Abstract
Introduction
In several claims-based studies, major depressive disorder (MDD) has been associated with increased risk of hospitalization due to acute infections. It remains unclear if this is a causal effect, and if it generalizes to an increased susceptibility to infections.
Methods
We used data of the BiDirect (n = 925) and the HaBIDS (n = 1007) cohort studies to estimate the effect of MDD on self-reported infections, which were assessed with identical infection susceptibility questionnaires in both studies. We used the Center for Epidemiologic Studies Depression Scale (CES-D) to examine if there was a dose–response relationship between depressive symptom severity and self-reported infections.
Results
BiDirect participants with MDD diagnosis (48%) had a higher risk of lower respiratory tract infections (incidence rate ratio 1.32, 95% confidence interval [1.00–1.75]), gastrointestinal infections (1.68 [1.30–2.16]) and fever (1.48 [1.11–1.98]) after adjusting for confounders identified by a directed acyclic graph approach. There was a dose–response relationship, i.e. individuals with higher CES-D scores reported more infections. Effect sizes were similar in HaBIDS (4% individuals with MDD).
Conclusion
We found increased risks of mild infections in patients with MDD diagnosis and a dose–response relationship between depressive symptom severity and infection frequency. While causal immunological pathways remain unclear, the results of our study might contribute to a change in prevention strategies, e.g. by recommending vaccination against influenza and S. pneumoniae to MDD patients because observed effect sizes in our study are similar to those of patients with cardiovascular and metabolic diseases for which the respective vaccinations are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorders (MDDs) have a profound and pervasive impact on physical health, for example, by causing immuno-inflammatory dysregulation [1]. Consequently, a link between MDD and susceptibility to infections has been proposed [2]. A register-based study from Denmark revealed MDD to be a risk factor for severe infections (e.g. hepatitis infection, lower respiratory tract infection, sepsis) leading to contact with the healthcare system [3]. In a systematic review, a variety of psychosocial factors (including the diagnosis of MDD) were found to be associated with acute respiratory tract infections (RTI) [4]. These findings are supported by more specific findings for different types of RTI. In patients with pneumonia, for example, MDD has been identified as a risk factor for hospitalization [5], mortality [6, 7], admission to an intensive care unit and the need for mechanical ventilation [7]. Similar analyses have been performed for other infectious diseases, such as gastrointestinal infections, which have as well been reported to be associated with MDD [8].
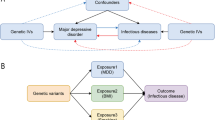
Previous studies have had specific limitations due to the use of data from health registers, hospitals or health insurances in most studies, leading to (i) the focus on more severe infectious diseases, (ii) missing information about MDD besides the ICD diagnosis (e.g. severity, medication intake) as well as (iii) a lack of a sufficiently large group of individuals with MDD to analyse different subgroups. The present study sought to investigate the effect of MDD on the occurrence of infections in a population-based setting. Specifically, this study aimed to investigate the incidence of mild infections, which do not necessarily lead to contact with the healthcare system, based on self-reports of depressed and non-depressed individuals; to investigate whether there is a dose–response relationship between the severity of depressive symptoms and risk of infections and to estimate incidence rate ratios by adjusting for confounders identified via a directed acyclic graph (DAG) approach.
Materials and methods
Study design and population
The BiDirect Study [9, 10] is an observational, prospective cohort study originally designed to investigate the bidirectional relationship between depression and (subclinical) arteriosclerosis. Between 2010 and 2013, BiDirect enrolled participants aged 35 to 66 years into three distinct cohorts: (1) patients hospitalized due to an acute episode of depression at the time of recruitment (N = 999), (2) patients shortly after myocardial infarction or an acute coronary event at the time of recruitment (N = 347) and (3) population-based control subjects randomly invited from the registry of the city of Münster, Germany (N = 912). The thorough assessment of depressive symptoms is a key element of BiDirect: For patients from cohort 1, assessment of depression was conducted during recruitment. For participants from cohorts 2 and 3, diagnosis of depression took place during the baseline examination at the study centre [10]. BiDirect participants underwent up to three follow-up examinations, with an average of two to three years between examinations. The third follow-up examination between 2018 and 2020, which included a questionnaire on the infection data analysed here, was completed by 1024 participants from the BiDirect cohorts 1 and 3 (participants from the myocardial infarction cohort were excluded from our analyses). In our analysis, we restricted all analyses to this population and then excluded all participants with self-reported intake of systemic immune-modulating medication (n = 32) based on ATC codes (i.e. corticosteroids for systemic use, antineoplastic agents, immunostimulants, immunosuppressants).
The HaBIDS study (Hygiene and Behaviour Infectious Diseases Survey) was a longitudinal online panel aiming to assess hygiene practices and behaviour regarding various infectious diseases in the federal state of Lower Saxony, Germany. The detailed description of the applied methodology is presented elsewhere [11, 12]. In brief, almost 27,000 males and females between 15 and 69 years of age were invited to participate in the panel. Potential participants were randomly selected from the population registries in urban (Braunschweig, Salzgitter and Wolfenbüttel) and rural areas (Vechta). Each month, participants completed questionnaires on different aspects of infectious diseases. Among 2379 individuals who had consented to participate in the panel (8.9% initial response rate) 1151 filled in both a questionnaire about medical history (in summer 2015) and a questionnaire about frequency of infections and infection-associated symptoms within the preceding 12 months (in spring 2014). In addition, HaBIDS participants were asked in May 2015 (873/1151) and in May 2016 (788/1151) about the frequency of RTI (e.g. cold, flu or otitis media) within the preceding 12 months, respectively. We refer to these variables as RTI from the seasons 2014/15 and 2015/16.
Definition of exposure
The primary exposure of interest was life-time diagnosis of MDD at baseline. In BiDirect, all participants in cohort 1 were exposed (N = 361) as well as participants in cohort 3 who had received an MDD diagnosis at baseline (N = 79). In HaBIDS, exposure classification was determined by the self-report of MDD in the medical history survey. To investigate the effect of recent depressive symptom severity on infectious diseases, we used in BiDirect the German version of the Center for Epidemiologic Studies Depression Scale (CES-D [13]) as well as treatment with antidepressant medication. The CES-D summary score was categorized in four different categories as described in the literature [14]: not depressed (< 10 points), mild depression (10–15 points), moderate depression (16–24 points) and severe depression (≥ 25 points). Classification of the CES-D score was independent of the life-time diagnosis of MDD, i.e. a participant without life-time diagnosis of MDD could be categorized as “depressed” if the recent CES-D score was above 9 points. Treatment with antidepressant medication was coded by ATC codes (i.e. non-selective monoamine reuptake inhibitors, selective serotonin reuptake inhibitors, non-selective monoamine oxidase inhibitors, monoamine oxidase A inhibitors, lithium, other antidepressants).
Definition of outcome
The outcomes of interest were self-reported infectious diseases and infection-related variables within the 12 months preceding the respective questionnaire [15]. The BiDirect questionnaire was filled in during the third follow-up examination, and included upper respiratory tract infections (URTI), lower respiratory tract infections (LRTI), cystitis, gastrointestinal (GI) infections, fever and intake of antibiotics. From the HaBIDS study, we used information on the frequency of URTI, LRTI, RTI from the seasons 2014/15 and 2015/16, as well as on cystitis, long-lasting coughing, fever, diarrhea and herpes labialis in spring 2014. For all variables of BiDirect, except for intake of antibiotics, and for all variables of HaBIDS, except for RTI in the 2014/15 and 2015/16 seasons, the questionnaire included seven answer options. For the analysis of all answers including a range of values, we used the lower one (all used values in brackets): “Never” (0), “Once” (1), “Twice” (2), “3 to 4 times” (3), “5 to 6 times” (5), “More than 6 times” (7) and “Don’t know” (Missing). We created the variable “any RTI” as the sum of URTI and LRTI. For the intake of antibiotics in BiDirect and for RTI from 2014/15 and 2015/16 in HaBIDS, we used the following values: “Never” (0), “Once” (1), “Twice” (2), “3 to 4 times” (3), “More than 4 times” (5) and “Don’t know” (Missing). We excluded participants (BiDirect: N = 62; HaBIDS: N = 144) who had any missing value in the outcome variables (except for RTI in 2014/15 and 2015/16 because they were, by design, only completed by a subset of participants). If the outcome variable has missing values, then the incomplete cases do not contain any information about the exposure coefficient; in these cases, complete case analysis is recommended [16].
Definition of covariables
Confounders of the effect of MDD diagnosis on self-reported infections were identified by using a DAG (Online Resource 1). Whether a variable or an arrow between two variables was included and how these arrows were directed were decided based on published study results (Online Resource 2). There was evidence for bidirectional associations between five pairs of variables. For the DAG, we decided on one direction (as reported in Online Resource 2). Using the R package dagitty (version 0.3–1, code available at zivgitlab.unimuenster.de/ruebsame/mdd_infection_germany_bidirect_habids) [17], we identified the following minimal sufficient adjustment set (MSAS): age, sex, socioeconomic status, body mass index (BMI), household size, smoking, alcohol intake, physical activity, nutritional status, stress, intake of proton pump inhibitors, asthma bronchiale, chronic obstructive pulmonary lung disease (COPD), diabetes mellitus, heart failure, chronic kidney disease, stroke and cancer. The MSAS did not change if the direction of the five arrows in question was reversed in the DAG. We checked whether the assumptions encoded in the DAG were consistent with the data using dagitty’s function localTests.
In the analysis of the BiDirect data, we adjusted for age, sex, socioeconomic status (years of education, marital status, income), BMI, household size, smoking, alcohol intake (g/d), physical activity (IPAQ-level [18]), stress (PSS-14 [19]), intake of proton pump inhibitors, chronic lung disease (covering asthma bronchiale and COPD), diabetes mellitus, heart failure, chronic kidney disease and stroke. We did not adjust for nutritional status because it was measured with uncertainty. We adjusted for cancer by excluding all participants with intake of systemic immune-modulating medication as described above. In HaBIDS, the dataset included the following variables to adjust for age, sex, socioeconomic status (education level, marital status, income), household size, smoking, alcohol intake (AUDIT-C [20]), stress (PSS-4 [19]) and comorbidities (i.e. chronic lung disease, diabetes mellitus, heart failure, chronic kidney disease, stroke). We were not able to adjust for BMI, intake of proton pump inhibitors, physical activity, nutritional status and cancer in the analysis of the HaBIDS study.
Statistical analysis
All analyses were performed with R version 4.1.3 (www.R-project.org). We used negative binomial regression models to estimate incidence rate ratios (IRR) and 95% confidence intervals (CI). P value functions were calculated by using the R package pvaluefunctions (version 1.6.2) [21]. To evaluate differences of infection frequency in participants with vs. without antidepressant treatment, we categorized BiDirect participants into three groups: no MDD (reference group; n = 485), MDD without medication (n = 198) and MDD with medication (n = 242). Missing values in confounder variables were imputed (R package mice [22] version 3.14.0) with five multiple imputations. Regression coefficients were estimated in each imputed dataset separately and pooled afterwards. Missing values in outcome variables were not imputed as discussed above.
Quantitative bias analysis
As MDD diagnosis was ascertained by trained staff in BiDirect, but based on self-report in HaBIDS, we explored whether misclassification of this exposure biased our estimate of effect in HaBIDS. We assumed that any misclassification would be non-differential, i.e. not dependent on the outcome, so we used a wide range of values for sensitivity and specificity (10–100%) to correct the observed estimate of effect with the method suggested by Fox et al. [23].
The outcome could be misclassified due to mood-congruent memory, i.e. that individuals with depression recall more negative biographical events than controls without depression [24]. Using the BiDirect data (where we could adjust for nearly all confounders), we explored three different scenarios: 1) that all participants with recent CES-D score ≥ 16 points; 2) that 50% and 3) that 90% of all participants with MDD diagnosis reported too many infections. In each scenario, we reclassified the respective participants to the adjacent lower outcome category (e.g. from “Twice” to “Once”) and re-estimated the IRR as described above. We did not reclassify participants from “Once” to “None” because we assumed that participants with MDD diagnosis would falsely report the number of infections, but not their actual occurrence. The R code for the bias analyses is available at zivgitlab.uni-muenster.de/ruebsame/mdd_infection_germany_bidirect_habids.
Results
Of all 925 participants of the BiDirect cohort study included into our analysis, 440 participants (48%) had received a diagnosis of MDD in the baseline examination (Table 1). Forty-one of all 1007 participants of the HaBIDS study with information about medical history reported a diagnosis of depression in their lifetime at baseline (4%) (Table 1). Most assumptions encoded in the DAG were consistent with the data (Online Resource 3).
In BiDirect, all estimates were consistently greater than one (Table 2), albeit some 95% CI included “no association” (i.e. IRR = 1). The p value functions (Online Resource 4) showed that there was the same amount of evidence for “no association” as there was for increased incidence rates (counternull IRR between 1.11 for URTI and 2.18 for fever). In HaBIDS, all estimates except for LRTI and RTI in season 2014/2015 were consistently greater than one (Table 2); the confidence intervals, however, were wide. The p value functions (Online Resource 4) showed that there was the same amount of evidence for “no association” as there was for increased incidence rates of most outcomes (counternull IRR between 1.31 for LRTI and 3.13 for fever) except for RTI in season 2014/2015 (counternull IRR = 0.72) and URTI (counternull IRR = 0.98).
For all outcome variables (except for URTI) we found a dose–response relationship between depressive symptom severity (measured by CES-D) and risk of infection (Table 3), i.e. the IRR increased when depressive symptom severity increased from mild over moderate to severe.
Intake of antidepressant medication was consistent with MDD being protective, except for GI infections (Table 4), but 95% CI were widely overlapping.
Quantitative bias analysis
If the specificity of the MDD diagnoses in HaBIDS was 100%, then the corrected estimates with any sensitivity < 100% would be greater than the observed estimates. If both sensitivity and specificity were smaller than 100%, then the corrected estimates would be smaller than the observed estimates.
If all participants with recent CES-D score ≥ 16 points reported too many infections, then the corrected estimates would decrease in magnitude, but would still be consistently above one (Online Resource 5). The same would be true if 50% of all participants with MDD diagnosis reported too many infections (Online Resource 5). Only if 90% of all participants with MDD diagnosis reported too many infections, the corrected estimates of URTI, any RTI, cystitis and intake of antibiotics would change to below one (Online Resource 5).
Discussion
MDD was associated with an increased incidence of different mild infections and infection-related variables in the BiDirect cohort study as well as in the HaBIDS study. While we found a non-null effect of MDD on the risk of LRTI, this was not true for URTI. Previous studies with similar results [3, 5, 25] had different limitations. While these studies mostly used routine data sources from health insurances companies, health registers or hospitals and therefore focused on severe outcome variables (e.g. mortality, hospitalization) or ICD diagnoses, our study is the first one using self-reported infections as outcomes in a population-based setting. Self-reported acute infections (bronchitis, ear infection, sinus infection, Streptococcal pharyngitis) have so far only been investigated among US college students aged 18–24 years [2]. We were able to show susceptibility to infection in two cohort studies covering a broad age range (15–74 years). This is important because MDD is a chronic disease and changes in the immune system may occur also in advanced age.
We found a monotone dose–response relationship between self-reported infections during the past 12 months and self-reported depressive symptom severity during the past seven days measured by the CES-D. The CES-D covers only the past seven days, but can still be used as a good proxy for MDD because of the chronicity of MDD and the long duration of depressive episodes. The CES-D has a very good validity, discriminating well between individuals with depression and healthy control subjects [13]. Other studies have also investigated the dose–response relationship, but did not use the CES-D score: Adams et al. found a dose–response relationship between feeling exhausted and acute infectious diseases among US college students [2]. Andersson et al. found no linear relationship between the amount of depressive episodes and infection frequency due to the lack of sufficient data of individuals with several depressive episodes and infection diagnosis [3]. Using the CES-D, a more depression-related instrument than measuring exhaustion, we were able to show a monotone relationship between the sum of a broad spectrum of typical depressive symptoms during the past seven days and infection frequency, adding more evidence to answering the question of a possible dose–response relationship between MDD diagnosis and infection.
The analysis of self-reported medication intake during the past four weeks shows an elevated risk of infections in MDD patients both with and without antidepressant medication compared to individuals without MDD; however, infection frequency was reduced in MDD patients with medication compared to MDD patients without medication so that treating MDD with antidepressants seems to reduce the risk of infections. While this effect of antidepressant medication on infectious diseases has not previously been studied, there are several findings of an anti-inflammatory effect of antidepressant treatment on the level of cytokine production [26]. One exception is the frequency of gastrointestinal infections which is higher in MDD patients with antidepressant medication compared to MDD patients without. Since gastrointestinal infections are in general difficult to differentiate from other causes of gastrointestinal symptoms (i.e. diarrhea, vomiting), gastrointestinal side effects of antidepressants might add a source of bias to the self-report of infections.
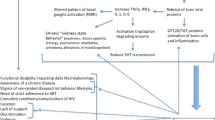
There are different mechanisms that can explain the effect of depression on infectious diseases. First, a dysregulation of the immune system in individuals with depression has been reported in several studies [27, 28]. A further link between the immune system and depression is reported on the level of the gut microbiome [26]: There are findings of specific bacterial taxa whose changed occurrence is associated with depression as well as with changes of anti-inflammatory signals, e.g. Coprococcus bacteria that are active in the dopamine pathway [29]. In addition, other factors as poor self-care in patients with depression or reduced compliance in general medication intake could as well contribute to the observed effects [30].
If our results are replicated in other studies, infection prevention strategies, like vaccination recommendations, might need to be extended to individuals with depression. The German National Public Health Institute (Robert Koch Institute) recommends vaccination against influenza and Streptococcus pneumoniae for individuals with various comorbidities, including those with type 2 diabetes [31], who showed in a large matched cohort study an elevated risk of LRTI (1.40 [1.38–1.43]) and pneumonia (1.58 [1.53–1.64]) [32]. These results are on the same level as our findings for LRTI (1.32 [1.00–1.75]), indicating a similar relative risk of LRTI in individuals with depression and diabetes type 2.
Strengths and limitations
For the first time, this study (i) adjusted for all confounders identified by the systematic approach of a DAG, (ii) describes a monotone dose–response effect and (iii) shows the effect of antidepressant treatment on infection frequency. Although some assumptions encoded in the DAG were not consistent with the data, we decided against changing the DAG post-hoc to avoid “overfitting” the DAG to the specific dataset that the tests were performed on. We present justifications for all arrows in the DAG, and the composition of the MSAS was insensitive to changes in the direction of the few arrows of bidirectional relationships.
The BiDirect cohort study provides data of high quality to examine changes in individuals with MDD. Using the CES-D in addition to the MDD diagnosis from the baseline examination enabled us to show higher risk of infections for depressed individuals with two different definitions of depression. We assume that there is no misclassification of the MDD diagnosis in BiDirect because of the thorough assessment of depressive symptoms in this study. MDD might, however, been misclassified in HaBIDS. Our bias analysis indicates that as long as all non-depressed participants do not self-report any MDD diagnosis (specificity = 100%), misclassification would result in a bias towards null. If misclassification affected both depressed and non-depressed participants, our results would overestimate the true effect. The consistent results in the BiDirect study indicate that misclassification might have been low in HaBIDS.
Using self-reported infections as an outcome variable is both a strength and a limitation of our study. It reduces bias based on differential health seeking behaviour of depressed and non-depressed individuals. Although our results are supported by other studies not using self-reported infections, they might be influenced by information bias. For instance, mood-congruent memory bias describes the fact that individuals with depression recall more negative biographical events than healthy controls [24]. Depression is also associated with memory impairments [33] due to reduced hippocampal volume and other physiological changes [34]. Thus, the number of infections being remembered might be influenced by different effects leading to either higher or lower numbers. Our bias analysis indicates that overreporting of the number of infections by many depressed participants could have resulted in false-positive associations between MDD and some, but not all outcomes; the main findings regarding LRTI, GI infections, and fever cannot be explained by information bias alone.
For all infection variables we used the lower of two numbers in cases where answer possibilities included a range of numbers. That means that our analysis is likely to underestimate the true effect on the infection variables.
While we were able to adjust for most confounders proposed by the DAG in the regression model of BiDirect data, there were several confounders not available in the HaBIDS dataset. Self-selection of the participants, e.g. by loss to follow-up, could have biased our results, but the two studies probably exhibit different mechanisms of selection due to their varying study designs (long study period and in-person examinations in BiDirect versus short study period and online questionnaires in HaBIDS). However, results in both cohorts point into the same direction, underlining the reliability of our findings.
Conclusions
Depression increases the susceptibility to different mild infectious diseases in two German cohort studies. Further research is needed to replicate this finding and to determine causal immunological pathways in more detail and to differentiate the components of the bidirectional relationship between depression and infection. The inclusion of depression into infection prevention strategies, like vaccination recommendations, should be part of further discussion.
Data/code availability
The R code is available at zivgitlab.uni-muenster.de/ruebsame/mdd_infection_germany_bidirect_habids. The data used in this analysis are available from the corresponding author upon reasonable request.
References
Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N (2013) Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med 11:129
Adams TB, Wharton CM, Quilter L, Hirsch T (2008) The association between mental health and acute infectious illness among a national sample of 18- to 24 year-old college students. J Am Coll Health 56:657–663
Andersson NW, Goodwin RD, Okkels N, Gustafsson LN, Taha F, Cole SW et al (2016) Depression and the risk of severe infections: prospective analyses on a nationwide representative sample. Int J Epidemiol 45:131–139
Falagas ME, Karamanidou C, Kastoris AC, Karlis G, Rafailidis PI (2010) Psychosocial factors and susceptibility to or outcome of acute respiratory tract infections. Int J Tuberc Lung Dis 14:141–148
Davydow DS, Hough CL, Zivin K, Langa KM, Katon WJ (2014) Depression and risk of hospitalization for pneumonia in a cohort study of older Americans. J Psychosom Res 77:528–534
DeWaters AL, Chansard M, Anzueto A, Pugh MJ, Mortensen EM (2018) The association between major depressive disorder and outcomes in older veterans hospitalized with pneumonia. Am J Med Sci 355:21–26
Kao L-T, Liu S-P, Lin H-C, Lee H-C, Tsai M-C, Chung S-D (2014) Poor clinical outcomes among pneumonia patients with depressive disorder. PLoS ONE 9:e116436
Nudel R, Appadurai V, Schork AJ, Buil A, Bybjerg-Grauholm J, Børglum AD et al (2020) A large population-based investigation into the genetics of susceptibility to gastrointestinal infections and the link between gastrointestinal infections and mental illness. Hum Genet 139:593–604
Wersching H (2012) Berger K [New cohorts. The BiDirect study]. Bundesgesundheitsblatt 55:822–823
Teismann H, Wersching H, Nagel M, Arolt V, Heindel W, Baune BT et al (2014) Establishing the bidirectional relationship between depression and subclinical arteriosclerosis - rationale, design, and characteristics of the BiDirect Study. BMC Psychiatry 14:1–9
Rübsamen N, Akmatov MK, Castell S, Karch A, Mikolajczyk RT (2017) Comparison of response patterns in different survey designs: a longitudinal panel with mixed-mode and online-only design. Emerg Themes Epidemiol 14:1–11
Rübsamen N, Akmatov MK, Castell S, Karch A, Mikolajczyk RT (2017) Factors associated with attrition in a longitudinal online study: results from the HaBIDS panel. BMC Med Res Methodol 17:132
Radloff L (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401
Kim J, Chung H, Askew RL, Park R, Jones SMW, Cook KF et al (2017) Translating CESD-20 and PHQ-9 scores to PROMIS depression. Assessment 24:300–307
Hassenstein MJ, Aarabi G, Ahnert P, Becher H, Franzke C-W, Fricke J et al (2020) Self-reported infections in the German National Cohort (GNC) in the context of the current research landscape. Bundesgesundheitsblatt 63:404–14
Hughes RA, Heron J, Sterne JAC, Tilling K (2019) Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol 48:1294–1304
Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTTH (2016) Robust causal inference using directed acyclic graphs: The R package “dagitty.” Int J Epidemiol Narnia 45:1887–1894
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE et al (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395
Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24:385–396
Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA (1998) The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med 158:1789–1795
Infanger D, Schmidt‐Trucksäss A (2019) P value functions: an underused method to present research results and to promote quantitative reasoning. Stat Med 38:4189–4197
van Buuren S, Groothuis-Oudshoorn K (2011) mice: multivariate imputation by chained equations in R. J Stat Softw 45:1–67
Fox MP, MacLehose RF, Lash TL (2021) Applying quantitative bias analysis to epidemiologic data. Springer International Publishing, Cham
Watkins PC (2002) Implicit memory bias in depression. Cogn Emot 16:381–402
Davydow DS, Ribe AR, Pedersen HS, Vestergaard M, Fenger-Grøn M (2016) The association of unipolar depression with thirty-day mortality after hospitalization for infection: a population-based cohort study in Denmark. J Psychosom Res 89:32–38
Beurel E, Toups M, Nemeroff CB (2020) The bidirectional relationship of depression and inflammation: double trouble. Neuron 107:234–256
Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS et al (2017) Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135:373–387
Leighton SP, Nerurkar L, Krishnadas R, Johnman C, Graham GJ, Cavanagh J (2018) Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry 23:48–58
Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY et al (2019) The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4:623–632
Katon WJ (2003) Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry 54:216–226
Ständige Impfkommission (2020) [Recommendations of the Standing Committee on Vaccination at the Robert Koch Institute 2020/2021]. Epid Bull 2020(34):1–68
Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG (2018) Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 41:513–521
Burt DB, Zembar MJ, Niederehe G (1995) Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 117:285–305
MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT et al (2005) Course of illness, hippocampal function, and hippocampal volume in major depression. Focus (Madison) 3:146–155
Acknowledgements
The BiDirect Study is funded by the German Federal Ministry of Education and Research (Klaus Berger, Grant Numbers 01ER0816, 01ER1506).
Funding
Open Access funding enabled and organized by Projekt DEAL. The BiDirect Study is funded by the German Federal Ministry of Education and Research (Klaus Berger, Grant Numbers 01ER0816, 01ER1506).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The BiDirect Study was approved by the Ethics Committee of the University of Münster (No. 2009–391-f-S) and the Westfalian Chamber of Physicians in Münster, North-Rhine-Westfalia, Germany. The HaBIDS study was approved by the Ethics Committee of Hannover Medical School (No. 2021–2013) and by the Federal Commissioner for Data Protection and Freedom of Information in Germany.
Consent to participate
Written informed consent was obtained from all participants in case of the BiDirect and HaBIDS cohort studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elpers, H., Teismann, H., Wellmann, J. et al. Major depressive disorders increase the susceptibility to self-reported infections in two German cohort studies. Soc Psychiatry Psychiatr Epidemiol 58, 277–286 (2023). https://doi.org/10.1007/s00127-022-02328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00127-022-02328-5