Abstract
Purpose
We aimed to explore the multidimensional nature of social inclusion (mSI) among patients diagnosed with schizophrenia spectrum disorder (SSD), and to identify the predictors of 3-year mSI and the mSI prediction using traditional and data-driven approaches.
Methods
We used the baseline and 3-year follow-up data of 1119 patients from the Genetic Risk and Outcome in Psychosis (GROUP) cohort in the Netherlands. The outcome mSI was defined as clusters derived from combined analyses of thirteen subscales from the Social Functioning Scale and the brief version of World Health Organization Quality of Life questionnaires through K-means clustering. Prediction models were built through multinomial logistic regression (ModelMLR) and random forest (ModelRF), internally validated via bootstrapping and compared by accuracy and the discriminability of mSI subgroups.
Results
We identified five mSI subgroups: “very low (social functioning)/very low (quality of life)” (8.58%), “low/low” (12.87%), “high/low” (49.24%), “medium/high” (18.05%), and “high/high” (11.26%). The mSI was robustly predicted by a genetic predisposition for SSD, premorbid adjustment, positive, negative, and depressive symptoms, number of met needs, and baseline satisfaction with the environment and social life. The ModelRF (61.61% [54.90%, 68.01%]; P =0.013) was cautiously considered outperform the ModelMLR (59.16% [55.75%, 62.58%]; P =0.994).
Conclusion
We introduced and distinguished meaningful subgroups of mSI, which were modestly predictable from baseline clinical characteristics. A possibility for early prediction of mSI at the clinical stage may unlock the potential for faster and more impactful social support that is specifically tailored to the unique characteristics of the mSI subgroup to which a given patient belongs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social inclusion is a fundamental human right that has posed serious challenges for patients with severe mental illness [1], constituting 5% of the global population [2]. Social inclusion has been described as “a multidimensional state where prevailing conditions enable full and active participation in all aspects of daily life” [3,4,5], where “multidimensional” is vaguely unrestricted to individual characteristics [6], relationships with other individuals and group environments [3, 6], subjective and objective living environments [7,8,9,10] and social-political rights [6]. Research on social inclusion is essential, as limited healthcare expenditures are spent on psychiatric disorder management (e.g., 0.47% of the Dutch total healthcare expenditures in 2017) [11,12,13], leaving problems other than clinical remission unattended. Such situation also applies to schizophrenia spectrum disorder (SSD) which accounts for one in five individuals with mental disorders [14, 15]. Guided SSD management elevates social inclusion, thus benefiting clinical and social recoveries, patients’ quality of life, and reducing burdens on healthcare organizations, and patient families. Therefore, investigating social inclusion for an indicative purpose, and identifying its predictive factors in SSD are crucial for promoting recoveries and preserving social inclusion in SSD.
Quantifying social inclusion and related concepts such as social capital, social participation and so forth [16] have faced challenges due to its multidimensional nature, ambiguous scope, conceptual complexity, lack of validated instruments and consequently absent application [16, 17]. We previously adopted a unidimensional construct of social inclusion through social functioning [18]. However, low social inclusion has been recognized in diversifying forms of low socio-economic status (e.g., unemployment, low education), a lack of functional recoveries, low social engagement, and quality of life (QoL), which have been studied as standalone conventional outcomes [19,20,21,22]. Therefore, as a broad holistic concept, social inclusion should be approximated by aggregating the aforementioned outcomes. In the present study, we expanded the uniform concept to multidimensional social inclusion by aggregating subscales of social functioning and additional QoL, to provide a more comprehensive evaluation of a patient’s social inclusion level. Hence, it remains to be studied whether patients’ subgroups of individuals experiencing similar mSI and the predictors of mSI subgroups are applicable.
Previous observational studies have identified factors and predictors of outcomes that are highly relevant to mSI, including socio-economic status (e.g., age, ethnicity, education, marriage status) [18, 21, 23, 24], genetic predisposition for SSD [18], early-life factors (e.g., premorbid adjustment and childhood trauma) [18, 24], disease profiles (e.g., types of diagnoses, cognition, symptoms, global functioning) [18, 21, 23,24,25,26,27], baseline work status [23], unmet needs [28], living environment [21, 29], and medication and substance use [18, 21]. Traditional approaches, such as regressions, have identified these (predictive) factors but have not been used in social inclusion prediction. In addition, as individual-level risk can maximize the utilities of prediction models and the intricate nature of mSI may benefit from a relaxed assumption of linearity, one solution is data-driven approaches. Due to their methodological advantages and focus on prediction accuracy, they have been increasingly applied in predicting SSD onset among ultra-high-risk populations [30, 31], psychosis outcomes including symptoms, treatment, relapsing and hospitalization [21, 32,33,34,35,36], and more recently, social aspects such as social recoveries, vocation, education and QoL [21, 36], with common algorithms such as support vector machine, decision tree and random forests. However, evidence has shown that their performance compared to standard approaches varies, depending on evaluation metrics relevant to the research question and/or clinical requirements [37,38,39,40]. Therefore, we hypothesized that mSI clusters exist within the SSD cohort. We also hypothesized that the data-driven approach might not perform worse and identify distinct predictors compared to the standard approach. We aimed to evaluate the predictability of mSI clusters by employing and comparing the performance of standard and data-driven approaches. We addressed firstly how many mSI subgroups can be identified in SSD. Secondly, what are the predictors of mSI, and which of the standard and data-driven approaches performs most accurately in predicting patients’ mSI at 3-year follow-up concerning model accuracy and discriminability.
Methods
Study design and population
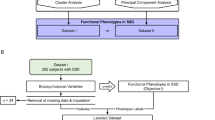
The overall study design is illustrated in Fig. 1. To identify subgroups of mSI, K-means clustering was applied. To predict mSI subgroups, prediction models were built using multinomial logistic regression (ModelMLR) and random forest (ModelRF), which were then internally validated. Lastly, to compare the two models, simulations and individual-level inspections were conducted.
We used the Dutch-nationwide database Genetic Risk and Outcome in Psychosis (GROUP) project, data release 8.0. Details on the GROUP project structure, participant recruitment, data collection and ethical approval have been published elsewhere [41]. In brief, 1119 patients were recruited at baseline across 36 partner mental health institutes including four university medical centres in Amsterdam, Groningen, Maastricht, and Utrecht. The inclusion criteria were: (1) age of 16–50 years; (2) fluent Dutch speaking; (3) diagnosis of a non-affective psychotic disorder according to DSM-IV; (4) the first contact with mental health care service no longer than 10 years ago; and (5) being able and willing to give written consent. The measurements taken at baseline and 3-year follow-up were used. Loss of follow-up was not applicable due to the naturalistic design of the GROUP project. As data was collected when a patient was visiting the clinics.
Outcome
We defined mSI by the clusters derived from 13 subscales from two surveys: Social Functioning Scales (SFS) [42] and World Health Organization Quality of Life (WHOQOL-BREF) [43] (Supplementary Table S1), based on literature and data availability. These two validated well-developed self-reported questionaries were used to cover direct and indirect aspects of social inclusion in SSD. The baseline mSI was not available as SFS was not collected.
Predictors
Potential predictors were included at baseline and/or 3-year follow-up based on literature, experts’ opinions and data availability. The inclusion of the 3-year follow-up in the analysis was mainly attributed to inspecting the predictability of a predictor at the baseline on the outcome mSI after the 3-year follow-up [44]. Covariables include domains of WHOQOL-BREF measured at baseline (physical, psychologic, social and environmental domains) [43]. The variables conceptually overlapping with the outcome mSI were considered predictors to avoid complicating and invalidating the mSI outcome conceptualized on the two validated questionnaires. For detailed predictors and explanations see Table 1.
Data analysis and statistical modeling
To construct outcome mSI, all subscales of SFS and WHOQOL-BREF were standardized and used to cluster patients with K-means clustering (Fig. 1, Objective 1). The assumption of K, symbolizing the number of subgroups, was premised on statistical indexes (i.e., silhouette, duda, pseudot2, Hartigan and gap indexes with the Euclidean distance) [45] and supplemented with clinical knowledge. Subgroup (between clusters) difference was assessed by Kruskal–Wallis tests. The two-group comparison was examined by Dunn’s Kruskal–Wallis Multiple Comparison tests and Bonferroni adjustment.
Prediction models were parallelly constructed via multinomial logistic regression (ModelMLR, standard approach) and random forest (ModelRF, data-driven approach) (Fig. 1, Objective 2). In the ModelMLR, the subgroup with the best mSI level was chosen as the reference group because of our particular interest in the groups with relatively worse mSI levels under the probable mimicry of the subgroup with the best mSI to healthy controls. We presented odds ratios (ORs), confidence interval (95% CI), model performance including accuracy (i.e., 1-misclassification rate) and its 95% CI derived from bootstrapping. As for the ModelRF, we reported the variable importance of the identified predictors (i.e., derived from a variable-specific out-of-bag decrease in accuracy averaged over all trees after permutation), where a higher value indicates higher usefulness of a variable in prediction. We also reported the model performance metric, including accuracy in both training and testing and the P value of the one-sided binomial test. In addition, considering the imbalanced outcome, P values of the one-sided binomial test in both models were reported to examine if the model accuracy is significantly better than no information rate (NIR), suggesting if the model can allocate a patient into the right outcome group significantly better than classification by chance. Finally, complete-case sensitivity analyses were conducted and reported in Supplementary Results.
Two models were compared by accuracy and mSI-cluster discriminability (Fig. 1, Objective 3). We conducted simulations with 1000 repeats using a random draw of 30%, 50%, 70%, 80% and 90% of the total sample. Furthermore, the individual-level prediction accuracy and mSI-cluster discriminability of both models were also examined by scatterplots and confusion matrix. Such evaluation shares similarities with the use of the Area Under the Curve (AUC), which is typically employed for binary outcomes.
The data analyses were conducted using R version 1.4.1103[46]. Technical details including outlier inspection, missingness and imputation, statistical power, justification of the chosen algorithm and model constructions were illustrated in Supplementary Methods.
Results
Clusters of multidimensional social inclusion
Figure 2 demonstrates the centroid features of five subgroups identified: (1) “very low (social functioning)/very low (QoL)” cluster (VLL, 8.58% of patients); (2) “low/low” cluster (LL, 12.87%); (3) “high/low” cluster (HL, 49.24%); (4) “medium/high” cluster (MH, 18.05%); and (5) “high/high” cluster (HH, 11.26%) (Statistical indexes see Supplementary Table S2). Differences in 13 subscales among 5 groups were observed (Supplementary Table S3). To gain statistical power, we merged VLL and LL due to their similarities in low social functioning, ending up with four multinomial subgroups: “low/low” (LL, 21.45%), “high/low” (HL, 49.24%), “medium/high” (MH, 18.05%), and “high/high” (HH, 11.26%). Patient characteristics of all included patients and patients in each mSI cluster are shown in Table 1.
Centroid feature of subgroups of multidimensional social inclusion. Green color represents the seven subscales from SFS (Social Functioning Scale), and purple color represents the six subscales from WHOQOL-BREF (the abbreviated version of World Health Organization Quality of Life). VLL “very low/very low” mSI subgroup characterized by the lowest levels of social functioning and quality of life while the quality of life is even worse, LL “low/low” mSI subgroup featured by low levels of social functioning and quality of life but moderately better quality of life, HL “high/low” mSI subgroup with a high social functioning but low quality of life, MH “medium/high” mSI subgroup with a medium level social functioning but a relatively high level of quality of life, HH “high/high” mSI subgroup featured by the highest level of both social functioning and quality of life
Multinomial logistic regression model
The ModelMLR included 22 predictors (Table 2), among which PAS (LL vs HH, 1.66 [1.22, 2.26]; HL vs HH, 1.43 [1.07, 1.92]), PRSSCZ (HL, 0.95 [0.91, 0.99]; MH, 0.93 [0.88, 0.98]), presence of positive (MH, 0.91 [0.86, 0.96]), core negative symptoms (LL, 1.08 [1.02, 1.15]; HL, 1.07 [1.01, 1.13]), and frequency of depressive symptoms (MH, 0.58 [0.37, 0.91]), symptomatic remission (MH, 3.19 [1.90, 5.36]; HL, 0.37 [0.26, 0.54]), number of met needs (MH, 0.86 [0.78, 0.95]), baseline WHOQOL-BREF environment and social domains (MH, 3.09 [1.79, 5.35] and 1.55 [1.06, 2.27] respectively) were identified as some of the more important predictors. Overall, the model accuracy was 59.16% (bootstrapping 95% CI [55.75%, 62.58%]; P = 0.994).
Random forest model
The ModelRF identified 22 predictors, among which WHOQOL-BREF domain scores and CTQ-total contributed the most to predicting the mSI group (Fig. 3). Observing the feature importance indices, other important factors were age, PRSSCZ, PAS-overall, symptom severity (positive, core negative and depressive) and the number of met needs. The accuracies on training and testing sets, respectively, were 70.46% ± 2.03% and 61.61% (95% CI [54.90%, 68.01%]; P =0.013).
Variable importance provided by the random forest model. WHOQOL-BREF the abbreviated version of World Health Organization Quality of Life, CTQ Childhood Trauma Questionnaire, Dutch Version, GAF Global Assessment of Functioning, PAS Premorbid Adjustment Score, PRSSCZ polygenic risk score for schizophrenia
Model comparison
For the simulation of 30% (up to 90%) of the observed patients, the mean accuracy of ModelMLR was 59.12% ± 2.50% (59.13% ± 1.20%) and the mean of ModelRF was 92.29% ± 1.34% (92.26% ± 0.67%; Supplementary Table S4). In the comparison of observed and predicted mSI subgroups among 1119 patients, 662 patients (59.16%) and 1033 patients (92.31%) were correctly predicted by ModelMLR and ModelRF correspondingly. We saw a similar pattern and percentage of mSI subgroup partition between the observations and RF-predictions, and contrarily, differences occurred between the observations and MLR-predictions (Fig. 4a). For example, a distinctly higher HL percentage with a (62.73% vs 49.24% observed, 48.88% RF-predicted, Fig. 4b).
Individual level of prediction results. The figure used the complete data of 1119 patients to assess the model on an individual level. a plotted pattern overview from the observed, MLR-predicted, and RF-predicted mSI clusters. b demonstrated the overall model accuracy (i.e., secondary diagonal sum) and the percentage of each mSI cluster which was correctly and incorrectly predicted by the ModelMLR and MdoelRF compared to the observations. From the figure below, we observed comparable distributions between the observed and RF-predicted mSI clusters. Dissimilarly, the MLR-predicted mSI clusters displayed a higher proportion in HL in trade of apparent lower percentages in LL and HH. LL mSI subgroup with both low social functioning and quality of life, HL mSI subgroup with high social functioning and low quality of life, MH mSI subgroup with medium social functioning and high quality of life, HH mSI subgroup with both high social functioning and quality of life, MLR multinomial logistic regression, RF random forest, obs observations
Discussion
We identified five mSI subgroups, including VLL, LL, HL, MH and HH. The ModelMLR and ModelRF consistently regard PRSSCZ, premorbid adjustment, symptoms, baseline environment, social domains and numbers of met needs as paramount predictors for mSI at 3-year follow-up. Comparatively, the ModelRF is cautiously considered better for its discriminability of all mSI subgroups. The mSI clusters intend to preliminarily define and bring awareness to social inclusion, an understudied but crucial outcome in SSD. The mSI prediction models should be further improved and externally validated for possible profound clinical and policy implications.
Clusters of multidimensional social inclusion among patients with schizophrenia spectrum disorder
Among the five mSI subgroups, the “very low (social functioning)/very low (QoL)” (VLL) and “low/low” (LL) indicated a low mSI level. VLL demonstrated the lowest social functioning and QoL in the spectrum with an even worse QoL, while LL exhibited relatively low levels of social functioning and QoL but slightly higher QoL. The VLL and LL combined, totalling 21.45%, presented more prominent core negative symptoms and worse premorbid adjustment compared to other mSI subgroups. Given that approximately 80% of the participants were diagnosed with schizophrenia [47] and a recent meta-analysis has indicated a 32.19% global prevalence in deficit schizophrenia [48], characterized by primary and persistent negative symptoms that distinguish it from non-deficit schizophrenia [49], the amalgamated group might align with deficit schizophrenia. Furthermore, VLL and LL were merged for the analysis, yet disparities exist. Patients in VLL, compared to LL, were more likely to be non-Caucasian, genetically vulnerable for SSD, have affective psychosis, depressed, have lower IQ, have higher levels of childhood trauma and symptoms, and showed less remission. This may eventually lead to restricted access to the labor market, recreational activities, and social engagement with simultaneously affected QoL and mSI.
The “high/low” (HL) and “medium/high” (MH) implied a medium mSI level. Compared to LL, HL showed a better level of social functioning, particularly in the areas of independence performance, interperson, recreation and prosociality. This improvement could be attributed to their higher premorbid adjustment, slightly less severe symptoms (especially core negative symptoms), and higher rates of symptomatic remissions. However, LL and HL had similar levels of QoL (moderately lower than the average QoL of all patients with SSD), with HL displaying a distinctively lower satisfaction toward general health, environment, and physical conditions. The disparities between LL and HL might suggest that the patients in HL probably experience increased social exposure through recreational and social activities along with psychologic impacts such as internalized stigma, and a systematically perceived low QoL [50]. Differently, MH exhibited an average social functioning level and mildly higher QoL than the average QoL of all patients with SSD, which could be resulted from better IQ, less severe symptoms, stable remission over six months and fewer met needs. No significant difference in occupation was observed among LL, HL and MH which corroborates with previous studies [51, 52]. Contrarily, HL and MH with better function were likely to stay at a job. The final mSI subgroup, “high/high” (HH), showed a high mSI level with the highest levels of social functioning and QoL, suggesting that this subgroup mimics healthy controls, albeit on an overall lower-level contrary to healthy controls. Notably, patients with medium-to-high mSI (HL, MH and HH) demonstrated fewer core negative symptoms, better premorbid adjustment and a higher rate of symptomatic remission over six months. These characteristics could correspond to diagnostic categories such as acute and transient psychotic disorders (ATPD) and non-affective acute remitting psychosis (NARP), marked by abrupt onset of psychotic symptoms within two weeks and early, complete remission [53,54,55].
Therefore, mSI is essential, as a holistic approach, to provide a comprehensive overview of social inclusion of an individual. The subgroup characteristics could also guide intervention strategies. Patients in VLL and LL may require more psychosocial interventions to manage symptoms and improve social functioning. Priority should be given to their eligibility for protected living when independent living is not achievable. While patients in HL could be targeted with current programs aiming for long-term remission and stigma reduction, patients in MH and HH could benefit from training for advanced skills and opportunities for more challenging job positions, which enhance self-esteem and self-actualization, ultimately improving mSI.
Common factors predictive of multidimensional social inclusion
The ModelMLR identified predictors such as gender, ethnicity, current urbanicity, antipsychotic dose, among others. In contrast, the ModelRF selected predictors including baseline all QoL domains, childhood adversity, and change of total week with cannabis consumption in the past 12 months among others. Noteworthily, eight important common predictors were shared between both models. Congruent with previous studies [18, 56,57,58,59], in ModelMLR, more severe core negative symptoms increased the risk of having low-to-medium mSI (LL and HL relative to HH). Premorbid adjustment had the highest negative effect on low-to-medium mSI in ModelMLR (LL and HL relative to HH). Thus, premorbid adjustment showed moderate predictability of the 3-year mSI. This is aligned with earlier studies which have shown that worse premorbid adjustment may lead to poorer social outcomes later in the course of SSD [60,61,62,63,64]. Therefore, premorbid adjustment is undoubtfully vital and can be potentially used for screening of low mSI. Yet, it has not caught enough attention in the field given the limited literature.
Surprisingly, worse positive symptoms significantly predicted good mSI (i.e., MH relative to HH) with a mild protective effect. Other studies have found that positive symptoms do not contribute much to QoL or social cognition [65,66,67], although the cross-sectional symptomatic remission [68] can significantly improve social functioning [69]. A higher genetic vulnerability toward SSD displayed a significant protective effect on good mSI (HL and MH relative to HH). This is possibly due to the single comparison in a relatively low sample size of HH in a multivariate model, which may occupy the variability of mSI concerning PRSSCZ, and consequently yielded a dubious finding. Therefore, the relationship between genetic predisposition and mSI should be independently investigated in well-powered research. Counterintuitively, we found that the more often an SSD patient experienced depressive symptoms, the more likely the patient was to be in high mSI (HH) than in medium mSI (HL) in the ModelMLR, which is discordant with previous studies [70,71,72]. One possible explanation is that in the diagnostic categories of SSD, patients with a higher level of depressive symptoms are more likely to have affective symptoms and to be associated with affective dysregulation, which results in a better outcome (mSI in our case) than the one of the patients with non-affective symptoms such as withdrawal in HL [73]. Specifically, the early detection and interventions of depressive symptoms could be essential to help patients with SSD, which might further impact their lives and subsequent mSI. All the aforesaid factors were confirmed informative in the ModelRF as well.
Abundant studies have emphasized the importance of occupation (and thus financial income and opportunities for acquiring new skills) and social relationships [74, 75], secure stable housing, family support [76] and inclusive and accessible support systems across sectors such as transportation [76, 77] for social inclusion. On the other hand, a higher level of fulfilled needs significantly distinguished MH and HH only in ModelMLR. Particularly in the ModelRF, the number of met needs was considered necessary. Therefore, with a growing emphasis on extramural care [78], the local communities and mental health organizations need to incorporate the heterogeneous environmental and social needs, beyond the medical needs, of patients with SSD at different mSI levels.
In contrast to the univariable analysis, we identified the duration of psychosis as a predictor with limited contribution in the ModelMLR or even with zero importance in the ModelRF for predicting mSI. This might be influenced by the presence of the other important predictors exemplified by QoL-related factors and childhood trauma (Fig. 3). The exclusion of variables related to duration of psychosis such as age at baseline and age of onset from the ModelRF did not improve the low importance of duration of psychosis. While previous studies have suggested an association between a shorter duration of psychosis and favorable changes in symptomatic remission and social functioning [79, 80], limited and conflicting evidence hinders confirmation of the association between chronicity and symptom severity, functioning and QoL [80, 81]. Therefore, the duration of psychosis might be less relevant, resulting in a modest contribution to predicting mSI at 3 years in multivariable models.
Despite many common factors that were selected in the both models, PRSSCZ, positive, negative, depressive symptoms, premorbid adjustment, baseline environment and social-domain satisfactions and the number of met needs were found to be crucially predictive of mSI.
Model performance in prediction
The predictivities of ModelMLR and ModelRF were fair and comparable. We inferred the ModelRF outperforms as it allocated individuals to the correct mSI cluster significantly better than chance alone, suggesting caution in applying the ModelMLR as some predictors in the ModelMLR were indiscriminative to mSI subgroups except for the HL cluster due to the imbalanced mSI outcome. Furthermore, although ModelRF did not perform as well as expected, earlier studies using data-driven methods have reported similar accuracies of 60%-75%, highlighting the complexity of SSD. The holistic mSI measure (compared to a single clinical outcome) and a longer time interval of 3-year (compared to 1 year) may be influenced vastly by various factors and their interactions, making mSI prediction challenging. To be integrated into clinical practices through electronic health records (EHR) (Supplementary Clinical Illustration) [82], the ModelRF requires external validation through international data-sharing efforts [83]. Despite its replicability, the model implementation may encounter a prolonged journey. Aligned with the minority of readily implementable psychiatry prediction models, the ModelRF requires inputs easily obtainable in clinical settings [84]. However, addressing the challenge of accurately and efficiently computing PRSSCZ from available and affordable genotype data remains essential, especially when considering the opportunity costs of assisting individuals facing poor social inclusion [83].
Future perspectives
Future studies should continue working on the conceptualization of mSI and examining its applicability across diagnoses. Meanwhile, developing a validated composite score could enable longitudinal monitoring. Methodologically, future studies should take extra steps in modeling procedures such as outer cross-validation and different feature-selection algorithms and give opportunities to the latest interpretable machine learning algorithms for exertion to pick up next-level clinical utility. Clinically, when building prediction models, future studies should test the utilities of potential factors that measure similar clinical outcomes but with slight variations in submodels and understand the multidimensional mechanisms hidden under the effect sizes such as premorbid adjustment. Furthermore, investigations on the genetic effects on behaviors and mSI along the course of SSD could be necessary for early screening. Statistically, other observable and non-observable factors, such as personality traits, coping strategies, diversity of community residents, community social-economic status, relationship with caregivers, and so forth, might simultaneously be more essential for improving the mSI prediction. With growing awareness of social inclusion and the development of sophisticated prediction models for mSI, along with personalized interventions and supporting policies, patients with SSD would be able to acquire necessary skills and receive essential resources. This would consequently aid them in managing their conditions and achieving great inclusion in society.
Study strengths and limitations
We quantified the multidimensional nature of social inclusion by combining thirteen subscales of the self-reported SFS and WHOQOL-BREF without the intention of developing a validated questionnaire. Our conceptualization emphasizes the multidimensionality that provides a comprehensive overview of an individual’s status of social inclusion through a broad range of activities, the perception of an individual, and the exploitation of the existing large cohort and standardly collected data. The longitudinal measurements in the cohort were utilized. We also compared standard and data-driven models to examine the robustness and enhance the credibility of the factors and predictability of mSI. However, no baseline mSI was available to investigate mSI changes over time. The models require external validation and could be improved with more non-clinical data.
Despite previous efforts in developing conceptual frameworks for social inclusion, it is vital to acknowledge that the constitution of social inclusion is nebulous, implying a variable boundary of this multifaceted construct. The available data do not provide much flexibility or balance the eligibility and validity of the elements used for the construct. No interview was conducted to preliminarily determine the most relevant scope of social inclusion.
Conclusion
We introduced mSI which is backboned by social functioning and quality of life, resulting in five identified subgroups including “very low/very low”, “low/low”, “high/low”, “medium/high”, and “high/high”. We found that genetic predisposition for SSD, premorbid adjustment, positive, negative and depressive symptoms, number of met needs and baseline satisfaction with the environment and social life were robust factors predictive of mSI in SSD. We cautiously concluded that the ModelRF offered a better prediction, compared to the ModelMLR, of the 3-year mSI among patients with SSD due to its better discriminability. Yet, continuous model refinement and external validation are still required.
Our findings indicate that mSI is applicable and offers possibilities for personalized treatment strategies and policymaking tailored for patients with SSD at different mSI levels. Our study emphasizes the special proposition of mSI as an imperative goal for patients with SSD and a possible solution to expensive healthcare and societal harmony.
References
United Nations (2006) Convention on the Rights of Persons with Disabilities (CRPD). United Nations, New York
Charlson F, Van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S (2019) New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet 394(10194):240–248. https://doi.org/10.1016/s0140-6736(19)30934-1
Nhunzvi C, Langhaug L, Mavindidze E, Harding R, Galvaan R (2019) Occupational justice and social inclusion in mental illness and HIV: a scoping review protocol. BMJ Open 9(3):e024049. https://doi.org/10.1136/bmjopen-2018-024049
Pereira RB, Whiteford GE (2013) Understanding social inclusion as an international discourse: implications for enabling participation. Br J Occup Ther 76(2):112–115. https://doi.org/10.4276/030802213x13603244419392
Cobigo V, Stuart H (2010) Social inclusion and mental health. Curr Opin Psychiatry 23(5):453–457. https://doi.org/10.1097/YCO.0b013e32833bb305
Simplican SC, Leader G, Kosciulek J, Leahy M (2015) Defining social inclusion of people with intellectual and developmental disabilities: an ecological model of social networks and community participation. Res Dev Disabil 38:18–29. https://doi.org/10.1016/j.ridd.2014.10.008
Izquierdo A, Cabello M, Leal I, Ayora M, Rodriguez-Jimenez R, Ibáñez Á, Díaz-Marsá M, Bravo-Ortiz M-F, Baca-García E, Madrigal JLM, Fares-Otero NE, Díaz-Caneja CM, Arango C, Ayuso Mateos JL, Leza JC, Molina-García M, Andreu-Bernabeu Á, Dompablo M, Sánchez-Pastor L, López-Torres I, León-Quismondo L, Muñoz Sanjose A, Vidal-Villegas M-P, Puras-Rico P, Albarracín-García L (2021) How does neighbourhood socio-economic status affect the interrelationships between functioning dimensions in first episode of psychosis? A network analysis approach. Health Place 69:102555. https://doi.org/10.1016/j.healthplace.2021.102555
Izquierdo A, Cabello M, Leal I, Mellor-Marsá B, Ayora M, Bravo-Ortiz M-F, Rodriguez-Jimenez R, Ibáñez Á, Macdowell KS, Malpica N, Díaz-Marsá M, Baca-García E, Fares-Otero NE, Melero H, López-García P, Díaz-Caneja CM, Arango C, Ayuso-Mateos JL, García-Albea J, Saiz-González D, Durán-Cutilla M, Merchán-Naranjo J, Mediavilla-Torres R, Muñoz-Sanjosé A, Sanchez-Pastor L, Dompablo M, Fernández-Martín P, León-Quismondo L, Carlos Leza J, Puras-Rico P, Albarracin-García L (2021) The interplay between functioning problems and symptoms in first episode of psychosis: an approach from network analysis. J Psychiatr Res 136:265–273. https://doi.org/10.1016/j.jpsychires.2021.02.024
Fong P, Cruwys T, Haslam C, Haslam SA (2019) Neighbourhood identification and mental health: how social identification moderates the relationship between socioeconomic disadvantage and health. J Environ Psychol 61:101–114. https://doi.org/10.1016/j.jenvp.2018.12.006
Eaton S, Harrap B, Downey L, Thien K, Bowtell M, Bardell-Williams M, Ratheesh A, Mcgorry P, O’Donoghue B (2019) Incidence of treated first episode psychosis from an Australian early intervention service and its association with neighbourhood characteristics. Schizophr Res 209:206–211. https://doi.org/10.1016/j.schres.2019.04.017
Kovács G, Almási T, Millier A, Toumi M, Horváth M, Kóczián K, Götze À, Kaló Z, Zemplényi AT (2018) Direct healthcare cost of schizophrenia—European overview. Eur Psychiatry 48(1):79–92. https://doi.org/10.1016/j.eurpsy.2017.10.008
VZinfo.nl (2017) Schizofrenie Kosten Zorguitgaven. Volksgezondheidenzorg.info. https://www.volksgezondheidenzorg.info/onderwerp/schizofrenie/kosten/zorguitgaven. Accessed 18 April 2021
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, Mcgrath JJ, Whiteford HA (2018) Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull 44(6):1195–1203. https://doi.org/10.1093/schbul/sby058
Boardman J (2011) Social exclusion and mental health—how people with mental health problems are disadvantaged: an overview. Ment Health Soc Incl 15(3):112–121. https://doi.org/10.1108/20428301111165690
Krishnan L (2015) Social exclusion, mental health, disadvantage and injustice. Psychol Dev Soc 27(2):155–173
Cordier R, Milbourn B, Martin R, Buchanan A, Chung D, Speyer R (2017) A systematic review evaluating the psychometric properties of measures of social inclusion. PLoS ONE 12(6):e0179109. https://doi.org/10.1371/journal.pone.0179109
Curran C, Burchardt T, Knapp M, Mcdaid D, Li B (2007) Challenges in multidisciplinary systematic reviewing: a study on social exclusion and mental health policy. Soc Policy Adm 41(3):289–312. https://doi.org/10.1111/j.1467-9515.2007.00553.x
Tiles-Sar N, Habtewold TD, Liemburg EJ, van der Meer L, Bruggeman R, Alizadeh BZ (2023) Understanding Lifelong Factors and Prediction Models of Social Functioning After Psychosis Onset Using theLarge-Scale GROUP Cohort Study. Schizophr Bull. 49(6):1447–1459. https://doi.org/10.1093/schbul/sbad046
Menezes NM, Arenovich T, Zipursky RB (2006) A systematic review of longitudinal outcome studies of first-episode psychosis. Psychol Med 36(10):1349–1362. https://doi.org/10.1017/s0033291706007951
Leighton SP, Krishnadas R, Chung K, Blair A, Brown S, Clark S, Sowerbutts K, Schwannauer M, Cavanagh J, Gumley AI (2019) Predicting one-year outcome in first episode psychosis using machine learning. PLoS ONE 14(3):e0212846. https://doi.org/10.1371/journal.pone.0212846
Leighton SP, Upthegrove R, Krishnadas R, Benros ME, Broome MR, Gkoutos GV, Liddle PF, Singh SP, Everard L, Jones PB, Fowler D, Sharma V, Freemantle N, Christensen RHB, Albert N, Nordentoft M, Schwannauer M, Cavanagh J, Gumley AI, Birchwood M, Mallikarjun PK (2019) Development and validation of multivariable prediction models of remission, recovery, and quality of life outcomes in people with first episode psychosis: a machine learning approach. Lancet Digital Health 1(6):e261–e270. https://doi.org/10.1016/s2589-7500(19)30121-9
Krupa T, Woodside H, Pocock K (2010) Activity and social participation in the period following a first episode of psychosis and implications for occupational therapy. Br J Occup Ther 73(1):13–20
Chua YC, Abdin E, Tang C, Subramaniam M, Verma S (2019) First-episode psychosis and vocational outcomes: a predictive model. Schizophr Res 211:63–68. https://doi.org/10.1016/j.schres.2019.07.009
Griffiths SL, Birchwood M, Khan A, Wood SJ (2021) Predictors of social and role outcomes in first episode psychosis: a prospective 12-month study of social cognition, neurocognition and symptoms. Early Interv Psychiatry 15(4):993–1001. https://doi.org/10.1111/eip.13056
Wright AC, Mueser KT, McGurk SR, Fowler D, Greenwood KE (2020) Cognitive and metacognitive factors predict engagement in employment in individuals with first episode psychosis. Schizophr Res Cogn 19:100141. https://doi.org/10.1016/j.scog.2019.100141
Pothier W, Cellard C, Corbière M, Villotti P, Achim AM, Lavoie A, Turcotte M, Vallières C, Roy M-A (2019) Determinants of occupational outcome in recent-onset psychosis: the role of cognition. Schizophr Res Cogn 18:100158. https://doi.org/10.1016/j.scog.2019.100158
Baksheev GN, Allott K, Jackson HJ, McGorry PD, Killackey E (2012) Predictors of vocational recovery among young people with first-episode psychosis: findings from a randomized controlled trial. Psychiatr Rehabil J 35:421–427. https://doi.org/10.1037/h0094574
Slade M, Leese M, Taylor R, Thornicroft G (1999) The association between needs and quality of life in an epidemiologically representative sample of people with psychosis. Acta Psychiatr Scand 100(2):149–157. https://doi.org/10.1111/j.1600-0447.1999.tb10836.x
Hastings PD, Serbin LA, Bukowski W, Helm JL, Stack DM, Dickson DJ, Ledingham JE, Schwartzman AE (2020) Predicting psychosis-spectrum diagnoses in adulthood from social behaviors and neighborhood contexts in childhood. Dev Psychopathol 32(2):465–479
Sanfelici R, Dwyer DB, Antonucci LA, Koutsouleris N (2020) Individualized diagnostic and prognostic models for patients with psychosis risk syndromes: a meta-analytic view on the state of the art. Biol Psychiat 88(4):349–360. https://doi.org/10.1016/j.biopsych.2020.02.009
Bjornestad J, Tjora T, Langeveld JH, Stain HJ, Joa I, Johannessen JO, Friedman-Yakoobian M, Velden Hegelstad W (2022) Exploring specific predictors of psychosis onset over a 2-year period: a decision-tree model. Early Interv Psychiatry 16(4):363–370. https://doi.org/10.1111/eip.13175
De Nijs J, Burger TJ, Janssen RJ, Kia SM, Van Opstal DPJ, De Koning MB, De Haan L, Alizadeh BZ, Bartels-Velthuis AA, Van Beveren NJ, Bruggeman R, De Haan L, Delespaul P, Luykx JJ, Myin-Germeys I, Kahn RS, Schirmbeck F, Simons CJP, Van Amelsvoort T, van Os J, Van Winkel R, Cahn W, Schnack HG (2021) Individualized prediction of three- and six-year outcomes of psychosis in a longitudinal multicenter study: a machine learning approach. NPJ Schizophr 7(1):34. https://doi.org/10.1038/s41537-021-00162-3
Koutsouleris N, Kahn RS, Chekroud AM, Leucht S, Falkai P, Wobrock T, Derks EM, Fleischhacker WW, Hasan A (2016) Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatry 3(10):935–946. https://doi.org/10.1016/S2215-0366(16)30171-7
Fond G, Bulzacka E, Boucekine M, Schürhoff F, Berna F, Godin O, Aouizerate B, Capdevielle D, Chereau I, D’Amato T, Dubertret C, Dubreucq J, Faget C, Leignier S, Lançon C, Mallet J, Misdrahi D, Passerieux C, Rey R, Schandrin A, Urbach M, Vidailhet P, Leboyer M, Boyer L, Llorca PM (2019) Machine learning for predicting psychotic relapse at 2 years in schizophrenia in the national FACE-SZ cohort. Prog Neuropsychopharmacol Biol Psychiatry 92:8–18. https://doi.org/10.1016/j.pnpbp.2018.12.005
Soldatos RF, Cearns M, Nielsen MØ, Kollias C, Xenaki L-A, Stefanatou P, Ralli I, Dimitrakopoulos S, Hatzimanolis A, Kosteletos I, Vlachos II, Selakovic M, Foteli S, Nianiakas N, Mantonakis L, Triantafyllou TF, Ntigridaki A, Ermiliou V, Voulgaraki M, Psarra E, Sørensen ME, Bojesen KB, Tangmose K, Sigvard AM, Ambrosen KS, Meritt T, Syeda W, Glenthøj BY, Koutsouleris N, Pantelis C, Ebdrup BH, Stefanis N (2021) Prediction of early symptom remission in two independent samples of first-episode psychosis patients using machine learning. Schizophr Bull 48(1):122–133. https://doi.org/10.1093/schbul/sbab107
Basaraba CN, Scodes JM, Dambreville R, Radigan M, Dachepally P, Gu G, Wang R, Dixon LB, Wall MM (2023) Prediction tool for individual outcome trajectories across the next year in first-episode psychosis in coordinated specialty care. JAMA Psychiat 80(1):49. https://doi.org/10.1001/jamapsychiatry.2022.3571
Lynam AL, Dennis JM, Owen KR, Oram RA, Jones AG, Shields BM, Ferrat LA (2020) Logistic regression has similar performance to optimised machine learning algorithms in a clinical setting: application to the discrimination between type 1 and type 2 diabetes in young adults. Diagn Progn Res 4(1):1–10. https://doi.org/10.1186/s41512-020-00075-2
Weng SF, Vaz L, Qureshi N, Kai J (2019) Prediction of premature all-cause mortality: a prospective general population cohort study comparing machine-learning and standard epidemiological approaches. PLoS ONE 14(3):e0214365. https://doi.org/10.1371/journal.pone.0214365
Li J, Zhou Z, Dong J, Fu Y, Li Y, Luan Z, Peng X (2021) Predicting breast cancer 5-year survival using machine learning: a systematic review. PLoS ONE 16(4):e0250370. https://doi.org/10.1371/journal.pone.0250370
Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B (2019) A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol 110:12–22. https://doi.org/10.1016/j.jclinepi.2019.02.004
Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L, Investigators G (2012) Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene–environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res 21(3):205–221
Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S (1990) The social functioning scale the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry 157(6):853–859. https://doi.org/10.1192/bjp.157.6.853
The WHOQoL Group (1998) Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 28(3):551–558. https://doi.org/10.1017/s0033291798006667
Bruins J, Pijnenborg GHM, Visser E, Castelein S (2021) The association of cannabis use with quality of life and psychosocial functioning in psychosis. Schizophr Res 228:229–234. https://doi.org/10.1016/j.schres.2020.11.059
Islam MA, Alizadeh BZ, van den Heuvel ER, Bruggeman R, Cahn W, de Haan L, Kahn RS, Meijer C, Myin-Germeys I, van Os J, Wiersma D (2015) A comparison of indices for identifying the number of clusters in hierarchical clustering: a study on cognition in schizophrenia patients. Commun Stat Case Stud Data Anal Appl 1(2):98–113. https://doi.org/10.1080/23737484.2015.1103670
RStudio Team (2020) RStudio: integrated development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
Verweij KHW, Derks EM (2013) The association between intelligence scores and family history of psychiatric disorder in schizophrenia patients, their siblings and healthy controls. PLoS ONE 8(10):e77215. https://doi.org/10.1371/journal.pone.0077215
López-Díaz Á, Valdés-Florido MJ, Palermo-Zeballos FJ, Pérez-Romero A, Menéndez-Sampil C, Lahera G (2022) The relationship between human development and prevalence of deficit schizophrenia: Results from a systematic review and meta-analysis. Psychiatry Res 114910. https://doi.org/10.1016/j.psychres.2022.114910
Kirkpatrick B, Mucci A, Galderisi S (2017) Primary, enduring negative symptoms: an update on research. Schizophr Bull 43(4):730–736. https://doi.org/10.1093/schbul/sbx064
Dong M, Lu L, Zhang L, Zhang Y-S, Ng CH, Ungvari GS, Li G, Meng X, Wang G, Xiang Y-T (2019) Quality of life in schizophrenia: a meta-analysis of comparative studies. Psychiatr Q 90(3):519–532. https://doi.org/10.1007/s11126-019-09633-4
Henderson M, Harvey SB, Overland S, Mykletun A, Hotopf M (2011) Work and common psychiatric disorders. J R Soc Med 104(5):198–207. https://doi.org/10.1258/jrsm.2011.100231
Greenberg N, Tarn M, Tracy D (2022) Lessons from the pandemic: why having a good understanding of occupational psychiatry is more important now than ever before. Br J Psychiatry 221(4):589–590. https://doi.org/10.1192/bjp.2022.49
Fusar-Poli P, Salazar De Pablo G, Rajkumar RP, López-Díaz Á, Malhotra S, Heckers S, Lawrie SM, Pillmann F (2022) Diagnosis, prognosis, and treatment of brief psychotic episodes: a review and research agenda. Lancet Psychiatry 9(1):72–83. https://doi.org/10.1016/s2215-0366(21)00121-8
López-Díaz Á, Ayesa-Arriola R, Ortiz-García de la Foz V, Crespo-Facorro B, Ruiz-Veguilla M (2020) S101. DIFFERENTIATING NON-AFFECTIVE ACUTE REMITTING PSYCHOSIS (NARP) FROM SCHIZOPHRENIA IN FIRST-EPISODE PSYCHOSIS: FINDINGS FROM A 3-YEAR LONGITUDINAL STUDY IN THE PAFIP COHORT. Schizophr Bull 46(Supplement_1):S72-73
Susser E, Finnerty MT, Sohler N (1996) Acute psychoses: a proposed diagnosis for ICD-11 and DSM-V. Psychiatr Q 67(3):165–176. https://doi.org/10.1007/bf02238949
Pazoki R, Lin BD, Van Eijk KR, Schijven D, De Zwarte S, Guloksuz S, Luykx JJ (2021) Phenome-wide and genome-wide analyses of quality of life in schizophrenia. BJPsych Open 7(1):e13. https://doi.org/10.1192/bjo.2020.140
Maat A, Fett A-K, Derks E (2012) Social cognition and quality of life in schizophrenia. Schizophr Res 137(1):212–218. https://doi.org/10.1016/j.schres.2012.02.017
Brill N, Levine SZ, Reichenberg A, Lubin G, Weiser M, Rabinowitz J (2009) Pathways to functional outcomes in schizophrenia: the role of premorbid functioning, negative symptoms and intelligence. Schizophr Res 110(1–3):40–46. https://doi.org/10.1016/j.schres.2009.02.016
Desalegn D, Girma S, Abdeta T (2020) Quality of life and its association with psychiatric symptoms and socio-demographic characteristics among people with schizophrenia: a hospital-based cross-sectional study. PLoS ONE 15(2):e0229514. https://doi.org/10.1371/journal.pone.0229514
Klorman R, Strauss JS, Kokes RF (1977) Premorbid adjustment in schizophrenia: III. The relationship of demographic and diagnostic factors to measures of premorbid adjustment in schizophrenia. Schizophr Bull 3(2):214–225. https://doi.org/10.1093/schbul/3.2.214
Shapiro DI, Marenco S, Spoor EH, Egan MF, Weinberger DR, Gold JM (2009) The Premorbid Adjustment Scale as a measure of developmental compromise in patients with schizophrenia and their healthy siblings. Schizophr Res 112(1):136–142. https://doi.org/10.1016/j.schres.2009.04.007
Jeppesen P, Petersen L, Thorup A, Abel M-B, Øhlenschlæger J, Christensen ØT, Krarup G, Jørgensen P, Nordentoft M (2008) The association between pre-morbid adjustment, duration of untreated psychosis and outcome in first-episode psychosis. Psychol Med 38(8):1157–1166. https://doi.org/10.1017/s0033291708003449
Bailer J, Bräuer W, Rey E-R (1996) Premorbid adjustment as predictor of outcome in schizophrenia: results of a prospective study. Acta Psychiatr Scand 93(5):368–377. https://doi.org/10.1111/j.1600-0447.1996.tb10662.x
Velthorst E, Reichenberg A, Kapra O, Goldberg S, Fromer M, Fruchter E, Ginat K, de Haan L, Davidson M, Weiser M (2016) Developmental trajectories of impaired community functioning in schizophrenia. JAMA Psychiat 73(1):48–55
Galuppi A, Turola M, Nanni M, Mazzoni P, Grassi L (2010) Schizophrenia and quality of life: how important are symptoms and functioning? Int J Ment Heal Syst 4(1):31. https://doi.org/10.1186/1752-4458-4-31
Landeen J, Pawlick J, Woodside H, Kirkpatrick H, Byrne C (2000) Hope, quality of life, and symptom severity in individuals with schizophrenia. Psychiatr Rehabil J 23:364–369. https://doi.org/10.1037/h0095142
Svirskis T, Korkeila J, Heinimaa M, Huttunen J, Ilonen T, Ristkari T, Hietala J, Syvälahti E, McGlashan T, Vahlberg T, Salokangas RKR (2007) Quality of life and functioning ability in subjects vulnerable to psychosis. Compr Psychiatry 48(2):155–160. https://doi.org/10.1016/j.comppsych.2006.10.008
Helldin L, Kane JM, Hjärthag F, Norlander T (2009) The importance of cross-sectional remission in schizophrenia for long-term outcome: a clinical prospective study. Schizophr Res 115(1):67–73. https://doi.org/10.1016/j.schres.2009.07.004
Brissos S, Dias VV, Balanzá-Martinez V, Carita AI, Figueira ML (2011) Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophr Res 129(2):133–136. https://doi.org/10.1016/j.schres.2011.04.001
Van Rooijen G, Van Rooijen M, Maat A, Vermeulen JM, Meijer CJ, Ruhé HG, De Haan L, Alizadeh BZ, Bartels-Velthuis AA, Van Beveren NJ, Bruggeman R, Cahn W, Delespaul P, Myin-Germeys I, Kahn RS, Schirmbeck F, Simons CJP, Van Amelsvoort T, Van Haren NE, Van Os J, Van Winkel R (2019) Longitudinal evidence for a relation between depressive symptoms and quality of life in schizophrenia using structural equation modeling. Schizophr Res 208:82–89. https://doi.org/10.1016/j.schres.2019.04.011
Subodh BN, Sandeep G (2020) Depression in schizophrenia: Prevalence and its impact on quality of life, disability, and functioning. Asian J Psychiatr 54:102425. https://doi.org/10.1016/j.ajp.2020.102425
Reininghaus U, Priebe S, Bentall RP (2012) Testing the psychopathology of psychosis: evidence for a general psychosis dimension. Schizophr Bull 39(4):884–895. https://doi.org/10.1093/schbul/sbr182
van Os J, Kapur S (2009) Schizophrenia. Lancet 374(9690):635–645. https://doi.org/10.1016/s0140-6736(09)60995-8
Killaspy H, White S, Lalvani N, Berg R, Thachil A, Kallumpuram S, Nasiruddin O, Wright C, Mezey G (2014) The impact of psychosis on social inclusion and associated factors. Int J Soc Psychiatry 60(2):148–154. https://doi.org/10.1177/0020764012471918
Turner N, Ferguson L, Hill M, Nesbitt T, O’Callaghan E, O’Mahony P, Clarke M (2017) An exploratory study of the extent of social inclusion among people with psychosis and psychotic-related conditions. Int J Soc Psychiatry 63(3):195–202. https://doi.org/10.1177/0020764017691551
Filia K, Jackson H, Cotton S, Killackey E (2019) Understanding what it means to be socially included for people with a lived experience of mental illness. Int J Soc Psychiatry 65(5):413–424. https://doi.org/10.1177/0020764019852657
Hall T, Kakuma R, Palmer L, Minas H, Martins J, Kermode M (2019) Social inclusion and exclusion of people with mental illness in Timor-Leste: a qualitative investigation with multiple stakeholders. BMC Public Health 19(1):1–13. https://doi.org/10.1186/s12889-019-7042-4
Netherlands Organization for Applied Scientific Research (TNO) (2020) Vision of extramural care in 2030: The right care with the right logistics. TNO. https://www.tno.nl/nl/aandachtsgebieden/mobiliteit-logistiek/roadmaps/smart-and-safe-traffic-and-transport/smart-mobility-and-logistics/visie-extramurale-zorg-2030/. Accessed 3 Jan 2023
de Winter L, Couwenbergh C, van Weeghel J, Hasson-Ohayon I, Vermeulen JM, Mulder CL, Boonstra N, Klaver KM, Oud M, de Haan L, Veling W (2022) Changes in social functioning over the course of psychotic disorders—a meta-analysis. Schizophr Res 239:55–82. https://doi.org/10.1016/j.schres.2021.11.010
Altamura AC, Serati M, Buoli M (2015) Is duration of illness really influencing outcome in major psychoses? Nord J Psychiatry 69(6):1685–1699. https://doi.org/10.3109/08039488.2014.990919
Harding CM, Zubin J, Strauss JS (1987) Chronicity in schizophrenia: fact, partial fact, or artifact? Psychiatr Serv 38(5):477–486
Lee TC, Shah NU, Haack A, Baxter SL (2020) Clinical implementation of predictive models embedded within electronic health record systems: a systematic review. Informatics 7(3):25. https://doi.org/10.3390/informatics7030025
Fusar-Poli P (2021) New electronic health records screening tools to improve detection of emerging psychosis. Front Psych 12:698406
Salazar De Pablo G, Studerus E, Vaquerizo-Serrano J, Irving J, Catalan A, Oliver D, Baldwin H, Danese A, Fazel S, Steyerberg EW, Stahl D, Fusar-Poli P (2021) Implementing precision psychiatry: a systematic review of individualized prediction models for clinical practice. Schizophr Bull 47(2):284–297. https://doi.org/10.1093/schbul/sbaa120
Cannon-Spoor HE, Potkin SG, Wyatt RJ (1982) Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 8(3):470–484. https://doi.org/10.1093/schbul/8.3.470
Arntz A, Wessel I (1996) Jeugd trauma vragenlijst [Dutch version of the childhood trauma questionnaire]. The Netherlands
Galderisi S, Mucci A, Dollfus S, Nordentoft M, Falkai P, Kaiser S, Giordano GM, Vandevelde A, Nielsen MØ, Glenthøj LB, Sabé M, Pezzella P, Bitter I, Gaebel W (2021) EPA guidance on assessment of negative symptoms in schizophrenia. Eur Psychiatry 64(1):1–91. https://doi.org/10.1192/j.eurpsy.2021.11
van Os J, Verdoux H, Hanssen M (1999) CAPE (Community Assessment of Psychic Experiences). University of Maastricht, Maastricht
Endicott J (1976) The global assessment scale. Arch Gen Psychiatry 33(6):766. https://doi.org/10.1001/archpsyc.1976.01770060086012
Phelan M, Slade M, Thornicroft G, Dunn G, Holloway F, Wykes T, Strathdee G, Loftus L, McCrone P, Hayward P (1995) The Camberwell Assessment of Need: the validity and reliability of an instrument to assess the needs of people with severe mental illness. Br J Psychiatry 167(5):589–595. https://doi.org/10.1192/bjp.167.5.589
Acknowledgements
We sincerely thank the GROUP recruited participants for the time spared, efforts made and generosity of sharing data for research. Furthermore, we would like to thank all research personnel involved in the GROUP project, in particular: Joyce van Baaren, Erwin Veermans, Ger Driessen, Truda Driesen, Erna van ’t Hag and Jessica de Nijs. Finally, we would like to express our appreciation to GROUP Investigators for their valuable feedback: Lieuwe de Haan (Department of Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Arkin Institute for Mental Health, Amsterdam, The Netherlands), Frederike Schirmbeck (Department of Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands), Claudia J. P. Simons (Department of Psychiatry and Neuropsychology, Maastricht University Medical Centre, School for Mental Health and Neuroscience, Maastricht, The Netherlands; GGzE, Institute for Mental Health Care, Eindhoven, The Netherlands), Therese van Amelsvoort (Department of Psychiatry and Psychology, Maastricht University Medical Centre, School for Mental Health and Neuroscience, Maastricht, The Netherlands), Wim Veling (Department of Psychiatry, University Medical Centre Groningen, University of Groningen, Groningen, The Netherlands).
Funding
This work was supported by Geestkracht programme of the Dutch Health Research Council (Zon-Mw) (10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck; AstraZeneca; Eli Lilly and Janssen Cilag) and Universities and Mental Health Care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Centre and the mental health institutions: GGZ Ingeest; Arkin, Dijk en Duin; GGZ Rivierduinen; Erasmus Medical Centre and GGZ Noord Holland Noord. Groningen: University Medical Centre Groningen and the mental health institutions: Lentis, GGZ Friesland; GGZ Drenthe; Dimence; Mediant; GGNet Warnsveld; Yulius Dordrecht and Parnassia psycho-medical centre The Hague. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGZ Eindhoven en De Kempen; GGZ Breburg; GGZ Oost-Brabant; Vincent van Gogh voor Geestelijke Gezondheid; Mondriaan; Virenze riagg; Zuyderland GGZ; MET ggz; Universitair Centrum Sint-Jozef Kortenberg; CAPRI University of Antwerp; PC Ziekeren Sint-Truiden; PZ Sancta Maria Sint-Truiden; GGZ Overpelt and OPZ Rekem. Utrecht: University Medical Center Utrecht and the mental health institutions: Altrecht; GGZ Centraal and Delta). The sponsors have no role in designing the study, in the collection, analysis, and interpretation of data, in the writing of the report and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
J.H. and B.Z.A. formulated the research questions and designed analysis plan. R.B. contributed to the formulation of the research questions. J.H. conducted data analysis and checked for reproducibility. J.H. drafted and revised the manuscript. N.T.S., T.D.H., E.J.L., GROUP Investigators, R.B., L.M. and B.Z.A. assisted with results interpretation and provided constructive comments on the manuscript. All authors read and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The GROUP protocol was approved by the Medical Ethics Review Committee (Medisch Ethische Toetsingscommissie [METC]) of the University Medical Centre Utrecht. The protocol was conducted in accordance with the Helsinki Declaration that was revised in 2008. Thereof, no further ethical approval is required for this study.
Consent to participate
All GROUP participants have given written informed consent.
Availability of data and material
Due to privacy, we are not permitted to share the data. Any researcher interested in acquiring the dataset can submit an application form to the GROUP committee via e-mail.
Additional information
The members of Genetic Risk and Outcome of Psychosis (GROUP) Investigators are listed in Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hao, J., Tiles-Sar, N., Habtewold, T.D. et al. Shaping tomorrow’s support: baseline clinical characteristics predict later social functioning and quality of life in schizophrenia spectrum disorder. Soc Psychiatry Psychiatr Epidemiol (2024). https://doi.org/10.1007/s00127-024-02630-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00127-024-02630-4