compared to stipes of T. equestre. The median values of the quotient of Hg content in cap to Hg content in stipe (QC/S) varied from 1.1 to 1.8 (Table 1). In this study, for seven of the sites examined Hg content of caps ranged from 0.71 ± 0.11 to 1.3 ± 0.7 μg/g dw (median values were from 0.72 to 0.99 μg/g dw) and for two other sites in one case in caps was 0.25 ± 0.07 μg/g dw (median 0.24 μg/g dw) and in another (in a whole fruiting body) was 0.23 ± 0.06 μg/g dw (median 0.21 μg/g dw) (Table 1). In some earlier studies of T. equestre, the specimens examined contained Hg in caps of 0.94 ± 0.74 μg/g dw (n = 3; 1994) and 1.7 ± 0.9 μg/g dw (n = 14; 1996–1997) (Falandysz et al. 2001b, 2003). Based on data on total Hg in T. equestre in this and earlier studies it can be concluded that a site to site variation of Hg content over years can be up to ten-fold, while the upper mean and median content in caps did not exceed 2.0 μg/g dw.
Mercury accumulated in fruiting bodies of mushrooms might pose health risk for consumers. In countries where T. equestre grows, it could be eaten by some individuals in relatively large amount within a week. Intoxication was noted after eating 1,200 g (fresh weight) fruiting bodies of T. equestre in 5 days by a boy of age 5 years (Chodorowski et al. 2003). Locally, in autumn in pine forests T. equestre can be very abundant. This mushroom, after blanching (boiled, pickled) or without blanching (salted) are usually eaten at a rate of around 300–400 g daily – during 3–4 days in a week.
The Hg reference dose (RfD) of 0.0003 mg/kg body weight/day and provisional tolerable weekly intake value of 0.004 mg/kg bw for person of 70 kg bw can be applied to assess health risk to consumers due to Hg taken from foods (JECFA 2010; US EPA 1987).
A meal made of 300 or 400 g fresh caps or whole fruiting bodies of T. equestre containing 0.24–0.25 μg Hg/g dw on the average (two sites, Table 1) could provide from 0.0072–0.0075 to 0.096–0.010 mg Hg (assuming 90 % of moisture in fresh mushrooms). For eight other sites, where the mean value of Hg in caps were higher, i.e. between 0.71 and 1.3 μg Hg/g dw, a mushroom dish of similar volume could provide from 0.021–0.039 to 0.028–0.052 mg Hg, on the average. These values, calculated for a person of 60-kg body weight gave a range of 0.00035 and 0.00087 mg/kg bw, which are doses exceeding the RfD of Hg and show elevated risks from the consumption of this mushroom. On the other site Hg intake from such meals eaten seven times in a week will provide from 0.149 to 0.199 and from 0.27 to 0.36 mg Hg, and these are intakes equivalent to 2.1–2.8 and 3.9–5.2 μg Hg/kg bw respectively. For some of the sites examined (Table 1) Hg intake solely from caps of T. equestre is close to or above the PTWI of Hg.
As reported in Table 1, the Yellow Knights, even if they emerged at sites unpolluted with Hg, are mushroom species that are usually contaminated with elevated concentrations of this element, which is a feature common to certain other edible wild mushrooms (Falandysz et al. 2002, 2004, 2007a, b).
Data on methylmercury (CH3Hg+) content and proportion to total Hg in T. equestre are not available. Selenium in foods is considered as element that to some degree is also able to reduce or eliminate toxicity from Hg and CH3Hg+. Mercury can form species hardly soluble in water such as HgSe and HgS but nothing is known on such Hg compounds in mushrooms. Knowledge of Se and Hg interactions in flesh of mushrooms is also non-existing. Selenium content of T. equestre is known only from a single study and the reported Se concentration of 2.8 ± 0.3 (2.5–3.2; n = 4) μg/g dw seems overestimated (cited after Falandysz 2008).
References
Chodorowski Z, Anand J, Grass M (2003) Acute poisoning with Tricholoma equestre of five-year old child. Przegląd Lekarski 60:309–310
Chudzyński K, Bielawski L, Falandysz J (2009) Mercury bio-concentration potential of Larch Bolete, Suillus grevillei, mushroom. Bull Environ Contam Toxicol 83:275–279
Chudzyński K, Jarzyńska G, Stefańska A, Falandysz J (2011) Mercury content and bio-concentration potential of Slippery Jack, Suillus luteus, mushroom. Food Chem 125:986–990
Drewnowska M, Jarzyńska G, Kojta AK, Falandysz J (2012a) Mercury in European Blusher, Amanita rubescens, mushroom and soil. Bioconcentration potential and intake assessment. J Environ Sci Health Part B 47:466–474
Drewnowska M, Jarzyńska G, Sąpór A, Nnorom IC, Sajwan KS, Falandysz J (2012b) Mercury in Russula mushrooms: bioconcentration by Yellow-ocher Brittle Gills Russula ochroleuca. J Environ Sci Health Part A 47:1577–1591
Falandysz J (2008) Selenium in edible mushrooms. J Environ Sci Health Part C 26:256–299
Falandysz J (2012) Comments on “Determination of mercury, cadmium, lead, zinc, selenium and iron by ICP-OES in mushroom samples from around thermal power plant in Muğla, Turkey”. Bull Environ Contam Toxicol 88:651–653. doi:10.1007/s00128-011-0357-1
Falandysz J, Gucia M, Frankowska A, Kawano M, Skwarzec B (2001a) Total mercury in wild mushrooms and underlying soil substrate from the city of Umeå and its surroundings, Sweden. Bull Environ Contam Toxicol 67:763–770
Falandysz J, Szymczyk K, Ichihashi H, Bielawski L, Gucia M, Frankowska A, Yamasaki S (2001b) ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit Contam 18:503–513
Falandysz J, Gucia M, Skwarzec B, Frankowska A, Klawikowska K (2002) Total mercury in mushrooms and underlying soil from the Borecka forest, northeastern Poland. Arch Environ Contam Toxicol 42:145–154
Falandysz J, Brzostowski A, Kawano M, Kannan K, Puzyn T, Lipka K (2003) Concentrations of mercury in wild growing higher fungi and underlying substrate near Lake Wdzydze, Poland. Water Air Soil Poll 148:127–137
Falandysz J, Jędrusiak A, Lipka K, Kannan K, Kawano M, Gucia M, Brzostowski A, Dadej M (2004) Mercury in wild mushrooms and underlying soil substrate from Koszalin North-central Poland. Chemosphere 54:61–466
Falandysz J, Frankowska A, Mazur A (2007a) Mercury and its bioconcentration factors in King Bolete (Boletus edulis) Bull. Fr. J Environ Sci Health Part A 42:2089–2095
Falandysz J, Gucia M, Mazur A (2007b) Content and bioconcentration factors of mercury by Parasol Mushroom Macrolepiota procera. J Environ Sci Health Pt B 42:735–740
Falandysz J, Widzicka E, Kojta AK, Jarzyńska G, Drewnowska M, Danisiewicz-Czupryńska D, Dryżałowska A, Lenz E, Nnorom IC (2012a) Mercury in common Chanterelles mushrooms: Cantharellus spp. update. Food Chem 133:842–850
Falandysz J, Kojta AK, Jarzyńska G, Drewnowska A, Dryżałowska A, Wydmańska D, Kowalewska I, Wacko A, Szlosowska M, Kannan K, Szefer P (2012b) Mercury in bay bolete Xerocomus badius: bioconcentration by fungus and assessment of element intake by humans eating fruiting bodies. Food Addit Contam A 29:951–963
Grgić J, Mandić M, Grgić Z, Manić Z (1992) Diva i arsen u samoniklim jestivim i nejestivim gljivama. Farm Glas 48:183–189
Jarzyńska G, Falandysz J (2011) The determination of mercury in mushrooms by CV-AAS and ICP-AES techniques. J Environ Sci Health Part A 46:569–573
JECFA (2010) Joint FAO/WHO expert committee on food additives. Seventy-second meeting. Rome, 16–25 February 2010. Summary and conclusions. JECFA/72/SC. Food and Agriculture Organization of the United Nations World Health Organization. Issued 16th March 2010
Læssøe T, Del Conte A, Lincoff G (1996) The mushroom book, 1st American ed. A DK Publishing Book, London, UK
Melgar MJ, Alonso J, Garcia MÁ (2009) Mercury in edible mushrooms and soil. Bioconcentration factors and toxicological risk. Sci Total Environ 407:5328–5334
Stijve T, Roschnik R (1974) Mercury and methyl mercury content of different species of fungi. Trav Chim Aliment Hyg 65:209–220
Suchara I, Sucharová J (2002) Distribution of sulphur and heavy metals in forest floor humus of the Czech Republic. Water Air Soil Pollut 136:289–316
US EPA (1987) United States Environmental Protection Agency peer review workshop on mercury issues. summary report. Environmental criteria and Assessment Office. Cincinnati, U.S. EPA, OH, 26–27 Oct 1987
Acknowledgments
Technical assistance by Anna Chylicka, Karolina Lubiejewska, Maja Mackiewicz, Aleksandra Maternik, Dominika Milewska and Alina Pękacka is acknowledged. This study was supported by the Ministry of Science and Higher Education under project DS-8130-4-0092-1.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Maćkiewicz, D., Falandysz, J. Total Mercury in Yellow Knights (Tricholoma equestre) Mushrooms and Beneath Soils. Bull Environ Contam Toxicol 89, 755–758 (2012). https://doi.org/10.1007/s00128-012-0757-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0757-x