Abstract
Purpose
Existing evidence on the mental health consequences of disadvantaged areas uses cross-sectional or longitudinal studies with short observation periods. The objective of this research was to investigate this association over a 69-year period.
Methods
Data were obtained from the MRC National Survey of Health and Development (NSHD; the British 1946 birth cohort), which consisted of 2125 participants at 69 years. We assessed longitudinal associations between area disadvantage and mental health symptoms at adolescence and adulthood with use of multilevel modelling framework.
Results
After adjustment for father’s social class, for each one percentage increase in area disadvantage at age 4, there was a 0.02 (95% CI 0.001, 0.04) mean increase in the total score of the neuroticism scale at age 13–15. After adjustment for father’s social class, adult socio-economic position, cognitive ability and educational attainment, a one percentage increase in change score of area disadvantage between age 4 and 26 was associated with a mean increase in the total Psychiatric Symptom Frequency score (MD 0.06; 95% CI 0.007, 0.11). Similar associations were observed with change scores between ages 4, 53, 60 and total General Health Questionnaire-28 score at age 53 (MD 0.05; 95% CI 0.01, 0.11) and 60–64 (MD 0.06; 95% CI 0.009, 0.11).
Conclusions
Cohort members who experienced increasing area disadvantage from childhood were at increased risk of poor mental health over the life course. Population-wide interventions aiming at improving social and physical aspects of the early neighbourhood environment could reduce the socio-economic burden of poor mental health.
Similar content being viewed by others
Introduction
The life course approach highlights the importance of timing, duration and temporal ordering of effects between exposure and outcomes [1, 2]. The timing of an exposure may be particularly important for mental health during social transitions from childhood to adulthood [3, 4] and for the duration of lifestyle and environmental risk factors which tend to accumulate over the life course [5].
However, these mental health determinants do not only cluster temporally but also spatially [6,7,8,9,10] and there are potential reasons why such a spatio-temporal relationship may exist [3]. Living in a disadvantaged area could impact mental health via scarcity of community resources, such as adverse built environment exposures (e.g., noise [11], air pollution [12]) and through stress exposures (e.g., crime [13]) and these impacts could be modified by individual’s socio-economic status [14, 15]. A non-causal explanation could be selection or ‘social drift’, where people with mental health problems may relocate to disadvantaged areas. These processes may interact to form a chain of cumulative risk [16]. Nevertheless, five systematic reviews [6,7,8,9,10] over the last decade investigating area socioeconomic conditions and mental health concluded that evidence is still inconclusive and that longitudinal designs with extensive area histories are warranted [6, 17].
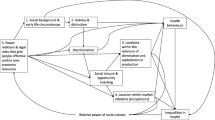
The aim of this study was, therefore, to investigate whether area disadvantage is associated with mental health over a 69-year period using prospectively collected residential addresses from the MRC National Health Survey of Health and Development (NHSD), the British 1946 birth cohort. We investigated the following four hypotheses (Fig. 1): (i) Early area level disadvantage will be associated with poorer adolescent and adult mental health [6]; (ii) Area disadvantage at different stages in adulthood will be associated with poorer adult mental health [18]; (iii) Increasing area level disadvantage over time from childhood will be associated with poorer mental health [3]; (iv) these associations will be exacerbated by participant’s social disadvantage [15].
Potential pathways of neighbourhood effect on mental health over the life course. Black pathways refer to potential associations between area disadvantage and mental health. Blue pathways refer to potential continuity in area characteristics. Red pathways refer to continuity in affective symptoms. PSE Total score of Present State Examination at age 36. PSF Total score of Psychiatric Symptom Frequency scale at age 45 GHQ-28: Total score of 28 item General Health Questionnaire at ages 53, 60–64 and 69
Methods
Study population and design
The MRC NSHD is a socially stratified sample originally consisting of 5362 singleton births during one week in March 1946. Cohort members have been followed up 24 times since birth and a wealth of medical and socioeconomic data has been collected throughout their life. At 69 years, this sample consisted of 2125 men and women (61% of the original cohort) still alive and living in England, Scotland and Wales. The sampling procedure and follow-up have been described in detail elsewhere [19] and the cohort socioeconomic profile was broadly similar to a census reference population at age 60–64 [20]. The most recent ethical approval was granted by the National Research Ethics Service Committee London Queen Square and by the Scotland Research Ethics Committee (REC) (14/LO/1073) and Scotland A REC (14/SS/1009). All study members gave written informed consent and did not receive financial reimbursement. Weights were applied to the analysis to account for the sampling procedure. The sample was distributed geographically in proportion to the national population. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Life course area disadvantage
At every data collection, the address of the current place of residence of each study member was recorded. Place of residence at four different ages was chosen to represent area in different age periods: in childhood (age 4 years—1950), early adulthood (age 26 years—1972) and middle adulthood (age 53 years—1999 and age 60 years—2006) and to be close to census years (eFigure S1–see online supplement). The overall process of linking residential addresses to area level measures (local authority district in England and Wales; counties in Scotland which is approximately linked to a population of 110 000, similar to county level data in the US) was a two-step one and is described in detail elsewhere [21].
In brief, automated matching, county administrative diagrams and manual methods of assignment was carried out on addresses in order to allocate to each place of residence a grid coordinate [22]. Second, these generated coordinates were used to link area data from the closest census: 1951 data for local government districts for 1950, 1971 data for districts for 1972, 2001 data for districts or unitary authorities for 1999 and 2011 data for census local authorities for 2011 [21, 22]. For 1951 and 1971, Scottish addresses had to be linked to data for counties and the four main cities, since district data were not available [21, 22].
We linked MRC NSHD participants at Local Authority level to two measures of area disadvantage: (i) the proportion of employed persons at ages 4, 26, 53 and 60 in each area with occupations that were semi-skilled or unskilled. This was defined according to the UK Registrar General and selected as the primary marker of area socioeconomic disadvantage, since this was previously shown to be the most consistent and appropriate available census variable in NSHD across all study years [22] (ii) the change score in these proportions of area level disadvantage over time. Specifically, change scores in percentage of area disadvantage were estimated by the difference in percentages of employed persons with occupations that were semi-skilled or unskilled in each area–difference in the percentage between age 4 and 26, difference in percentage between age 4 and 53 and difference in percentage between age 4 and 60. These differences could either be due to the participant moving area or the area itself changing; however, we could not disentangle this due to lack of extensive moving status information.
Measures of mental health
Self-reported mental health scale and teacher ratings at age 13–15
At age 13 years (1959), participants completed the Pintner Aspects of Personality Inventory [23, 24] which includes a 35-item neuroticism scale. At ages 13–15 years, teachers rated behaviour and emotionality using a forerunner of the Rutter A scale [25, 26]. Previous studies using this cohort have created summary measures of these problems by deriving global measures for each from factor analysis, then dividing scores for these into absent, mild and severe based on established centile cuts [27].
Interviews at age 36 and 43
At age 36 (1982), a short version of the Present State Examination (PSE), a clinically validated semi-structured interview administrated by trained nurses, was used [28]. A total symptom score was derived. At age 43 (1989), the Psychiatric Symptom Frequency (PSF) scale, an interview-based 23-item scale derived from the PSE, was administered. A total score was calculated [29] were higher scores indicates increased symptoms.
Self-reported questionnaire at age 53, 60–64 and 69
Study members completed the 28-item self-administered General Health Questionnaire (GHQ-28) [30] at ages 53 (1999), 60–64 (2006–2010) and 69 (2015). Each individual item was scored using a 4-point Likert scale, and a log-transformed total score of the 28-item GHQ was produced for each different age were higher scores indicate probable mental ill health.
Confounders
The following variables were treated as potential confounders: childhood cognitive ability [31] (age 15 years—1961), individual socioeconomic position (SEP) at 3 stages of life: age 4 (1950) using father’s occupational social class; age 36 (1982) and age 53 (1999) using participant’s occupational social class; and educational attainment (up to age 26, i.e., up to 1972). Individual SEP at the three stages of life were selected to match the years for which census data were available. Childhood SEP was based on father’s occupation when the cohort member was aged 4 years. SEP for each time point was fitted as categorical indicators of professional, intermediate, skilled (non-manual), skilled (manual), partly skilled and unskilled, based on the UK Registrar General classification. Childhood cognitive ability was represented at age 15 years by tests of verbal and non-verbal intelligence (the AH4 test), reading comprehension, and mathematics. Scores were summed to represent overall cognitive ability. Educational attainment was based on the highest educational qualifications and their training equivalents attained by 26 years and were classified as none, vocational only and ordinary secondary (O levels), advanced secondary (A levels), or degree level or equivalent.
A full timeline of data collected is presented in eFigure S1 in the online supplement.
Statistical analyses
First Hypothesis: Early area level disadvantage will be associated with poorer adolescent and adult mental health
Prospective associations between area disadvantage at age 4 and mental health at age 13–15, 36, 43, 53, 60–64 and 69 were examined by two-level linear and ordinal multilevel single time point models with individuals (level 1) nested within areas (level 2). Initially, models were fitted separately for each measure of mental health over time and area disadvantage at age 4 (model 1). Second, each model was adjusted for childhood SEP from the same year (Model 2), and then further adjusted for cognitive ability (Model 3).
These models where individuals are nested within area in a particular year assume that measuring area of residence at one point in the life course is sufficient to assess the association between area disadvantage and mental health. However, as exposure to area disadvantage can change over the life course, models including only a single time point could provide biased estimates of the effect of area on mental health [32, 33]. Cross-classified models are used when there is no strict hierarchical structure to higher level units and comprise individuals who are nested within a cross-classification of two or more differing hierarchies–in our case, participants nested within a cross-classification of neighbourhoods. In our model, we have up to four classifications relating to the areas (at age 4, 26, 53 and 60) at each census year (eFigure S2 in the online supplement).
Second Hypothesis: Area disadvantage at different stages in adulthood will be associated with poorer adult mental health
Cross-classified models were fitted separately to test prospective associations between area disadvantage (i) at age 26 and mental health at ages 36, 43, 53, 60–64 and 69 (ii) at age 53 and mental health at ages 60–64 and 69 (iii) at age 60 and mental health at age 69.
Third Hypothesis: Increasing area-level disadvantage over time will be associated with poorer adult mental health
Similar cross-classified models were fitted to test each association separately for change score in area disadvantage (i) between age 4 and 26 and mental health at ages 36, 43 (ii) between age 4 and 53 and mental health at age 53 (iii) between age 4 and 60 with mental health at ages 60–64 and 69.
Initially, area disadvantage and change scores in area disadvantage were modelled separately for each year (Model 1). Then, each model was adjusted for adult SEP at age 36 or age 53 (Model 2); further adjusted for childhood SEP (Model 3); further adjusted for educational attainment up to age 26 and cognitive ability at age 15 (Model 4); further adjusted for area disadvantage at previous age (Model 5); further adjusted for mental health at previous age (Model 6) (eTable A).
Fourth Hypothesis: Associations will be exacerbated by participant’s social disadvantage
We also tested possible effect modification of the association between mental health and area disadvantage by individual SEP with the inclusion of an interaction term in the above-mentioned models.
We repeated our statistical analyses using the STATA routine ice, an implementation in STATA of the multiple imputations using chained equations (MICE) and compared our results with the original analysis under the missing at random (MAR) assumption [34]. Data analyses were performed using STATA 14.1 and MLwin 3.04.
A table which summarises the above-mentioned models is provided in the supplemental material (eTable A).
Results
Our final sample for the analysis was 4873 at age 4, 4231 at age 13–15, 3293 at age 36, 3187 at age 43, 2902 at age 53, 2190 at age 60–64 and 2125 at age 69. The average area disadvantage (percentage of persons employed in semi-skilled or unskilled occupations) of the areas in which cohort members lived was highest in 1950, at 29.3% (IQR, 24.1–35.1), and declined steadily to 25.1% (IQR, 21.0–29.5) in 1972, 19.7% (IQR, 17.6–21.6) in 1999 and 15.5% (12.7, 18.0) in 2006. Descriptive statistics of the sample are presented in Table 1.
First Hypothesis: Is early area level disadvantage associated with poorer adolescent and adult mental health?
There was evidence of a prospective association between area disadvantage at age 4 and the total score of neuroticism scale at age 13–15 (Table 2). After adjusting for childhood SEP and cognitive ability at age 15 (Model 3), for each one percentage increase in area disadvantage at age 4, there was a 0.02 (95% CI 0.001, 0.04) increase in the score of the neuroticism scale.
At age 60–64, there was evidence for a negative association between area disadvantage at age 4 and the total score of GHQ-28 (MD -0.06; 95% CI -0.12, -0.01) after adjusting for childhood SEP at age 4, adult SEP at age 36 and 53, cognitive ability at age 15 and educational attainment (eTable S3-see online supplemental material). This pattern of a negative association, although weaker, between area disadvantage at age 4 and mental health was also observed at ages 36 (eTable S1), 43 (eTable S1) and 53 (eTable S2). No evidence of associations was seen with teacher-rated emotional or conduct problems at ages 13–15 (Table 2) and total score of GHQ-28 at age 69 (eTable S4).
Second Hypothesis: Is area disadvantage at adult stages of the life course associated with poorer adult mental health?
There was no evidence for an association between area disadvantage at age 26 and PSE score at age 36, total PSF score at age 43 (eTable S1–see online supplemental material) and GHQ-28 total score at age 53 (eTable S2). However, a one percentage increase in area disadvantage at age 26 was associated with increased GHQ-28 total score at age 60–64 (MD 0.06; 95% CI 0.01, 0.10) and age 69 (MD 0.05; 95% CI 0.01, 0.12) (eTable S3 and eTable S4).
There was no evidence for an association between area disadvantage at age 53 and the total GHQ-28 score at age 53, 60–64 and 69 (eTable S2, eTable S3 and eTable S4-see online supplement). Nor there was evidence for an association between area disadvantage at age 60 and total GHQ-28 score at age 60–64 and 69 (eTable S3 and S4–see online supplement).
Third Hypothesis: Is increasing area-level disadvantage over time associated with poorer adult mental health?
There was evidence of an association between change score in area disadvantage between age 4 and 26 and total score of the PSF at age 43, but not at age 36 (Fig. 2 and Table S1). For example, when adjusting for childhood SEP at age 4, adult SEP at age 36, educational attainment and cognitive ability at age 15 (Fig. 2, model 2), there was a 0.06 (95% CI 0.007,0.11) mean increase in the total score of the PSF per one percentage change score increase in area disadvantage.
Associations of change score in area disadvantage and adult mental health. Mean Difference (MD) and 95% Credible Intervals (CrI) represent a difference in mental health scores (PSE-age 36; PSF-age 43, GHQ-age 53, age 60–64 and age 69) per one percentage change score increase in area disadvantage between age 4 and 26 (for PSE and PSF), age 4 and 53 (for 28-item GHQ at age 53), age 4 and 60 (for 28-item GHQ at age 60–64 and age 69). In model 1, results were unadjusted; in model 2, results were adjusted for childhood socioeconomic position (SEP) at age 4 and adult SEP (age 4 or age 36 or age 53); model 3 was further adjusted for educational attainment up to age 26 and cognitive ability at age 15, PSE Total score of Present State Examination, PSF Total score of Psychiatric Symptom Frequency Scale GHQ Total score of 28-item General Health Questionnaire
In addition, there was evidence of a mean increase (in the total score of the 28-item GHQ at age 53 MD: 0.05; 95% CI 0.01, 0.11) for one percentage change score increase of area disadvantage between age 4 and age 53 (eTable S2). A one percentage change score increase in area disadvantage between age 4 and 60 was also associated with GHQ-28 total score at age 60–64 (MD 0.06; 95% CI 0.009, 0.11) but not with GHQ-28 total score at age 69 (eTable S3 and S4).
Fourth Hypothesis: Are associations between area disadvantage and mental health exacerbated by participant’s social disadvantage?
Effect modification by individual SEP (manual vs non-manual class) of the association between area disadvantage and mental health at ages 13–15 and 69 were observed (eTable S5-see online supplemental material). There were mean differences between individuals from manual and non-manual social classes of 0.017 (0.005, 0.024) and 0.001 (0.0001, 0.003) with mental health outcomes at age 13–15 and age 69, respectively, per one percentage increase in area disadvantage–thus, the association between area disadvantage and mental health outcomes was stronger for those in a manual compared with a non-manual social class.
In addition, effect estimates (OR and MD) and corresponding 95% confidence intervals did not change substantially when we replicate our analyses with the use of the MICE procedure (eTable S6).
Discussion
This is the first population-based study to utilise geographical data linked to prospectively collected address information in childhood, early adulthood and middle adulthood and subsequently link these exposures to mental health in childhood, early adulthood, middle adulthood and later life during a 69-year period. In relation to our first hypothesis, we found that residence in disadvantaged area in childhood (age 4) was associated with poorer mental health outcomes in early adolescence (age 13), controlling for father’s occupational social class; in contrast, no consistent effect and even a negative effect was detected for early adulthood, middle adulthood or later life. In addition, partly consistent with our second hypothesis, we found poorer mental health in middle adulthood (age 60–64) and later life (age 69) for study members who reside in a disadvantaged area as young adults (age 26). We were partly consistent with our third hypothesis, that cohort members that continued to reside or moved into areas which became more disadvantaged between early life and adulthood had poorer mental health in adulthood. Finally, in regards to our fourth hypothesis, we found that the effects of area disadvantage on mental health were more pronounced in participants from a manual social class, providing evidence of greater vulnerability to area disadvantage in early adolescence (age 13–15) and later life (age 69).
Strengths and limitations of this study
Strengths of the study include an unusually novel and long follow-up period, and prospective data obtained from a national general population birth cohort based on representative sampling across different socioeconomic areas. We included an extensive range of potential mental health outcomes (repeated collection of mental health outcomes from adolescence and spanning 6 decades) linked with geocoded area data for nearly all study members using linkage with census data. Furthermore, combination of repeated collection of neighbourhood exposure preceding measurement of mental health and vice versa is particularly important to address temporal ambiguity and reverse causality issues observed in cross-sectional studies. People may select into or remain against their desire in certain neighbourhoods due to life history, health or simply in the hope that a different school, neighbourhood, or proximity to specialist health care clinicians might improve their child’s well-being [7, 17, 17, 35].
These strengths should, however, be considered alongside important limitations. A key limitation is that different measures of mental health were used within the NSHD which may specifically impair comparability at ages 13–15 to 69–although, there is no reason to anticipate that this would have changed the pattern of associations observed a the thresholds for case-level symptoms were either clinically validated, or, in the case of the adolescent assessments, consistent with a previous percentile-based cut for the most severe symptoms [36]. In addition, at age 13–15, mental health measures were rated by the teacher which could lead to misclassification and potential bias if participants had emotional problems that were unrecognised by teacher, although there is evidence of consistency between teacher ratings and self-reports of psychiatric disorders [37].
In spite of the population-based sampling, there was selective sample attrition of those less socially advantaged and less healthy and of those with poorer mental health itself [38, 39]. However, comparisons with the census data of the sample successfully contacted at 53 years and weighted to adjust for the initial sampling procedure, show that the sample was representative of the general population of similar age in terms of sex and social class profiles, similar to the 2001 England Census and Integrated Household Survey (IHS) [19, 20]. The greatest overall attrition occurred in the early adult years. In addition, multiple imputation showed very similar associations for the models employed.
The current study utilises administrative boundaries which change over time which may be an imperfect proxy for a person’s true residential area. Furthermore, percentage in skilled vs. unskilled manual occupations, although an adequate proxy in this study [22], does not address all aspects of area disadvantage (such as quality and proximity of amenities and crime) and future studies are needed to identify appropriate conceptualization of place [40] and different area definitions and measurement processes lead to different analytical results [41].
Although we examined a large number of associations and the significance level at 5% is purely nominal and the likelihood of type I error is inflated, the majority of our findings were consistent in terms of the direction of the association between area disadvantage and mental health. In addition, the use of change scores as a measure of area disadvantage should be interpreted with caution as there is a danger of conditioning for variables that are on the causal pathway and further analytical approaches (e.g., causal mediation) should be considered [42].
Comparison to other studies and discussion of potential mechanism
Our findings provide empirical support to the notion that the child’s environment has a measurable effect on adolescence mental health, in line with previous observational [43,44,45,46] and quasi-experimental [47, 48] studies conducted both in the UK, Europe and US.
Our study also indicated a time-lasting association of area disadvantage with mental health that was observed in early adulthood, midlife and later life. Our findings are partly consistent with a Swedish population cohort study of 1.4 million participants where children and adolescents with stability (as number of movers) in their residential environments were less likely to experience psychotic disorders in early adulthood [49]. In addition, higher rates of poor mental health in more deprived, socially fragmented urban environments might be a consequence of social drift [50,51,52,53] as many people may move into cheaper and more disadvantaged areas [54, 55]. The vulnerability hypothesis that we tested provides a framework for exploring plausible mechanisms linking area disadvantage with poor mental health. Individuals of lower SEP may be exposed to more stressors such as air [56] and noise [57] pollution, crime [58] and perceived and actual neighbourhood disorder [59, 60] and may have fewer personal resources to cope with these stressors, which may put them at greater risk of poor mental health. We also observed a consistent counterintuitive association between early life area disadvantage and improved mental health outcomes in adulthood (ages 36, 43, 53, 60–64 and 69) (in contrast with findings in adolescence) which was hard to explain and requires replication.
Although overall our effect sizes and 95% confidence intervals might seem small, we need to highlight that associations were observed for a one percentage increase in area disadvantage. If we considered a 10% increase in area disadvantage—which is plausible based on the changes that took place within that time period—our effect sizes and 95% confidence intervals are consistent with similar studies on the topic [6].
Conclusion
Our results are consistent with a lifelong association of area disadvantage with poor mental health, where a disadvantaged environment could increase the risk of poor mental health; and these risks could be more pronounced in participants from more disadvantaged individual socioeconomic background. Improving neighbourhood environments is a tractable, though complex issue [7] and therefore measures to fund and facilitate area based interventions, such as jobs skill training seminars or psychosocial support for vulnerable individuals [61] may represent a potentially impactful primary health measure for the prevention of poor population mental health. This study also highlights the importance of efforts that are now required to examine the reasons via causal pathway analysis and appropriate geographical levels and provides evidence to direct future interventions and healthcare services in targeting specific vulnerable populations.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available. Cohort data comply with ESRC data sharing policies, readers can access data via the UK Data Archive (www.data-archive.ac.uk), through a formal request.
References
Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C (2003) Life course epidemiology. J Epidemiol Commun Health 57:778–783
Pickles A, Maughan B, Wadsworth M (2007) Epidemiological methods in life course research. Oxford University Press
Clarke P, Morenoff J, Debbink M, Golberstein E, Elliott MR, Lantz PM (2014) Cumulative exposure to neighborhood context: consequences for health transitions over the adult life course. Res Aging 36:115–142
Hatch SL, Jones PB, Kuh D, Hardy R, Wadsworth ME, Richards M (2007) Childhood cognitive ability and adult mental health in the British 1946 birth cohort. Soc Sci Med 64:2285–2296
Sir Michael M (2005) The social environment and health. Clin Med (Lond) 5:244–248
Jivraj S, Murray ET, Norman P, Nicholas O (2019) The impact of life course exposures to neighbourhood deprivation on health and well-being: a review of the long-term neighbourhood effects literature. Eur J Public Health 30:922–928
Kim D (2008) Blues from the neighborhood? Neighborhood characteristics and depression. Epidemiol Rev 30:101–117
Mair C, Diez Roux AV, Galea S (2008) Are neighbourhood characteristics associated with depressive symptoms? A review of evidence. J Epidemiol Commun Health. 62:940–946
Oakes JM, Andrade KE, Biyoow IM, Cowan LT (2015) Twenty years of neighborhood effect research: an assessment. Current epidemiol report 2:80–87
Paczkowski MM, Galea S (2010) Sociodemographic characteristics of the neighborhood and depressive symptoms. Curr Opin Psychiatry 23:337–341
Clark C, Crumpler C, Notley H (2020) Evidence for environmental noise effects on health for the United Kingdom policy context: a systematic review of the effects of environmental noise on mental health, wellbeing, quality of life, cancer, dementia, birth, reproductive outcomes, and cognition. Int J Environ Res Public Health 17:393
Newbury JB, Stewart R, Fisher HL, Beevers S, Dajnak D, Broadbent M, Pritchard M, Shiode N, Heslin M, Hammoud R (2021) Association between air pollution exposure and mental health service use among individuals with first presentations of psychotic and mood disorders: retrospective cohort study. British J Psychiatr. 219:1–8
Pearlin LI, Schieman S, Fazio EM, Meersman SC (2005) Stress, health, and the life course: Some conceptual perspectives. J Health Soc Behav 46:205–219
Winkleby M, Cubbin C, Ahn D (2006) Effect of cross-level interaction between individual and neighborhood socioeconomic status on adult mortality rates. Am J Public Health 96:2145–2153
Boylan JM, Robert SA (2017) Neighborhood SES is particularly important to the cardiovascular health of low SES individuals. Soc Sci Med 188:60–68
Rutter M (1989) Pathways from childhood to adult life. J Child Psychol Psychiatr 30:23–51
Van Ham M, Manley D (2012) Neighbourhood effects research at a crossroads. ten challenges for future research introduction. Environ Plann A 44:2787–2793
Jivraj S, Norman P, Nicholas O, Murray ET (2019) Are there sensitive neighbourhood effect periods during the life course on midlife health and wellbeing? Health Place 57:147–156
Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, Friberg P, Ghosh AK, Harwood N, Hughes A, Macfarlane PW (2011) Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol 40:e1–e9
Stafford M, Black S, Shah I, Hardy R, Pierce M, Richards M, Wong A, Kuh D (2013) Using a birth cohort to study ageing: representativeness and response rates in the National Survey of Health and Development. Eur J Ageing 10:145–157
Murray ET, Ben-Shlomo Y, Tilling K, Southall H, Aucott P, Kuh D, Hardy R (2013) Area deprivation across the life course and physical capability in midlife: findings from the 1946 british birth cohort. Am J Epidemiol 178:441–450
Murray ET, Southall H, Aucott P, Tilling K, Kuh D, Hardy R, Ben-Shlomo Y (2012) Challenges in examining area effects across the life course on physical capability in mid-life: Findings from the 1946 British Birth Cohort. Health Place 18:366–374
Pintner R, Forlano G (1938) Four retests of a personality inventory. J Educ Psychol 29:93
Pintner R, Loftus JJ, Forlano G, Alster B (1937) Aspects of personality
Elander J, Rutter M (1996) Use and development of the Rutter parents' and teachers' scales. International Journal of Methods in Psychiatric Research
Rutter M, Tizard J, Whitmore K (1970) Education, health and behaviour. Longman Publishing Group
Xu MK, Jones PB, Barnett JH, Gaysina D, Kuh D, Croudace TJ, Richards M (2013) Adolescent self-organization predicts midlife memory in a prospective birth cohort study. Psychol Aging 28:958
Wing JK, Cooper JE, Sartorius N (1974) Present state examination. Cambridge University Press London:
Lindelow M, Hardy R, Rodgers B (1997) Development of a scale to measure symptoms of anxiety and depression in the general UK population: the psychiatric symptom frequency scale. J Epidemiol Commun Health 51:549–557
Goldberg DP, Hillier VF (1979) A scaled version of the General Health Questionnaire. Psychol Med 9:139–145
Douglas J, Ross J, Simpson H (1965) The relation between height and measured educational ability in school children of the same social class, family size and stage of sexual development. Human Biol 21:178–186
Curtis S, Southall H, Congdon P, Dodgeon B (2004) Area effects on health variation over the life-course: analysis of the longitudinal study sample in England using new data on area of residence in childhood. Soc Sci Med 58:57–74
Leyland AH, Næss Ø (2009) The effect of area of residence over the life course on subsequent mortality. J R Stat Soc A Stat Soc 172:555–578
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393
Woodhead C, Aschan L, Lynskey MT, Polling C, Goodwin L, Hatch SL (2015) Exploring evidence for a prospective relationship between common mental disorder and meeting residential mobility preferences. Health Place 32:19–28
Richards M, Abbott R, Collis G, Hackett P, Hotopf M, Kuh DPM, Jones P, Maughan B, Parsonage M (2009) Childhood mental health and life chances in post-war Britain. Insights from three national birth cohort studies. Sainsb Centre Mental Health. 32:18–28
Goodman R, Ford T, Simmons H (2002) Using the Strengths and Difficulties Questionnaire (SDQ) to Screen for Child Psychiatric Disorders in a Community Sample. Year Book Psychiatr Appl Mental Health 2002:37–38
Archer G, Kuh D, Hotopf M, Stafford M, Richards M (2018) Adolescent affective symptoms and mortality. Br J Psychiatry 213:419–424
Henderson M, Hotopf M, Shah I, Hayes RD, Kuh D (2011) Psychiatric disorder in early adulthood and risk of premature mortality in the 1946 British Birth Cohort. BMC Psychiatry 11:37
Matthews SA (2008) The salience of neighborhood: some lessons from sociology. Am J Prev Med 34:257–259
Visser K, Bolt G, Finkenauer C, Jonker M, Weinberg D, Stevens GW (2020) Neighbourhood deprivation effects on young people’s mental health and well-being: a systematic review of the literature. Soc Sci Med 21:113542
De Stavola BL, Daniel RM, Ploubidis GB, Micali N (2014) Mediation analysis with intermediate confounding: structural equation modeling viewed through the causal inference lens. Am J Epidemiol 181:64–80
Caughy MO, O’Campo PJ, Muntaner C (2003) When being alone might be better: neighborhood poverty, social capital, and child mental health. Soc Sci Med 57:227–237
Latkin CA, Curry AD (2003) Stressful neighborhoods and depression: a prospective study of the impact of neighborhood disorder. J Health Soc Behav 22:34–44
Sariaslan A, Larsson H, D’Onofrio B, Långström N, Fazel S, Lichtenstein P (2015) Does population density and neighborhood deprivation predict schizophrenia? A nationwide Swedish family-based study of 2.4 million individuals. Schizophr Bull 41:494–502
Elovainio M, Vahtera J, Pentti J, Hakulinen C, Pulkki-Råback L, Lipsanen J, Virtanen M, Keltikangas-Järvinen L, Kivimäki M, Kähönen M (2020) The contribution of neighborhood socioeconomic disadvantage on depressive symptoms over the adult lifetime: a 32-year prospective cohort study. Am J Epidemiol. https://doi.org/10.1093/aje/kwaa026
Leventhal T, Brooks-Gunn J (2003) Moving to opportunity: an experimental study of neighborhood effects on mental health. Am J Public Health 93:1576–1582
Kling JR, Liebman JB, Katz LF (2007) Experimental analysis of neighborhood effects. Econometrica 75:83–119
Price C, Dalman C, Zammit S, Kirkbride JB (2018) Association of residential mobility over the life course with nonaffective psychosis in 1.4 million young people in Sweden. JAMA Psychiat 75:1128–1136
Kirkbride JB, Jones PB, Ullrich S, Coid JW (2014) Social deprivation, inequality, and the neighborhood-level incidence of psychotic syndromes in East London. Schizophr Bull 40:169–180
Richardson L, Hameed Y, Perez J, Jones PB, Kirkbride JB (2018) Association of environment with the risk of developing psychotic disorders in rural populations: findings from the social epidemiology of psychoses in East Anglia study. JAMA Psychiat 75:75–83
Allardyce J, Gilmour H, Atkinson J, Rapson T, Bishop J, McCreadie R (2005) Social fragmentation, deprivation and urbanicity: relation to first-admission rates for psychoses. Br J Psychiatry 187:401–406
Jelleyman T, Spencer N (2008) Residential mobility in childhood and health outcomes: a systematic review. J Epidemiol Commun Health 62:584–592
Smith SJ, Easterlow D (2005) The strange geography of health inequalities. Trans Inst Br Geogr 30:173–190
Freeman H (1994) Schizophrenia and city residence. Br J Psychiatr 164:39–50
Bakolis I, Hammoud R, Stewart R, Beevers S, Dajnak D, MacCrimmon S, Broadbent M, Pritchard M, Shiode N, Fecht D (2020) Mental health consequences of urban air pollution: prospective population-based longitudinal survey. Soc Psychiat Psychiatr Epidemiol. 44:1–13
Rudolph KE, Shev A, Paksarian D, Merikangas KR, Mennitt DJ, James P, Casey JA (2019) Environmental noise and sleep and mental health outcomes in a nationally representative sample of urban US adolescents. Environ Epidemiol 3:e056
Peterson RD, Krivo LJ, Harris MA (2000) Disadvantage and neighborhood violent crime: do local institutions matter? J Res Crime Delinq 37:31–63
Polling C, Khondoker M, Hatch S, Hotopf M, SELCoH Study Team (2014) Influence of perceived and actual neighbourhood disorder on common mental illness. Soc Psychiatry Psychiatr Epidemiol 49:889–901
Polling C, Bakolis I, Hotopf M, Hatch SL (2019) Spatial patterning of self-harm rates within urban areas. Soc Psychiat Psychiatr Epidemiol 54:69–79
Moore T, Kapur N, Hawton K, Richards A, Metcalfe C, Gunnell D (2017) Interventions to reduce the impact of unemployment and economic hardship on mental health in the general population: a systematic review. Psychol Med 47:1062–1084
Acknowledgements
This paper represents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Ioannis Bakolis and Stephani Hatch are supported by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and Ioannis Bakolis by the NIHR Applied Research Centre at King’s College Hospital NHS Foundation Trust, King’s College London. Stephani Hatch is also supported by the ESRC Centre for Society and Mental Health at King’s College London (ESRC Reference: ES/S012567/1). The views expressed are those of the author(s) and not necessarily those of the ESRC or King’s College London. Marcus Richards is funded by the Medical Research Council (MC_UU_12019/1 and 3).
Funding
Funders supported the data collection stage by providing salaries for the researchers and had no role in study design, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and final responsibility for the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
IB and MR conceived the study and elaborated the design. EM linked the data. IB did the statistical analysis. IB and MR wrote the first draft, on which all authors commented. All authors have given their approval for the publication of this manuscript and agree to be accountable for all aspects of the work to ensure that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interests
We declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakolis, I., Murray, E.T., Hardy, R. et al. Area disadvantage and mental health over the life course: a 69-year prospective birth cohort study. Soc Psychiatry Psychiatr Epidemiol 58, 735–744 (2023). https://doi.org/10.1007/s00127-023-02427-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00127-023-02427-x