Abstract
The objective of this study was to examine the influence of different stressors, including cadmium (heavy metal), anthracene (polycyclic aromatic hydrocarbon—PAH) and chloridazon (herbicide), on population growth and biosynthesis of cytoplasmic HSP70 in Lemna minor (duckweed) in short (4 h)- and long (7 days)-term tests. A heat shock response was confirmed in Lemna exposed to high temperature: 35, 37.5, 40, or 42.5°C in short-term (4 h) treatments. The chemicals tested stimulated the biosynthesis of the cytoplasmic HSP70 protein in a concentration-dependent way (0.5–5 μM), higher in fronds exposed to lower doses of stressors. Additionally, production of HSP70 was greater after 4 h of incubation than after 7 days. The results suggest that HSP70 could be applied as a non-specific and sensitive detector of stress induced by different chemicals at concentrations below those that produce the type of response observed in classical cytotoxicity tests, such as growth inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Studies describing the phenomenon of toxicity induced by chemical pollutants in plants have mostly employed only individual or population level endpoints, such as growth, reproduction or mortality. For freshwater ecosystems, biotests with Lemna (EC 1999; OECD 2002; ISO 2004), a free floating aquatic macrophyte (duckweed), are used to supplement or replace the algal growth inhibition tests with Scenedesmus, a unicellular green microalgae (ISO 2004). At the same time, there is a steadily growing concern among toxicologists about more sensitive, specific and rapid detection of contaminants occurring in the aquatic environment. Therefore, in addition to traditional markers used in aquatic toxicity testing and risk assessment, many suborganismal endpoints (biomarkers or bioindicators) are commonly used, and new biochemical ones have been proposed (see the review of Torres et al. 2008). Biochemical biomarkers seem to be especially promising, since all organisms respond to stress at the cellular level, with a rapid synthesis of the so-called stress proteins and a simultaneous inhibition of normal protein synthesis. Published data indicate that for water plants the induction of heat shock proteins (HSPs), in particular HSP70 may be one such biomarker (Bierkens et al. 1998; Lewis et al. 1999, 2001; Ireland et al. 2004).
HSPs are a family of evolutionarily conserved proteins which play an important role in cell physiology under normal and stress conditions. It has been shown that some members of HSP class possess molecular chaperone activity involved in proper polypeptide folding, protein transport, proteolysis, and stress tolerance (Żylicz and Wawrzynów 2001), as well as immunoregulatory properties (Wieten et al. 2010). HSPs are classified into protein families based on their molecular weight and sequence homology; HSP40, HSP60, HSP70, HSP90, HSP100, and small HSPs (sHSPs) are the major families (Kampinga et al. 2009). HSP70 can be up-regulated in organisms proportionately to the degree of stress induced by different types of pollutants (Bierkens et al. 1998; Lewis et al. 2001; Ireland et al. 2004; Backor et al. 2006; Tukaj and Tukaj 2010), taking part in the recovery of the physiological state of the cells.
The aim of this study was to compare the intracellular biomarker HSP70 to the conventional growth response in Lemna minor following exposure to three different classes of chemicals. Lemna minor was chosen because it is a test species recommended for ecotoxicological assays by many authorities. Short (4 h)- and long (7 days)-term experiments were performed in which the organism was subjected to anthracene (aromatic hydrocarbon), cadmium (heavy metal) and chloridazon (triazine herbicide) applied at concentrations of 0.5–5 μM. In the present study, the influence of anthracene, cadmium and chloridazon applied at the same concentrations on the 7-day growth of Lemna was performed strictly according to the toxicological test protocol (Moody and Miller 2005).
Materials and Methods
Lemna minor L. clone St, was obtained from a certified culture collection of the Botanical Inst., University of Jena, Germany, and was cultured in Steinberg medium modified by Altenburger (ISO/DIS 20079 2004). Stock solutions of the compounds were prepared in distilled water. The final medium, without EDTA and FeCl3 (pH 5.5 ± 0.2) addition, was autoclaved for 20 min at 121°C. Stock solutions of EDTA and FeCl3 were sterilized by filtration and added to complete the autoclaved medium.
Fronds were cultured in 250-ml beakers filled with 100 ml of the culture medium, at 24 ± 2°C, under continuous fluorescent light (65–75 μmole PAR m−2 s−1). The pre-cultures were grown in 500 ml crystallizing dishes with about 300 ml of the nutrient medium for 7–10 days before experiments, under the same light and temperature conditions as in the test cultures. The vessels were covered by transparent food film to minimize evaporation and accidental contamination. The lateral walls of the vessels were also covered with black paper. Experimental cultures were started by inoculation of two 3-frond colonies (for growth measurement), or 10–12 colonies (for other analyses).
Three toxicants were used: a) anthracene (high purity, Aldrich Chemicals Co., USA) was dissolved in 0.1% (v/v) dimethyl sulfoxide (DMSO) (Acros Organics, Belgium) and supplied to the medium culture to a final concentration of 0.5, 1, 2, or 5 μM. DMSO had no significant effect on the growth of Lemna and HSP70 synthesis; b) cadmium (CdCl2*H2O, analytical grade; Merck, Germany) was dissolved in redistilled water and supplied to the medium culture to a final concentration of 0.5, 1, 2, or 5 μM; c) chloridazon (Redel de Hanen, Germany) was of commercial grade with 88% content of the active substance. The herbicide was dissolved in redistilled water and supplied to the medium culture to a final concentration of 0.5, 1, 2, or 5 μM.
A 4 h period at 35, 37.5, 40, or 42.5°C was chosen to demonstrate the heat shock response in Lemna fronds. Fronds were homogenized using a glass rod and quartz sand in a cold extraction buffer (25 mM Tris—HCl [pH 8.0], 1 mM β-mercaptoethanol, 10 mM EDTA and 2 mM phenylmethylsulfonyl fluoride—PMSF). Crude extracts were clarified by centrifugation (20,000 × g, 20 min at 4°C), and the supernatant was used for further analysis. The protein content was assayed as described by Bradford (1976).
Protein extracts were mixed with an equal volume of sample loading buffer, separated in 10% polyacrylamide gel under denatured condition (SDS—PAGE) in a Mini-Protean apparatus (Bio-Rad), and transferred onto nitrocellulose (in a buffer containing 200 mM glycine, 25 mM Tris and 20% methanol) at room temperature (RT) by 2 h (80 V). The membrane was blocked for 1 h (in RT) in a blocking buffer (0.1% Tween-20, 20 mM Tris–HCl [pH 7.5], 0.5 M NaCl). After the washing step in the buffer with a reduced amount of Tween-20 (0.05%), the membrane was incubated overnight in primary antibodies that recognize highly conserved fragments of the HSP70 protein in higher plants (rabbit polyclonal IgG anti-HSP70 (Agrisera, Sweden), in a dilution 1:1,000 in RT. Next, the membrane was incubated in secondary antibodies (goat anti-IgG rabbit coupled with HRP, Sigma, in dilution 1:2,000) for 1 h in RT. The membrane was washed and the substrate for HRP (3-3′ diaminobenzidine–DAB, Sigma) was added.
To quantify the levels of Western blotting products, a densitometric analysis was performed using a 1Dscan EX 3.0 program (Scanalytics, Rockville, MD, USA).
Statistical analyses were performed using SPSS 8.0 for Windows. Data are presented as the mean ± standard error (SEM). The means were compared statistically using one-way ANOVA with Dunnett’s post hoc test. The Results are considered significant at p < 0.05.
Results and Discussion
The contaminants chosen in this work belong to distinct chemical classes, i.e. exert their effect through different biochemical pathways. Cadmium, “heavy” metal, is a non-essential and non-beneficial element with a high toxic potential. Due to its great affinity to sulfhydryl groups, cadmium is considered to block functional groups in biomolecules resulting in the inhibition of different metabolic processes in cells (di Toppi and Gabbrielli 1999). Anthracene is recommended for study by the US EPA due to its high toxicity and wide occurrence in the environment (Bonnet et al. 2005). The toxicity of anthracene results from the fact that it is a strong photosensitizer and one of the most quickly photomodified hydrocarbons (Krylov et al. 1997). Its photomodification leads to the formation of both photostable and further photomodified products; most of them are quinones. Chloridazon (pyrazon) is an organochloride herbicide used to control broad-leaved weeds, particularly in sugar beet (Sanchez-Martin and Sanchez-Camazano 1991). Due to its binding to the D1 protein in the reaction center of PS II, this herbicide is an inhibitor of photosynthesis (Caux et al. 1995).
In the present study, the influence of anthracene, cadmium and chloridazon applied at the same concentrations (0.5–5 μM) on the 7-day growth of Lemna was performed strictly according to the toxicological test protocol (Moody and Miller 2005).
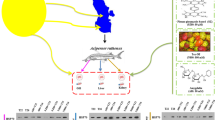
In the 7-day growth study, the number of Lemna fronds was not significantly affected by anthracene or chloridazon at concentrations of 0.5–5 μM (Fig. 1a, c), but was significantly reduced by cadmium (Fig. 1b) at all exposures in the same concentration range. Based on growth response, anthracene and chloridazon would be assumed to be nontoxic at the concentrations used in this study. Unfortunately, there are no available papers on chloridazon toxicity to freshwater plants. In the 7-day test, cadmium appeared to be the most toxic. Depending on concentration, the number of Lemna was reduced by ~25–42% compared to the controls (Fig. 1b). Tkalec et al. (2008) also showed in long-term experiments (6 and 12 days) that 10 μM cadmium significantly reduced Lemna growth, but also induced defense mechanisms. These included increased activities of antioxidant enzymes, as well as HSP70 synthesis. The dose-dependent growth reduction and increase in the concentration of sulfhydryl groups in the soluble fraction of protein obtained from Lemna trisulca fronds treated for 7 days with Cd (1–100 μM) suggest another adaptative mechanism counterbalancing Cd stress (Malec et al. 2010).
Levels of HSP70 in L. minor cells exposed to various concentrations (from 0.5 to 5 μM) of a anthracene (ANT), b cadmium (Cd) or c chloridazon (CHD), and cultured for 7 days. d An example of the western-blotting result showing HSP70 bands of a single representative experiment. The results are presented as mean (n = 3) values (±SEM). Results for HSP70 levels in plant extracts treated by chemicals were expressed relative to HSP70 levels detected by immunoblotting in untreated samples (0 μM) and indicated as 1 (=100%). Above the graphs presenting HSP70 levels, the effect of chemicals on number of fronds are presented. The results are presented as mean (n = 5) values (±SEM).Statistically significant differences (chemical-treated versus control cultures) are denoted with asterisks: * p < 0.05
It is clear that a useful biochemical biomarker should be a fast, dose-dependent and sensitive detector of the stress state, detectable before any growth response occurs. In addition, due to a variety of stressors having quite different properties, the time of their equilibrium adsorption should be also taken under consideration, when the toxicity is determined in short-term tests. The biosorption of chemicals, such as those used in the experiment reported herein, by Lemna appears to be a relatively fast process. For instance, Saygideger et al. (2005) found that a biosorption equilibrium for cadmium and other divalent ions by Lemna minor was reached after 40–60 min. The time needed to reach 90% of the steady state (T90) biosorption for anthracene by Lemna gibba was 1.8 h (Duxbury et al. 1997). There are no data available, but it seems is reasonable to assume that biosorption of chloridazon, a low-molecular-weight substance, also reaches the equilibrium state during the first several hours. For the above reasons, the intracellular level of HSP70 was monitored in the duckweed tissue after a 4 h exposure to chemicals, and compared to those after the 7-day exposure.
After 7 days, the highest over-production of HSP70 (about 2.4-fold) was induced by 0.5 μM chloridazon (p < 0.05), and a tendency to stimulate HSP70 by a higher concentration was observed (Fig. 1c). This is consistent with other observations (Bierkens et al. 1998), that HSPs are stimulated in cells exposed to stressors at sublethal concentration. Cadmium at a concentration of 0.5 μM significantly stimulated the synthesis of HSP70 (p < 0.05), but at higher concentrations (1, 2, or 5 μM) this effect was not statistically significant. Similar reaction to higher doses of cadmium (5 and 10 μM) in the lichen photobiont Trebouxia erici was observed by Backor et al. (2006). There was no statistically significant effect on HSP70 synthesis in L. minor exposed to anthracene in the long-term toxicology test (Fig. 1a).
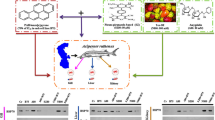
In short-term (4 h) tests, there was no effect of the three chemicals on the growth, frond size and morphology, but all stimulated the synthesis of HSP70 in L. minor cells in an inverse dose- dependent manner (Fig. 2). The highest expressions of HSP70 were induced by the lower 0.5 μM (p < 0.001) or 1 μM (p < 0.05) concentrations of anthracene, or chloridazon, with no effect occurring at their higher concentrations (2 or 5 μM) (Fig. 2a, c). To the best of our knowledge, this is the first such description showing an increased HSP70 expression in a higher plant (duckweed) exposed to anthracene and chloridazon. Cadmium stimulated HSP70 in L. minor in a similar manner to that for anthracene or chloridazon, but the highest concentration of cadmium (5 μM) showed a tendency to inhibit HSP70 expression (Fig. 2b). A similar tendency was observed by Ireland et al. (2004), however HSP70 stimulation in Lemna minor or Fucus serratus was observed up to 25 mM (Cd++), and higher cadmium concentrations significantly inhibited the expression of HSP70. This inhibition resulted probably from cadmium cytotoxicity, as previously suggested by di Toppi and Gabbrielli (1999). We and others (Bierkens et al. 1998; Lewis et al. 1999, 2001; Ireland et al. 2004; Tukaj and Tukaj 2010) postulate that heat shock proteins, in particular HSP70, may serve as useful biomarkers which are up-regulated by different types of pollutants (e.g., PAH, trace metals, herbicides) in many aquatic plant organisms proportionately to the degree of stress. Temperature-dependent (from 24 to 42.5°C) induction of HSP70 synthesis in L. minor was observed after 4 h of the heat treatment, as compared to controls (Fig. 2d). It is worth noting that we observed a statistically significant reduction in HSP70 level in fronds exposed to 35°C, in comparison to unheated plants. At present, we cannot explain this phenomenon. However, a question arises as to whether temperature of 24°C is optimal for L. minor recommended for toxicological standards.
Levels of HSP70 in L. minor cells exposed to various concentrations (from 0.5 to 5 μM) of a anthracene, b cadmium, c chloridazon or d exposed to higher temperatures: 35, 37.5, 40, or 42.5°C and cultured for 4 h. The results are presented as mean (n = 3) values (±SEM). Results for HSP70 levels in plant extracts treated by chemicals or high temperature were expressed relative to HSP70 levels detected by immunoblotting in untreated samples (0 μM/24°C) and indicated as 1 (=100%). Statistically significant differences (chemical/temperature-treated versus control cultures) are denoted with asterisks: * p < 0.05; *** p < 0.001
Summarizing, we have shown that HSP70 in Lemna minor can be induced by different chemicals. The chemicals tested stimulated the biosynthesis of the cytoplasmic HSP70 protein in a concentration-dependent way, higher in fronds exposed to lower doses of stressors. The results of our short-term experiments suggest that HSP70 induction is a sensitive and nonspecific indicator of cellular stress, and may serve as an adaptive function in water plants exposed mainly to low doses of contaminants.
References
Backor M, Gibalova A, Budova J, Mikes J, Solar P (2006) Cadmium-induced stimulation of stress-protein hsp70 in lichen photobiont Trebouxia erici. Plant Growth Regul 50:159–164
Bierkens J, Maes J, Plaetse FV (1998) Dose-dependent induction of heat shock protein 70 synthesis in Raphidocelis subcapitata following exposure to different classes of environmental pollutants. Environ Pollut 101:91–97
Bonnet JL, Guiraudb P, Dussera M, Kadrib M, Laffossea J, Steimanb R, Bohatier J (2005) Assessment of anthracene toxicity toward environmental eukaryotic microorganisms: Tetrahymena pyriformis and selected micromycetes. Ecotoxicol Environ Saf 60:87–100
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
EC (Environment Canada) (1999) Biological test method: test for measuring the inhibition of growth using the freshwater macrophyte, Lemna minor, Environmental Protection Service, Report EPS 1/RM/37, Ottawa, ON
Caux PY, Menard L, Kent A (1995) Comparative study of the effects of MCPA, butylate, atrazine and cyanazine on Selenastrum capricornutum. Environ Pollut 92:219–225
di Toppi S, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Duxbury CL, Dixon DG, Greenberg BM (1997) Effects of simulated solar radiation on the bioaccumulation of polycyclic aromatic hydrocarbons by the duckweed Lemna gibba. Environ Toxicol Chem 16:1739–1748
Ireland HE, Harding SJ, Bonwick GA, Jones M, Smith CJ, Williams JHH (2004) Evaluation of heat shock protein 70 as a biomarker of environmental stress in Fucus serratus and Lemna minor. Biomarkers 9:139–155
ISO (2004) Water quality: fresh algal growth inhibition test with unicellular green algae. ISO 8692:2004 (E)
ISO/DIS 20079 (2004) Water quality: determination of the toxic effect of water constituents and wastewater on duckweed (Lemna minor)—Duckweed growth inhibition test
Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111
Krylov SN, Huang XD, Zeiler LF, Dixon DG, Greenberg BM (1997) Mechanistic quantitative structure–activity relationship model for the photoinduced toxicity of polycyclic aromatic hydrocarbons: I. Physical model based on chemical kinetics in a two-compartment system. Environ Toxicol Chem 16:2283–2295
Lewis S, Handy RD, Cordi B, Billinghurst Z, Depledge MH (1999) Stress proteins (HSP’s): methods of detection and their use as an environmental biomarker. Ecotoxicology 8:351–368
Lewis S, Donkin ME, Depledge MH (2001) Hsp70 expression in Enteromorpha intestinalis (Chlorophyta) exposed to environmental stressors. Aquat Toxicol 51:277–291
Malec P, Maleva MG, Prasad MNV, Strzałka K (2010) Responses of Lemna trisulca L. (Duckweed) exposed to low doses of cadmium: thiols, metal binding complexes, and photosynthetic pigments as sensitive biomarkers of ecotoxicity. Protoplasma 24:1–4
Moody M, Miller J (2005) Lemna Minor growth inhibition test. Small-scale freshwater toxicity investigations. Springer, Netherlands
OECD (2002) Revised proposal for a new guideline 221: Lemna sp. growth inhibition test. OECD Guidelines for the Testing of Chemicals, Washington, DC
Sanchez-Martin MJ, Sanchez-Camazano M (1991) Adsorption of chloridazon by soils and their components. Weed Sci 39:417–422
Saygideger S, Gulnaz O, Istifli ES, Yucel N (2005) Adsorption of Cd(II). Cu(II) and Ni(II) ions by Lemna minor L.: effect of physicochemical environment. J Hazard Mater 126:96–104
Tkalec M, Prebeg T, Roje V, Pevalek-Kozlina B, Ljubešić N (2008) Cadmium-induced responses in duckweed Lemna minor L. Acta Physiol Plant 30:881–890
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution. A review. Ecotoxicol Environ Saf 71:1–15
Tukaj S, Tukaj Z (2010) Distinct chemical contaminants induce the synthesis of Hsp70 proteins in green microalgae Desmodesmus subspicatus: Heat pretreatment increases cadmium resistance. J Thermal Biol 35:239–244
Wieten L, van der Zee R, Goedemans R, Sijtsma J, Serafini M, Lubsen NH, van Eden W, Broere F (2010) Hsp70 expression and induction as a readout for detection of immune modulatory components in food. Cell Stress Chaperones 15:25–37
Żylicz M, Wawrzynów A (2001) Insights into the function of Hsp70 chaperones. IUBMB Life 51:283–287
Acknowledgments
This work was supported by a grant from the Polish Ministry of Science and Higher Education (No. N N304 457938).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tukaj, S., Bisewska, J., Roeske, K. et al. Time- and Dose-Dependent Induction of HSP70 in Lemna minor Exposed to Different Environmental Stressors. Bull Environ Contam Toxicol 87, 226–230 (2011). https://doi.org/10.1007/s00128-011-0339-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-011-0339-3