Abstract
To evaluate the fertility of porphyry mineralization in the Delamerian Orogen (South Australia), zircon and apatite from four prospects, including Anabama Hill, Netley Hill, Bendigo, and Colebatch, have been analyzed by LA-ICP-MS and electron microprobe. The zircon is characterized by heavy REEs enrichment relative to light REEs, high (Ce/Nd)N (1.3–45), and weak to moderate negative Eu/Eu* (0.2–0.78). The apatite has right-sloped REE patterns with variably negative to positive Eu anomalies. Low Mg (< 670 ppm) and Sr/Y ratios (< 5) in apatite likely illustrate fractional crystallization trends for the granitic melts in shallow crust. The Yb/Gb and Eu/Eu* in zircon reveal that intrusions at Anabama Hill, Netley Hill, and Bendigo underwent fractional crystallization controlled by amphibole (< 50–60%), garnet (< 15%), apatite (< 0.6%), and/or titanite (< 0.3%). These stocks have average fO2 values reported relative to fayalite-magnetite-quartz buffer (ΔFMQ), from 0.7 ± 0.9 to 2.1 ± 0.4, ascribed to prolonged magmatic evolution or sulfur degassing during post-subduction processes. Our data imply that both Anabama and Bendigo complexes experienced prevalent (garnet-) amphibole crystallization from hydrous melts that have moderately high oxidation (ΔFMQ + 1 to + 3) and elevated sulfur-chlorine components (Anabama, 37 ± 9 to 134 ± 83 ppm S and 0.30 ± 0.24 to 0.64 ± 0.89 wt% Cl; Bendigo, 281 ± 178 to 909 ± 474 ppm S and 0.45 ± 0.47 to 3.01 ± 1.54 wt% Cl). These are crucial ingredients to form porphyry Cu–Mo ± Au ores with economic significance, which provides encouragement for mineral exploration in this orogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Porphyry Cu–Mo ± Au deposits provide approximately 75% Cu, 50% Mo, 20% Au of the world’s resources, and significant amounts of Re, Ag, and other metal resources to support societal development (Sillitoe 2010). Porphyry Cu–Mo mineralization was recognized in the late 1960s to early 1970s in the Delamerian Orogen (Hosking 1970; Langsford 1971; Morris 1977), which is predominantly located in the southeast of South Australia (Fig. 1a). The Delamerian Orogen was proximal to an Andean-style convergent subduction belt active from middle Cambrian to early Ordovician along the eastern margin of the Archean-Proterozoic Australian craton (Preiss 2000; Kemp et al. 2009; Cayley 2011; Foden et al. 2020). No economically important porphyry-style deposits have been discovered in this early Paleozoic magmatic arc during the past five decades, largely due to limited exploration activity and deficits in geological understanding of the regional mineral systems. The recent discovery of the Thursday’s Gossan Cu–Mo ± Au deposit, associated with porphyritic intrusions in the adjacent Stavely Arc of Victoria (28.3 Mt at 0.75% Cu and 0.11 g/t Au; Stavely Minerals 2022), has led to renewed attention to explore porphyry-style ore deposits in the Delamerian greenfield province.

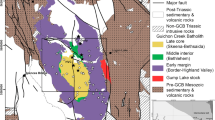
(modified from Gravestock et al. 2012). b-d. Sketch geologic maps for Delamerian porphyry prospects highlighting studied drill hole sites. b. Anabama Hill, adapted from McDonald (1992). c. Netley Hill, adapted from Hosking (1970). d. Bendigo, adapted from Langsford (1973). Inset showing the Orogenic belts of eastern Australia after Foden et al. (2020). Legend for b, c, and d are same as that included in Fig. d
a. Simplified geologic map of the Delamerian Orogen in South Australia.
During the past decades, increasing interest has been paid to in situ chemical analysis of magmatic minerals, including zircon and apatite, and its application to identifying the presence of, or potential for porphyry-style mineralization (e.g., Ballard et al. 2002; Belousova et al. 2006; Dilles et al. 2015; Bouzari et al. 2016; Lu et al. 2016, 2019; Mao et al. 2016; Nathwani et al. 2020; Parra-Avila et al. 2022). These minerals are defined as porphyry indicator minerals (Celis et al. 2013; Bouzari et al. 2016; Cooke et al. 2020). Trace and rare earth elements (REEs) in zircon such as Th, U, Y, Ce, Eu, Gd, and Yb, have been found to discriminate the metallogenic fertility of causative magmas genetically related to porphyry mineralization (Ballard et al. 2002; Chelle-Michou et al. 2014; Dilles et al. 2015; Shen et al. 2015; Lu et al. 2016; Lee et al. 2017, 2020; Loader et al. 2017; Nathwani et al. 2021; Paolillo et al. 2022; Parra-Avila et al. 2022). Apatite can directly archive the volatile signatures (e.g., S and Cl) of magmatic systems from which it crystallizes, which can distinguish mineralized intrusions from barren plutons (Chelle‑Michou and Chiaradia 2017; Richards et al. 2017; Zhu et al. 2018; Cao et al. 2021; Xu et al. 2021; Parra-Avila et al. 2022; Xie et al. 2023). Analyses of these minerals can provide crucial information to determine whether studied magmatic suites in a porphyry prospect are hydrous, oxidized, and sulfur-rich – all of which are prerequisites to exsolving fluids for extensive hydrothermal alteration and porphyry-style mineralization (Richards 2003, 2015; Seedorff et al. 2005; Sillitoe 2010; Cooke et al. 2014). Therefore, zircon and/or apatite chemistry are regarded as robust tools utilized on a district- to regional-scale at an early exploration stage, with the aim to target intermediate to felsic complexes or individual intrusions within such complexes (Bouzari et al. 2016; Loader et al. 2017; Cooke et al. 2020; Xing et al. 2020; Li et al. 2021; Nathwani et al. 2021; Parra-Avila et al. 2022), which are likely to host porphyry Cu–Mo ± Au ore deposits.
Geologic context
The Delamerian Orogen is the westernmost and earliest episode of the Tasmanides accretionary orogenic systems (~ 520–230 Ma; Fig. 1a) that form the eastern third of the Australian continent (Foster and Gray 2000; Preiss 2000; Kemp et al. 2009; Cayley 2011; Johnson et al. 2016; Foden et al. 2020). The orogen is best exposed in South Australia, known as the Adelaide Fold Belt, and extends eastwards to the Grampians-Stavely Belt in western Victoria (Fig. 1a; Foster and Gray 2000; Cayley 2011; Schofield 2018), and northeast to Loch Lilly-Kars-Koonenberry Belt in New South Wales (Fig. 1a; Greenfield et al. 2011; Johnson et al. 2016).
The Delamerian Orogen comprises Neoproterozoic to Cambrian sedimentary rock packages, with lesser intercalated volcanic units, which formed during successive phases of rift and sag events at the margin of the Archean-Proterozoic crust of southern Australia (Preiss 2000; Foden et al. 2006, 2020; Robertson et al. 2015; Lloyd et al. 2020). The Cambrian volcano-sedimentary rocks include the Normanville Group and succeeding Kanmantoo Group that were deposited in a deepening rift system on the eastern edge of the Neoproterozoic (Adelaidean) rift system (Fig. 1a; Preiss 2000; Foden et al. 2002, 2020; Betts et al. 2018; Lloyd et al. 2020, 2022). These Neoproterozoic to Cambrian rocks experienced multiple episodes of deformation and greenschist to amphibolite facies metamorphism from the middle to late Cambrian (i.e., the Delamerian Orogeny, 514–490 Ma; Foden et al. 2006), as a result of initial subduction of the Pacific oceanic crust and the imbrication and thickening of the Adelaide Superbasin during outboard volcanic arc development (Cayley 2011; Greenfield et al. 2011; Betts et al. 2018; Robertson et al. 2015; Schofield 2018; Foden et al. 2020). Syn-tectonic S- and I-type plutons and granitic sheets (~ 514–490 Ma) intruded the Neoproterozoic and Cambrian rocks during the early phases of the Delamerian Orogeny, while largely A-type granitic bodies were intruded late and subsequent to the main orogenic activity (490–470 Ma; Turner et al. 1992; Foden et al. 2006, 2020).
Porphyry and skarn Cu–Mo ± Au prospects recognized in the Delamerian Orogen (Fig. 1) include Anabama Hill, Blue Rose, Netley Hill, Cronje Dam, Bendigo, Colebatch, and Wild Horse in South Australia (Hosking 1970; Langsford 1971; Morris 1977). This porphyry Cu belt stretches southeast towards the Stavely Arc in Victoria, evidenced by the discovery of the Thursday’s Gossan and Junction porphyry Cu–Au deposits associated with porphyritic stocks from 505 to 498 Ma (Duncan et al. 2018; Stavely Minerals 2022). As opposed to Cambrian counterparts, Baatar et al. (2020) argued that Devonian monzonitic porphyries in the Loch Lilly-Kars Belt of New South Wales have the greater metallogenic potential for porphyry mineralization.
Detailed descriptions of hydrothermal alteration, vein stages, and mineralization regarding these prospects can be found in Hong et al. (2023), and concise summaries of related intrusive rocks, mineralization ages, hydrothermal alteration, and sulfide associations are listed in ESM2 Table S1. Granodiorite batholiths are the dominated intrusive phases, and diorite porphyry, monzonite, dacite porphyry and/or granite host sulfide mineralization and hydrothermal alteration (Fig. 1b-d). These intrusions have zircon U-Pb ages ranging from 490 to 465 Ma (Hong et al. 2023). Molybdenite Re-Os chronology revealed two porphyry Cu-Mo mineralization events at ca. 480 Ma and 470–460 Ma, respectively, with the later prevailing at Anabama Hill, Netley Hill, and Bendigo (Hong et al. 2023). Hydrothermal alteration and mineralization in the Delamerian prospects are comparable to typical porphyry Cu-Mo deposits, with Cu-bearing sulfides being hosted by multiple veins, stockworks, and minor breccias. These prospects are characterized by intense muscovite-quartz-pyrite assemblages (phyllic alteration) that commonly overprint weak to moderate K-feldspar ± biotite alteration (potassic) and chlorite-epidote ± albite alteration (propylitic; Fig. 1b-d). Zircon and apatite crystals were separated from drill core and hand specimen samples for in situ micro-analyses. These rocks include the granodiorite, diorite, monzogranite and dacite porphyry from Anabama Hill, granodiorite, diorite, biotite granite and K-feldspar granite from Netley Hill, and granodiorite, biotite granite and porphyritic diorite from Bendigo prospect, as well as the Colebatch granite.
Analytical techniques
SEM-BSE-CL imaging and EPMA analysis
Zircon and apatite grains were hand-picked from heavy mineral concentrates of crushed granitic rocks under a petrographic microscope. Selected mineral grains were placed on double-sided tape and then set into 2.5-cm diameter epoxy blocks. These mounts were polished using clean sandpaper and polishing cloths down to 1 μm diamond grade. Additional six samples from Anabama Hill and Bendigo were prepared as polished 2.5-cm rock blocks for apatite analyses (see details in ESM A1). In order to reveal internal textures and inclusions within individual grains prior to micro-analyses, cathodoluminescence (CL) and backscatter electron (BSE) images of investigated minerals were collected simultaneously on a Hitachi SU3800 SEM at Adelaide Microscopy in the University of Adelaide. For both minerals, the SEM was operated at voltage of 15 to 20 kV, spot intensity of 80, and sample depth of 10 to 15 mm.
Major element and halogen compositions for apatite were performed on a Cameca SX-Five electron probe microanalyzer (EPMA), equipped with five tunable wavelength-dispersive spectrometers at Adelaide Microscopy. The instrument runs PeakSite v6.2 software for microscope operation, and Probe for EPMA software (distributed by Probe Software Inc.) was used for all data acquisition and processing. The EPMA was operated with a 10-kV excitation voltage, 15–20 nA beam current, and 5-µm beam diameter during the apatite sessions. Analytical elements include Ca, P, Si, Fe, Mn, Na, F, Cl, and S. Oxygen was calculated by stoichiometry, assuming that all Fe was Fe2+. Matrix corrections of Armstrong-Love/Scott w(ρz) and Henke mass absorption coefficients were used for data reduction. Beam damage and alkali element migration in silicate analyses were minimized using a defocused electron beam, in addition to use of the mean atomic number background correction (Donovan et al. 2016) over traditional two-point background interpolation. This reduces the aforementioned effects of beam damage and element migration and their subsequent impact on the quality of analysis. Mobile elements (e.g., Na, F, and Cl) were analyzed first on the detector and the time-dependent intensity correction feature of Probe for EPMA was used, aimed to monitor and review post-analysis. All individual results for major and halogen elements of apatite are presented in Electronic Supplementary Material (ESM) A1.
LA-ICP-MS analysis
Both zircon and apatite trace element analyses were performed at Adelaide Microscopy, using an Agilent 7900x quadrupole ICP-MS with a RESOlution 193 nm ArF excimer laser system. Instrument mass bias and drift were corrected using standard materials analyzed after every 20–30 unknowns. For zircon session, the GJ zircon (Jackson et al. 2004) was used as the primary standard to correct for Pb/U and Pb/Th mass bias, down hole fractionation, and instrument drift, whereas zircons 91,500 and Plesovice were used as secondary standards. NIST610 glass was used as an external standard for trace element analysis (Pearce et al. 1997). Each zircon analysis began with a 30 s blank gas measurement followed by a further 30 s of analysis time when the laser was switched on. Zircons were analyzed with 30 μm spots and NIST610 glass with 43 μm spots, using a repetition rate of 5 Hz and an energy density of approximately 2 J/cm2 (3.5 J/cm2 for NIST610). A flow of He carrier gas (0.38 L/min) carried particles ablated by the laser out of the sample chamber to be mixed with Ar gas (1.01 L/min), before transport to the plasma torch. LA-ICP-MS data reduction was conducted using LADR software (https://norsci.com/ladr). Representative analytical zircon spots with CL images can be referred to Hong et al. (2023).
Zirconium was used as the internal standard with an assumed stoichiometric concentration of 49.29 wt%. 238U/206Pb age outliers apparently above or below the array of a 238U/206Pb age population are explained due to inheritance or Pb loss and thus were removed. Analyses with age discordance > 5% were discarded (discordance = 100 × [(207Pb/235Uage) – (238U/206Pbage)] / [207Pb/235Uage]). Silicon content > 185,000 ppm and U > 4,000 ppm are most likely due to micro-scale inclusions (e.g., silicates and/or U-rich minerals) within zircons. Results with Ti > 50 ppm were interpreted as ablation of Ti-Fe oxide inclusions. Lanthanum > 1 ppm commonly reflects apatite inclusions in zircon (e.g., Lu et al. 2016). Taking the approximately 0.2 ppm analytical uncertainty (2σ) into account, analyses with La < 1.2 ppm were considered free of apatite inclusions. Light rare earth element index (LREE-I = [Dy/Nd]+[Dy/Sm]) < 10 was used to screen out zircon grains that underwent apparent alteration (Bell et al. 2016). Individual trace element results for analyzed trace elements in standards and zircon samples, including U-Pb ages are tabulated in ESM A2.
For apatite session, the 401 apatite (Thompson et al. 2016) was used as the primary standard to correct for mass bias, down hole fractionation, and instrument drift. NIST612 glass (Pearce et al. 1997) was used as an external standard for trace element determination. Each apatite analysis began with a 30 s blank gas measurement followed by a further 40 s of analysis time when the laser was switched on. Apatite grains were analyzed with 30 μm spots and NIST612 glass with 43 μm spots, using a repetition rate of 5 Hz and an energy density of approximately 3.5 J/cm2. Related parameters for He and Ar gas flow are same to the zircon session described above. Representative analytical spots with CL-BSE images are presented in ESM1 Fig. S1.
Stable time-resolved LA-ICP-MS spectra were typically chosen to start at 35 s and end at 70 s using LADR. Spectra with anomalous spikes were interpreted as ablation of inclusions in the apatite and were then avoided. All apatite mineral data were normalized to 97.5 wt% oxides, with Ca used as the internal standard. Results with Ca of 36.5–40.5 wt% and P of 15.5–19.5 wt% are considered as apatite. High Si (normally > 1 wt%), Al (> 1000 ppm), Fe (> 2500 ppm), Mg (> 1000 ppm), Na (> 1500 ppm), Ti (> 100 ppm), and Zr (> 200 ppm) are interpreted as accidental ablation of mineral inclusions within apatite, and therefore these data have been screened out. A total of 305 apatite results were retained after discarding > 90 analyses due to the contamination of microscale inclusions. Individual results and detection limits for analyzed trace elements in apatite can be found in ESM A3.
Analytical results
Zircon composition
After data processing and screening a total of 360 analyses, 163 LA-ICP-MS results were retained. The chondrite-normalized (McDonough and Sun 1995) rare earth elements (REEs) in zircon from each intrusive phase show typical convex-upward patterns (Fig. 2), characterized by strongly enriched heavy REEs, depleted light REEs, prominent positive Ce anomalies (CeN/NdN = 1.3–45), and weak to moderate negative Eu anomalies (Eu/Eu* = 0.2–0.78). Granodiorite intrusions from the Anabama Hill, Netley Hill, and Bendigo prospects display similar REE patterns and compositions (Fig. 2a, c, and e), as do the porphyritic diorite plutons (Fig. 2a, c, and f). Zircons from the dacite porphyry at Anabama Hill and biotite porphyry from Bendigo have higher REE abundances than other intermediate-felsic counterparts, with medians of 1,827 and 2,936 ppm, respectively (ESM2 Table S2). Zircon from the A-type Colebatch granite is characterized by their slightly low Ce/Ce* values (12.9–23.7) and remarkably negative Eu anomalies (median Eu/Eu* 0.02–0.40; Fig. 2f; ESM2 Table S4).
Chondrite-normalized (McDonough and Sun 1995) rare earth element diagrams for zircon from each intrusive rock. Anabama Hill prospect: (a) granodiorite and diorite porphyry, (b) monzogranite and dacite porphyry. Netley Hill prospect: (c) granodiorite and diorite porphyry, (d) red K-feldspar granite and biotite porphyry. Bendigo: (e) granodiorite and biotite granite, and (f) diorite porphyry. Colebatch granite included in panel (f). Each line pattern represents an average value of analyses of each granite. The grey fields represent the maximum and minimum ranges
Ti-in-zircon thermometer of Ferry and Watson (2007) was used to calculate temperatures for each intrusive phase, assuming a TiO2 activity of 0.7 and SiO2 activity of 1 that reflect titanite and titanomagnetite saturation (Claiborne et al. 2010). The Ti-in zircon temperatures for the Anabama Hill granodiorite range from 846 to 674 °C (Fig. 3a), with a median of 735 ± 56 °C (ESM2 Table S4), whereas the plagioclase-phyric diorite is slightly hot and has temperature variations between 620 and 910 °C (median 765 ± 78 °C). The monzogranite at Anabama Hill appears to have a similar temperature range to the diorite, averaging at 762 ± 47 °C (706–854 °C). The dacite porphyry is slightly cool with temperatures from 870 to 694 °C (median 740 ± 46 °C). In the Netley Hill prospect, the temperatures for the red, K-feldspar-bearing granite (median 816 ± 57 °C) are shown 50–100 °C higher than those for the granodiorite (773 ± 58 °C), porphyritic diorite (770 ± 38 °C), and biotite-phyric granite (730 ± 41 °C). The Bendigo granodiorite (750 ± 47 °C) and biotite granite (780 ± 39 °C) appear to be slightly hotter than the diorite porphyry (median 728 ± 52 °C) from the same district (Fig. 3a). Ti-in-zircon thermometer estimates a temperature range of 865 to 650 °C (median 734 ± 66 °C) for the Colebatch granite.
Our analyses show that Hf in zircon from the Delamerian porphyry prospects mostly range from 8,500 to 16,000 ppm, Th of 50–2,000 ppm, and U of 75–3,000 ppm. Hafnium contents in zircon increase consistently with decreasing crystallization temperatures, Th/U ratios, and Eu/Eu* values (Fig. 3a, c, and e), and correlate positively with CeN/(Nd2/Sm)N and YbN/GdN ratios (Fig. 3d and f). Generally, 238U/206Pb ages in zircon decrease with Hf concentrations from 8,500 to 15,000 ppm, although there is a peak age of 510 Ma at a Hf value of ca. 11,000 ppm (Fig. 3b).
Apatite composition
BSE and CL images have been collected to reveal the internal textures and generations of the apatite crystals in this study and representative BSE-CL images for analyzed apatite crystals are presented in ESM1 Figure S1. Most of the apatite crystals are characterized by homogenous massive CL-bright domains (ESM1 Fig. S1) that may locally have thin oscillatory zoned rims (ESM1 Figs. S1 k and n), characteristic of igneous zoning features (Bouzari et al. 2016). A small amount of apatite crystals displays irregularly patchy zoning with dark CL features grown on CL-bright cores (ESM1 Fig. S1 i), crosscutting CL-dark cracks (ESM1 Figs. S1 a, f, g, and l), and/or porous textures filled with rare earth element-rich inclusions. These apatite crystals are classified as alteration apatite with hydrothermal origin (Bouzari et al. 2016) and were not analyzed for major and trace element compositions.
All apatite crystals have F abundances over 1.75 wt%, with the highest value to 4.5 wt%, and thus the apatite from the Delamerian prospects exclusively belongs to the fluorapatite group (Pasero et al. 2010). The excess F contents in apatite > 3.77 wt% (maximum values theoretically) could be ascribed to analysis facet parallel to apatite’s c-axis. Atom per formula (apfu) calculations are based on 12.5 oxygen equivalents and for those with excess F, calculated OH and Cl are omitted. At Anabama Hill, apatite crystals in the granodiorite and diorite have equivalent F (average ~ 4.02 wt%), Cl (~ 0.01 wt%), and SO3 (0.02 wt%), with XCl/OH of 0.02–0.04 and XF/OH of 10.3–15.1 (Fig. 4), whereas the monzogranite and dacite porphyry have lower F (average 3.42 and 3.79 wt%, respectively) and XF/OH of 6.9–11.4, but higher Cl (0.03 and 0.012 wt%) and SO3 (0.07 and 0.02 wt%) contents in apatite (Fig. 4a–c; ESM2 Table S3). There is a decreasing trend for F in apatite from granodiorite (3.88 ± 0.37 wt%; median XF/OH of 12.1), diorite porphyry (3.84 ± 0.42 wt%; median XF/OH of 7.82), to porphyritic aplite (3.63 ± 0.57 wt%; median XF/OH of 7.41) at Netley Hill, but their Cl and SO3 mostly fluctuate around 0.01 wt% and 0.02–0.03 wt%, respectively (Fig. 4a–b). At the Bendigo prospect, the apatite from the young diorite porphyry contains higher averaged Cl (~ 0.23 wt%; XCl/OH of 0.15) and SO3 (~ 0.14 wt%), and lower F contents (~ 3.02 wt%; XF/OH of 3.9) than from the granodiorite (averages of 0.01, 0.09, and 3.62 wt%) and biotite granite (averages of 0.02, 0.06, and 3.94 wt%; Fig. 4a–c). The Colebatch apatite shows relatively low F (3.30 wt%; XF/OH of 5.57) and high Cl (0.07 wt%; XCl/OH of 0.06) and SO3 (0.09 wt%) abundances compared to those from other prospects (Fig. 4a–c). The positive linear trend defined by XF/OH and XCl/OH ratios implies a volatile-undersaturated character for apatite from the Delamerian porphyry prospects (Fig. 4c; cf. Meng et al. 2021).
EMPA shows that MnO, SiO2, FeO, and Na2O in apatite from the Delamerian plutons are mostly below 0.41, 0.21, 0.22, and 0.13 wt%, respectively (Supplementary Material A1), and a large percentage of the total analyses are below respective detection limits (e.g., ~ 40% for MnO, 45% for SiO2, 50% for FeO). LA-ICP-MS analyses reveal Mn as the most abundant trace element in apatite, ranging from 110 to 10,900 ppm and averaging at 1,970 ppm (Fig. 5a; ESM2 Table S3). Yttrium in apatite ranges from 130 to 5,350 ppm and averages at 1,070 ppm (Fig. 5b). The light REEs (La to Gd) in apatite commonly have median values from 100 to 780 ppm, whereas heavy REEs (Tb to Lu) typically average 10–40 ppm (Fig. 6; ESM2 Table S3). The Sr, Fe, Na, Mg, and Si contents in apatite are typically few hundreds of ppm on average (Fig. 5d–h), and U, Th, As, V, and Pb concentrations typically several to tens of ppm (Fig. 5i–l). Other apatite trace elements, including Al, Ba, Cu, Ti, Rb, Zn, and Zr, commonly have median values below 0.65 ppm, whereas Cr, Hf, Mo, Nb, Ta, and W typically have concentrations < 0.05 ppm on average. Approximate 30–90% of LA-ICP-MS analyses on these elements are below detection limits (ESM A3).
Percentile box plots showing compositions and variations of representative trace elements in apatite from different intrusions of the Delamerian porphyry prospects. (a) Mn, (b) Y, (c) total REEs, (d) Sr, (e) Fe, (f) Mg, (g) Na, (h) Si, (i) U, (j) Th, (k) As, and (l) V. Host rock types and prospect names are labelled in panels (g) and (j), respectively
Rare earth element plots for apatite from the Delamerian porphyry prospects. Anabama Hill prospect: (a) granodiorite and diorite porphyry, and (b) monzogranite and dacite porphyry. Netley Hill prospect: (c) granodiorite and diorite porphyry. Bendigo prospect: (d) granodiorite, (e) biotite granite, and (f) diorite porphyry. (g) Colebatch granite. All LA-ICP-MS apatite results normalized to the Chondrite element values from McDonough and Sun (1995)
Overall, apatite crystals from the granodiorite, diorite porphyry and monzogranite at Anabama Hill and Netley Hill show similar abundances for each trace element (Fig. 5). Apatite crystals in the dacite porphyry at Anabama Hill are evidently depleted in Mn, Fe, Mg, Na, and Y, and enriched in Si, REEs, U, Th, As, V, and Pb relative to other granitoids from the two prospects (Fig. 5). At Bendigo, apatite Mn, Sr, and Y contents increase sequentially from the granodiorite, diorite porphyry, to the biotite granite, whereas Fe, Na, Si, REEs, U, Th, and V mostly decrease in concentration (Fig. 5). Apatite crystals from the Colebatch granite are characterized by relatively high Si, Y, U, As, and V, and low Mn, Sr, Fe, and Mg concentrations (Fig. 5).
Apatite from the Delamerian porphyry prospects displays similar REE fractionation patterns to magmatic apatite typically from unaltered rocks in porphyry Cu systems (Fig. 6; Bouzari et al. 2016; Parra-Avila et al. 2022). Apatite crystals from the granodiorite and diorite porphyry at Anabama Hill have comparable flat REE patterns (Fig. 6a), with variably negative to positive Eu anomalies (average Eu/Eu* = 0.72–1.24). The monzogranite and dacite porphyry have identical apatite REE patterns, but a wider REE composition range (690–4,200 ppm) than those from the granodiorite and diorite porphyry (Fig. 6a–b). Rare earth elements in apatite from the granodiorite and diorite at Netley Hill define a typical ‘V’-shaped pattern, with prominently negative Eu anomalies (average 0.35 ± 0.3) and slight enrichment of light REEs (Fig. 6c). Apatite crystals from the Bendigo Granite show distinctive right-inclined REE patterns, with light REE enrichments and negative Eu anomalies (Fig. 6d–f). The Colebatch apatite is characterized by a V-shaped REE pattern with prominent negative Eu anomalies (0.4 ± 0.1) and characteristic Y (900–5,350 ppm) and Lu (20–140 ppm) enrichments (Figs. 5b and 6g). No evident Ce anomalies occur in apatite from the Delamerian porphyry prospects (Fig. 6; ESM2 Table S4).
Discussion
Magmatic evolution
Zircon perspective
Correlations between increasing Hf contents and cooling temperatures in zircon have been used to record an elevated degree of magmatic evolution of intermediate-felsic magmas (Miller and Wooden 2004; Gagnevin et al. 2010; Dilles et al. 2015; Lee et al. 2017). Our data from the Delamerian Orogen indicate that decreasing crystallization temperatures, Eu/Eu*, and Th/U correlate systematically with increasing Hf content in zircon (Fig. 3). These data suggest that both the Anabama and Bendigo granitic complexes underwent an increased degree of magmatic evolution and fractionation, consistent with the presence of early intermediate compositions to later felsic compositions (Fig. 3a, c, and e).
The biotite granite at Netley Hill has the highest U and Th contents and lowest Th/U ratios (average 0.58; due to the higher partition coefficient for Th than U; Fig. 3e), which is consistent with a normal fractionation trend with Th and U enriched in the residual melts (e.g., Miller and Wooden 2004; Gagnevin et al. 2010). This is also supported by the increasing Yb/Gd ratios with Hf in zircon (Fig. 3f). However, in contrast with the normal fractional crystallization trend, zircon from the dacite porphyry at Anabama has similar U contents to the diorite and granodiorite, but relatively low Th and Th/U (Fig. 3e). This implies replenishing pulses or variable assimilation of the intermediate melts into the dacite porphyritic melt (cf. Gagnevin et al. 2010; Lee et al. 2017; Paolillo et al. 2022). Although limited in analyses, zircon crystals from the Bendigo diorite porphyry record higher Th, U, and Hf contents and lower Th/U than those in the granodiorite and biotite granite (Fig. 3e), illustrating a more evolved melt crystallized later under lower temperatures than other intrusions at Bendigo (Fig. 3a–b). Elevated Th and U and low Th/U generally with increasing Hf concentrations in the Colebatch zircon (Fig. 3e) are ascribed to prolonged plagioclase crystallization and is supported by their prominent negative Eu anomalies (average Eu/Eu* = 0.09; Fig. 3c).
To investigate fractional crystallization of different minerals and/or mineral assemblages equilibrated with magmatic melts from the Delamerian Orogen, empirical Rayleigh modelling has been performed as functions of Yb/Gb and Eu/Eu* in zircon (Fig. 7). The modelling started at the average composition of the Anabama granodiorite (see details in ESM A2). Partition coefficients between melt and plagioclase are from Dunn and Sen (1994), amphibole from Klein et al. (1997), apatite and zircon from Sano et al. (2002), titanite from Bachmann et al. (2005), and garnet from Burnham and Berry (2017). Single mineral and mixed mineral fractionation curves are calculated using formulas from Loader et al. (2017). Zircon populations in granodiorite from both the Anabama Hill and Netley Hill are characterized by low-moderate Yb/Gd (8.3–20) and high-intermediate Eu/Eu* ratios (> 0.4), implying initial high-temperature amphibole and apatite crystallization in the deep magma chamber (Fig. 7a–b). Increasing Yb/Gd (20–39.7) with decreasing Eu/Eu* (< 0.4) suggests a late titanite crystallization (up to 0.3%) with cooling (Fig. 7a–b). The diorite porphyries from both prospects have experienced distinct fractional crystallization controlled by amphibole (< 60%), garnet (< 15%), and minor apatite (< 0.6%; Fig. 7a–b). Monzogranite and K-feldspar granite phases from the Anabama Granite likely have undergone similar crystallization trends to their diorite counterpart (Fig. 7a–b). The dacite porphyry and biotite granite show limited variations in Eu/Eu* (0.3–0.5) and may have experienced minor apatite (< 0.4%) or titanite crystallization (< 0.15%; Fig. 7a–b) at Anabama Hill. The granodiorite, diorite porphyry, and biotite granite from the Bendigo prospect are consistently characterized by fractional crystallization dominated by amphibole (< 50%) under high temperature, with lesser influence due to apatite (< 0.8%) and/or titanite (< 0.15%) crystallization with cooling (Fig. 7c). In contrast to the Anabama and Bendigo granite suites, the A-type Colebatch granite is distinct by extensive plagioclase crystallization up to 90% (Fig. 7d).
Rayleigh fractionation modelling in terms of Yb/Gb and Eu/Eu* in zircon showing crystallization behaviors of different mineral or mineral assemblages in parental melt. (a) Anabama Hill, (b) Netley Hill, (c) Bendigo, and (d) Colebatch. Average values of the Anabama granodiorite are taken as the starting point of Rayleigh modelling. Partition coefficients between mineral and melt are from Klein et al. (1997; amphibole), Sano et al. (2002; apatite and zircon), Burnham and Berry (2017; garnet), Dunn and Sen (1994; plagioclase), and Bachmann et al. (2005; titanite). Single mineral and mixed mineral fractionation models are calculated using formulas from Loader et al. (2017). The numbers on each modelling curve represent crystallization percentages of the relevant mineral (black curve) or mixed mineral assemblage (blue curve). Abbreviations Amp = Amphibole, Ap = Apatite, Grt = Garnet, Pl = Plagioclase, Ttn = Titanite, Zrn = Zircon
Our zircon geochemical modeling reveals that garnet-amphibole crystallization dominated the fractionation of the intermediate to felsic components of the Anabama Granite, whereas amphibole crystallization prevailed during the fractionation of the Bendigo Granite, with additional minor apatite and/or titanite crystallization influencing this (Fig. 7). These characteristics are consistent with a number of hornblende phenocrysts observed by previous petrographic studies (Langsford 1971; McDonald 1992), both indicating that both Anabama and Bendigo granites contain high magmatic water content, a prerequisite that potentially generates porphyry Cu–Mo ± Au deposits (Sillitoe 2010; Richards 2011; Cooke et al. 2014; Loucks 2014).
Apatite perspective
Similar to zircon, apatite chemical composition is determined by melt composition, temperature-pressure conditions, oxidation state, and relative timing of its crystallization to other mineral phases (Belousova et al. 2002; Mao et al. 2016; Nathwani et al. 2020; Bromiley 2021; Parra-Avila et al. 2022). Apatite crystals from the Anabama Hill and Netley Hill prospects have average Mn concentrations of 900–2,740 ppm, as compared to a lower Mn content of 190–17,90 ppm (average = 545 ppm) from the Bendigo prospect (Fig. 5a). These values are consistent with porphyry Cu–Mo ± Au deposits that commonly have apatite with high Mn contents (MnAp) of 330–11,000 ppm (Mao et al. 2016). Experimental studies show that MnAp concentrations are controlled mainly by melt structure and composition (Stokes et al. 2019), and apatite that crystallizes from evolved magmas typically contains high Mn concentrations (Bromiley 2021). This increasing MnAp trend is recorded in the intermediate granodiorite, diorite porphyry, to felsic granite (except the Anabama dacite porphyry) of the Delamerian porphyry prospects (Fig. 8a). Magnesium contents in apatite correlate positively with Mn, but both Mg and Fe generally have lower concentrations in more felsic intrusions (e.g., Fig. 8a). The coherent decreases of Mg and Fe in apatite during the evolution of the Anabama porphyry systems (Fig. 5e–f) are likely ascribed to continuous amphibole crystallization (Parra-Avila et al. 2022). Consistent with the presence of amphibole (or hornblende) phenocrysts in the sulfide mineralization-hosting porphyritic diorite, apatite from this diorite porphyry records the highest Mg and Fe among intrusive suites at Bendigo (e.g., Figs. 5e–f and 8a).
The general negative correlation between Y and Sr in apatite (Fig. 8b) can be attributed to progressive Y enrichment and Sr depletion led by plagioclase crystallization during increasing magmatic fractionation (Bromiley 2021). This is contrasted with the zircon REE modelling that implies an amphibole-dominated crystallization at depth (Fig. 7). Perhaps, trace element compositions in apatite from the Delamerian porphyry prospects record the relatively extensive plagioclase crystallization in the shallow crust (Nathwani et al. 2020; Bromiley 2021). Consequently, Y increases and Sr decreases in apatite from intermediate to more felsic granite plutons at each prospect. There are a few exceptions such as from the Anabama and Bendigo granites that have high Sr and low Y (Fig. 8b), which are probably inherited from their primitive magmas at depth (cf. Nathwani et al. 2020).
Low Mg (< 670 ppm) and Sr/Y ratios (< 5) characterize the apatite crystals from Delamerian porphyry systems (Fig. 8c), implying that the intermediate-felsic host magmas experienced fractional crystallization principally in the shallow crust, since apatite crystallizing early from a near-liquidus melt in the lower crust typically contains high Mg (up to 4,000 ppm) and Sr/Y (up to 10; Nathwani et al. 2020). Plagioclase crystallization can lower Sr/Y and cause negative Eu anomalies during shallow crustal melt evolution, whereas amphibole ± garnet crystallization leads to elevated Sr/Y ratios and high Eu/Eu* under high magmatic water conditions (Richards et al. 2012; Loucks 2014). Accordingly, the low Sr/Y and Mg contents in apatite (Fig. 8c) may indicate that the Delamerian intrusive suites had experienced relatively extensive plagioclase crystallization in the shallow crust. However, the prominent Sr/Y increase with positive Eu/Eu* (Fig. 8d) and slight Sr/Y increase with decreasing Mg in apatite in both Anabama and Bendigo samples (Fig. 8c), mostly follow the trends defined by amphibole fractional crystallization. Potentially, these low Mg apatite crystals with high Sr/Y and Eu/Eu* values recorded primitive melt signatures with high Sr/Y and Eu/Eu*, driven by amphibole-dominated fractional crystallization at depth (cf. Nathwani et al. 2020). Extremely low Mg, Sr/Y, and Eu/Eu* in apatite from the A-type Colebatch granite highlight extensive plagioclase fractionation, in agreement with the zircon chemistry and trace-element modelling (Figs. 7 and 8c–d).
Low Mn, Y, Sr, Na, Fe, and Mg concentrations characterize apatite from the dacite porphyry at Anabama Hill (Fig. 5) and highlight that the dacite dikes may have greater metallogenic fertility than other igneous phases. These trace elements could have been exsolved from ore-associated magmas and progressively enriched in magmatic-hydrothermal fluids, causing their depletions in magmatic apatite that directly crystallized from ore-associated melts rather than replacement apatite modified by hydrothermal fluids (Cao et al. 2021). High concentrations of pathfinder elements in apatite, including high REEs, Si, Th, As, and V (Fig. 5c, h, j, k, and l), provide additional evidence for the porphyry mineralization potential of dacite dikes at Anabama Hill (cf. Mao et al. 2016; Xu et al. 2021; Parra-Avila et al. 2022). Elevated As and V contents might be incorporated into apatite at a relatively high oxygen fugacity state (presented as As5+ and V5+), whereas REEs, Th, and Si coupled with S6+ can substitute a small fraction of Ca and P into the apatite’s structure (cf. Parra-Avila et al. 2022).
Moderately high oxidation
Zircon Ce and Eu anomalies have been shown by experimental studies to relate to melt oxygen fugacity (Burnham and Berry 2012, 2014; Trail et al. 2012; Burnham et al. 2015), although the trace elements can also be affected by melt compositions, temperature, pressure, and co-crystallizing minerals (Burnham et al. 2015; Loader et al. 2017, 2022). Nevertheless, these studies indicate that more oxidized systems tend to have zircon with stronger positive Ce anomalies and intermediate negative Eu anomalies. These findings have important implications for exploration of porphyry-style Cu–Mo ± Au deposits that typically originate from hydrous, oxidized arc magmas (Candela and Piccoli 2005; Sillitoe 2010; Richards et al. 2012; Wilkinson 2013; Cooke et al. 2014; Loucks 2014). Much lower Ce4+/Ce3+ ratios (average 1.22 ± 0.51) were detected in zircon from barren Paleozoic granitoids of the Australia’s Lachlan Orogen (Belousova et al. 2006). Zircon from porphyry Cu deposits in the Central Asian Orogenic Belt has Ce4+/Ce3+ ratios mostly above 120, strengthening such a positive correlation between oxygen fugacity (represented as Ce4+/Ce3+ ratios) and Cu endowment (Shen et al. 2015). It is challenging to accurately determine the Ce anomalies (Ce/Ce* calculated as CeN/[(LaN×PrN)]0.5) due to La and Pr concentrations in zircon close to or below their detection limits (e.g., Belousova et al. 2006; Chelle-Michou et al. 2014; Shen et al. 2015), and Ce4+/Ce3+ ratios are complicated to calculate using the lattice-strain model by Ballard et al. (2002). Alternatively, chondrite-normalized Ce/Nd ratio in zircon was proposed to estimate the Ce anomaly (Chelle-Michou et al. 2014; Loader et al. 2017) and this approach is adopted in this study (Figs. 3d and 9a; ESM2 Table S4).
Selected zircon trace elements and ratios for magma fertility assessment for the Delamerian porphyry prospects. (a) CeN/(Nd2/Sm)N vs. Eu/Eu*, (b) Dy/Yb vs. Eu/Eu*, (c) (Ce/Nd)/Y vs. 1000 × (Eu/Eu*)/Y, and (d) (Ce/Nd)/Y vs. Eu/Eu*. The fields for fertile and barren magmas are from Lee et al. (2017) and Lu et al. (2016)
The Eu anomalies (Eu/Eu*) in zircon have been proposed to be a proxy for either magmatic oxidation state (Shen et al. 2015; Lee et al. 2017, 2020), hydration state (Lu et al. 2016), or SO2 degassing (Dilles et al. 2015). Distinctive Eu/Eu* ratios in zircon (> 0.4) characterize the ore-forming magmas associated with porphyry Cu–Mo ± Au deposits in Central Asia, Chile, Peru, and North America (Dilles et al. 2015; Shen et al. 2015; Lee et al. 2017, 2020). Lu et al. (2016) proposed that zircon from porphyry Cu–Mo ± Au systems have Eu/Eu* > 0.3. Based on a large global dataset, Lu et al. (2016) also proposed that zircons from fertile magmatic suites have collectively higher 1000×(Eu/Eu*)/Y (> 1) and (Ce/Nd)/Y (> 0.01), and lower Dy/Yb (< 0.3) ratios than barren pluton suites. More than 90% of zircon analyses from the Anabama Hill, Netley Hill, and Bendigo prospects plot in the fertile fields of porphyry mineralization in terms of Eu/Eu*, 1000×(Eu/Eu*)/Y, and (Ce/Nd)/Y (Fig. 9c–d), whereas only about 50% of zircon results plot into the fertile field defined by Dy/Yb and Eu/Eu* (Fig. 9b). The observed patterns in Ce and Eu anomalies in zircon from the three prospects are interpreted as a consequence of the prevalent garnet ± amphibole crystallization and suppression of plagioclase at hydrous and/or oxidized conditions (Richards 2011; Chelle-Michou et al. 2014; Loucks 2014; Dilles et al. 2015; Shen et al. 2015; Lu et al. 2016; Lee et al. 2017, 2020). Contrastingly, the A-type Colebatch granite is suggested to have a low potential for porphyry mineralization (Fig. 9).
Oxygen fugacity of the intrusive rocks from the Delamerian Orogen has been quantitively estimated using Ce, Ti, and initial U concentrations in zircon by adopting the approach of Loucks et al. (2020), irrespective of temperature, water content, and melt composition (Fig. 10a). The calculated oxygen fugacity values are presented in terms of fO2 relative to the fayalite-magnetite-quartz (FMQ) buffer. The granodiorite, porphyritic diorite, monzogranite, and dacite porphyritic dike at Anabama Hill have ΔFMQ values from 1.6 to 3.1 (average 2.1 ± 0.4), 0.87 to 2.62 (average 1.9 ± 0.5), 0.97 to 2.37 (average 1.8 ± 0.4), to -0.2 to 2.4 (average 1.3 ± 0.7; Fig. 10a). In the Netley Hill prospect, the granodiorite, K-feldspar granite, and biotite granite have near-identical ΔFMQ values, with averages of 1.3 ± 0.6 to 1.5 ± 0.6, whereas the ΔFMQ is slightly higher for the porphyritic diorite, with an average of 1.9 ± 0.5 ranging between 0.4 and 2.7 (Fig. 10a). The granodiorite and biotite granite from the Bendigo prospect have ΔFMQ compositions similar to comparable lithologies from the Anabama Hill prospect, with averages of 1.3 ± 0.4 and 1.2 ± 0.5 (ESM2 Table S4). The Bendigo diorite dikes have lower average ΔFMQ (0.7 ± 0.9), which fluctuates considerably from − 0.5 to 2.3 (Fig. 10a). The Colebatch granite from the Padthaway Ridge have the lowest ΔFMQ value of 0.4 ± 1.1 (-1.8 to 2.0). Except for the A-type Colebatch granite, all intrusions from the Delamerian porphyry prospects have ΔFMQ values into the range for subduction-related magmas typically defined by FMQ + 0.5 to + 2 (Rowe et al. 2009; Zimmer et al. 2010; Brounce et al. 2014; Richards 2015). These results highlight a moderate magmatic oxidation state of the Delamerian porphyry systems, which could be inherited from fO2 state of the parental magmas during post-subduction processes of the Delamerian Orogeny (ca. 514–490 Ma; Foden et al. 2006; Foden et al. 2020; Hong et al. 2023). About 24% of the zircon analyses yield high ΔFMQ values from 2 up to 3.1 (Fig. 10a). This is likely ascribed to prolonged magmatic evolution during cooling that can exert an important influence on magma redox state (cf. Burnham and Berry 2014; Burnham et al. 2015; Loader et al. 2017, 2022), or may have resulted from SO2 degassing to the exsolving magmatic-hydrothermal fluids accompanied with the reduction of S4+ from S6+ (sulfate), oxidizing the remnant melt (Dilles et al. 2015).
(a) Oxidation states plotted against temperatures estimated based on zircon geochemistry. The oxidation states are expressed in terms of fO2 relative to the fayalite-magnetite-quartz (FMQ) reference buffer. Zircon ΔFMQ is calculated from Loucks et al. (2020): ΔFMQ = 3.998 (± 0.124) × log [Ce/\(\sqrt{(Ui\times Ti)}\)] + 2.284 (± 0.101). Inset shows mean values of ΔFMQ and temperature with standard deviation uncertainty (error bar) for each intrusive rock. The ΔFMQ area for typical arc magmas from Zimmer et al. (2010) and Richards (2015). (b) Oxidation states plotted against temperatures estimated based on apatite Mn contents. Apatite saturation temperatures (AST) are calculated from whole-rock SiO2 and P2O5 concentrations using the equation of Piccoli and Candela (1994). Mn-in-apatite oxybarometer is using the equation of Miles et al. (2014): log fO2 = − 0.0022 (± 0.0003) Mn (ppm) – 9.75 (± 0.46). ASTs are 50–150 °C higher than Ti-in-zircon temperatures for respective rock type, in agreement with relatively early apatite crystallization in intermediate-felsic magmas (cf. Piccoli and Candela 1994)
Apatite Eu and Ce anomalies have also been argued to correlate with magmatic oxygen fugacity of the silicate melts from which it crystallizes (cf. Bromiley 2021). Under increasing oxidation conditions, negative Eu anomalies decrease progressively, in contrast to increasing negative Ce anomalies in apatite (Bromiley 2021). The narrow range of Ce/Ce* values in apatite from the Delamerian porphyry prospects (0.99–1.22; ESM2 Table S4) restricts the application to track magmatic oxidation. Apatite Eu/Eu* values range widely from 0.2 to 4 among the Delamerian samples (ESM2 Table S4), which are strongly affected by feldspar crystallization (Eu2+ incorporation) – they are more likely to highlight magmatic sources and evolution (cf. Belousova et al. 2002; Chu et al. 2009; Mao et al. 2016; Nathwani et al. 2020; Bromiley 2021). There has been increasing use of MnAp contents as an oxybarometer to quantitively estimate oxidation states of silicic magmas (Miles et al. 2014; Mao et al. 2016; Richards et al. 2017). The Mn-in-apatite oxybarometry yields fO2 results distinctly contrasting with the zircon oxybarometer for the Delamerian porphyry prospects, with the highest oxidation state of ΔFMQ + 3.5 on average for the A-type Colebatch (Fig. 10b). The Mn-in-apatite oxybarometry provides ΔFMQ values of 1.6 ± 1, 2.4 ± 0.4, and 1.8 ± 0.9 for the Bendigo biotite granite, granodiorite, and diorite porphyry, largely compatible with those yielded by zircon chemistry (Fig. 10b). However, this oxybarometer yields significantly lower mean ΔFMQ values, ranging from − 4.8 to + 0.5 for intrusions from the Anabama Hill and Netley Hill prospects (Fig. 10b). These discrepant results indicate the unreliability of the Mn-in-apatite oxybarometer, consistent with recent studies that MnAp contents are predominantly controlled by melt structure rather than magmatic redox (Stokes et al. 2019; Bromiley 2021).
Sulfur and chlorine contents
The formation of porphyry Cu–Mo ± Au deposits typically relate to magmatic-hydrothermal fluids exsolved from hydrous, moderately oxidized, intermediate to felsic melts that also contain high S and Cl concentrations. These halogen elements efficiently scavenge and enrich metals as complexing ligands in fluids (Richards 2003; Candela and Piccoli 2005; Seedorff et al. 2005; Sillitoe 2010; Cooke et al. 2014; Loucks 2014). Volatile elements may be incorporated into apatite lattice structure, and therefore S and Cl concentrations are commonly used to assess metallogenic fertility. Apatite Cl and S concentrations reportedly cannot distinguish mineralized magmas from barren intrusions in the Gangdese porphyry copper belt of Tibet (Li et al. 2021), and low SO3 contents (mean < 0.61 wt%) was revealed in magmatic apatite associated with the Huojihe porphyry Mo deposit of NE China (Xing et al. 2020). However, many workers have emphasized that higher Cl and S in apatite characterize well-mineralized porphyry Cu–Mo ± Au deposits, e.g., 0.25 ± 0.17 wt% SO3 in apatite from the Carmen de Andacollo Cu–Au deposit of northern Chile (Richards et al. 2017), high SO3 (0.28–0.32 wt% vs. 0.03–0.11 wt% in mineralized and barren intrusions) and Cl (0.82–1.47 wt% vs. 0.02–0.51wt.%) at Red Chris Cu–Au deposit of British Columbia (Zhu et al. 2018), higher Cl (average 0.17 wt% vs. 0.04 wt%) and SO3 (average 0.19 wt% vs. 0.10 wt%) from fertile magmas than barren suites from post-subduction porphyry provinces (Xu et al. 2021), and increasing Cl (1.5–3 wt%) and S concentrations (up to 0.7 wt% SO3) in apatite from the Pliocene Cu–Au porphyries at Tampakan of Philippines (Parra-Avila et al. 2022). Even for the Paleoproterozoic Haib porphyry Cu deposit in Namibia, relatively high S contents (0.11–0.27 wt%) were detected in apatite (Meng et al. 2021). In general, apatite crystals from Phanerozoic porphyry Cu systems have relatively high SO3 contents (average 0.30 ± 0.07 wt%), which are likely inherited from causative intermediate to felsic arc magmas with SiO2 > 52 wt% in Circum-Pacific ring (average 0.38 ± 0.07 wt% SO3 in apatite; Meng et al. 2021 and references therein).
Apatites from granodiorite, diorite porphyry, to dacite porphyry and aplite at Anabama Hill and Netley Hill, and from granodiorite, biotite granite to diorite porphyry at Bendigo yield Cl contents that increase positively with S and negatively with F (Fig. 4a–c). Overall, apatite from the porphyritic diorite at Bendigo has the highest Cl and S values (Fig. 4a–c). These trends are consistent with the argument that F is compatibly partitioned into co-existing silicate melt (Webster et al. 2014). The sulfur trend is inverse to the expected pattern of evolving from mafic to felsic composition, as S solubility decreases linearly with FeO and thus is expected to become depleted in felsic melt (Wallace and Carmichael 1992). Alternatively, S and Cl are more likely incorporated into a magmatic volatile phase (Zajacz et al. 2012; Webster et al. 2014), which are potentially exsolved into a magmatic-hydrothermal fluid, or high S contents in ore-associated felsic magmas can result from recharge by transcrustal S-rich mafic melts or evaporitic sulfate sources (cf. Keith et al. 1997). Sulfur contents in apatite fluctuate as a complex function of magmatic temperature, oxidation state, and sulfur fugacity (Peng et al. 1997; Parat and Holtz 2004; Konecke et al. 2017, 2019) and S concentrations in apatite are generally proportional to those in the magmatic melts. Using a revised approach by Meng et al. (2021), magmatic S contents are estimated to be 37.3 ± 9, 49.3 ± 17, 134 ± 83, and 65.8 ± 16 ppm on average for the Anabama granodiorite, diorite, monzogranite, and dacite porphyry, 345 ± 278, 281 ± 178, and 909 ± 474 ppm for the Bendigo granodiorite, biotite granite, and diorite porphyry (ESM2 Table S4).
Similarly, the Cl abundances in apatite are dependent on melt composition, Cl content, temperature and/or pressure of the system (Webster et al. 2014; Webster and Piccoli 2015; Li and Hermann 2017). The crystallization sequence of amphibole/hornblende and apatite is not well constrained for the intrusions from the Delamerian prospects, considering lack of petrographic evidence. However, Cl concentrations in melts calculated for the mineralized porphyries at Coroccohuayco porphyry-skarn deposit in Peru are 0.2–0.3 wt% based on Cl in apatite, higher than those based on Cl in amphibole (~ 0.10 wt%; Chelle‑Michou and Chiaradia 2017). The crystallization of hornblende/amphibole therefore does not significantly affect the Cl uptake into apatite in granitic magmas. Nevertheless, using Cl–OH exchange equations in apatite by Li and Hermann (2017), magmatic Cl contents in equilibrium with apatite are calculated to be 0.30 ± 0.24, 0.56 ± 0.85, 0.64 ± 0.89, and 0.36 ± 0.61 wt% on average for the Anabama granodiorite, diorite, monzogranite, and dacite porphyry, and 0.45 ± 0.47, 1.02 ± 1.13, and 3.01 ± 1.54 wt% for the Bendigo granodiorite, biotite granite, and diorite porphyry (ESM2 Table S4).
The S (0.02–0.56 wt% SO3) and Cl (0.01–0.58 wt% Cl) in apatite from the Delamerian porphyry systems are significantly lower than those from porphyry deposits in subducted magmatic arcs (normally 0.12–0.60 wt% SO3 and 0.2–3.0 wt% Cl). Recent studies have shown that zircon-hosted apatite inclusions prior to volatile exsolution commonly contain relatively high magmatic Cl and SO3 contents essential to potential porphyry Cu–Mo ± Au mineralization (Sun et al. 2023; Huang et al. 2024). All the apatite crystals analyzed in this study were separated and hand-picked from intrusive rocks and they probably occur as microphenocrysts in groundmass or as inclusions hosted by plagioclase and biotite. Consequently, these apatites could have lower Cl and SO3 contents than those hosted by zircon, due to fluid exsolution or re-equilibrium with post-exsolution melt (e.g., Sun et al. 2023; Huang et al. 2024). This is one of the likely reasons that apatites from the Delamerian porphyry prospects have significantly lower S and Cl signatures than those from typical porphyry Cu deposits in magmatic arcs. Although S and Cl compositions analyzed by apatite in this study might not best record the magmatic volatile budgets and evolution of the Delamerian porphyry systems, the low S and Cl signatures in magmas from the Delamerian porphyry prospects are, alternatively, consistent with their emplacement in a post-subduction setting, to which they have been ascribed (Cayley 2011; Johnson et al. 2016; Foden et al. 2020; Hong et al. 2023). In this environment, slab rollback and/or back-arc extension could have led to the development of magma from upwelling asthenosphere (sulfur-undersaturated), distal to the subduction arc with sufficient oxidized sulfur supply (Richards 2009; Wallace and Edmonds 2011; Richards et al. 2017). Magmas derived from a post-subduction setting may retain some arc geochemical signatures, but more likely, they have compositions similar to primitive intra-plate magmas with relatively poor S and/or Cl contents (Kimura and Yoshida 2006; Wallace and Edmonds 2011; Timm et al. 2012). Nevertheless, Cl and S in apatite illustrates gradual enrichment of Cl and S contents in causative magmas, which again identify the monzogranite and dacite porphyries at Anabama Hill and diorite porphyries at Bendigo as having good potential for porphyry-style mineralization.
Conclusions
Micro-analytical results of zircon and apatite from diverse granitic intrusions of four prospects have been used to evaluate the metallogenic fertility in terms of porphyry-style mineralization in the early Paleozoic Delamerian orogenic belt, South Australia. Zircon analyses from the Anabama Hill, Netley Hill, and Bendigo prospects have relatively high Eu/Eu* (> 0.3), 1000×(Eu/Eu*)/Y (> 1) and (Ce/Nd)/Y (> 0.01), and low Dy/Yb (< 0.3), which mostly fall into the fields defined by fertile magmas for porphyry Cu–Mo ± Au deposits globally. Key trace elements in zircon, e.g., Ti, Th, U, Hf, Gd, and Yb, imply elevated degrees of magmatic evolution from intermediate granodiorite to more felsic granite. Rayleigh fractionation modelling using Eu/Eu* and Yb/Gd ratios in zircon highlight that Anabama and Bendigo granite complexes had undergone prevalent (garnet ±) amphibole crystallization surpassing plagioclase crystallization under hydrous conditions, consistent with high REEs, Th, As, V, Sr/Y, and Eu/Eu*, and low Mg signatures detected in apatite. Zircon and/or apatite oxybarometers estimate a moderately high oxidation state ranging from ΔFMQ + 1 to + 3 for intrusive suites from these prospects. Apatite has elevated magmatic S and Cl components within monzogranite and dacite porphyries at Anabama Hill and diorite porphyry at Bendigo. Together, these data support the igneous phases of monzogranite and dacite porphyries at Anabama Hill and diorite porphyry at Bendigo as being the most fertile and likely to relate to Cu–Mo porphyry-style mineralization. Our study indicates that the prospects in Delamerian Orogen have positive indicators for porphyry-style mineralization and should encourage further exploration in this greenfield province.
References
Baatar B, Parra-Avila LA, Fiorentini ML, Polito P, Crawford AJ (2020) Porphyry Cu fertility of the Loch Lilly-Kars Belt, Western New South Wales, Australia. Aust J Earth Sci 67:75–87. https://doi.org/10.1080/08120099.2019.1637937
Bachmann O, Dungan M, Bussy F (2005) Insights into shallow magmatic processes in large silicic magma bodies: the trace element record in the Fish Canyon magma body, Colorado. Contrib Mineral Petrol 149:338–349. https://doi.org/10.1007/s00410-005-0653-z
Ballard JR, Palin MJ, Campbell IH (2002) Relative oxidation states of magmas inferred from ce (IV)/Ce (III) in zircon: application to porphyry copper deposits of northern Chile. Contrib Mineral Petrol 144:347–364. https://doi.org/10.1007/s00410-002-0402-5
Bell EA, Boehnke P, Harrison TM (2016) Recovering the primary geochemistry of Jack Hills zircons through quantitative estimates of chemical alteration. Geochim Cosmochim Acta 191:187–202. https://doi.org/10.1016/j.gca.2016.07.016
Belousova E, Griffin W, O’Reilly SY, Fisher N (2002) Apatite as an indicator mineral for mineral exploration: Trace-element compositions and their relationship to host rock type. J Geochem Explor 76:45–69. https://doi.org/10.1016/S0375-6742(02)00204-2
Belousova EA, Griffin WL, O’Reilly SY (2006) Zircon crystal morphology, trace element signatures and hf isotope composition as a tool for petrogenetic modelling: examples from eastern Australian granitoids. J Petrol 47:329–353. https://doi.org/10.1093/petrology/egi077
Betts MJ, Paterson JR, Jacquet SM, Andrew AS, Hall PA, Jago JB, Jagodzinski EA, Preiss WV, Crowley JL, Brougham T, Mathewson CP, García-Bellido DC, Topper TP, Skovsted CB, Brock GA (2018) Early Cambrian chronostratigraphy and geochronology of South Australia. Earth-Sci Rev 185:498–543. https://doi.org/10.1016/j.earscirev.2018.06.005
Bouzari F, Hart CJ, Bissig T, Barker S (2016) Hydrothermal alteration revealed by apatite luminescence and chemistry: a potential indicator mineral for exploring covered porphyry copper deposits. Econ Geol 111:1397–1410. https://doi.org/10.2113/econgeo.111.6.1397
Bromiley GD (2021) Do concentrations of Mn, Eu and Ce in Apatite reliably record oxygen fugacity in magmas? Lithos 384–385. https://doi.org/10.1016/j.lithos.2020.105900
Brounce M, Kelley K, Cottrell E (2014) Variations in Fe3+/∑Fe of Mariana arc basalts and mantle wedge fO2. J Petrol 55:2513–2536. https://doi.org/10.1093/petrology/egu065
Burnham AD, Berry AJ (2012) An experimental study of trace element partitioning between zircon and melt as a function of oxygen fugacity. Geochim Cosmochim Acta 95:196–212. https://doi.org/10.1016/j.gca.2012.07.034
Burnham AD, Berry AJ (2014) The effect of oxygen fugacity, melt composition, temperature and pressure on the oxidation state of cerium in silicate melts. Chem Geol 366:52–60. https://doi.org/10.1016/j.chemgeo.2013.12.015
Burnham AD, Berry AJ (2017) Formation of Hadean granites by melting of igneous crust. Nat Geosci 10:457–461. https://doi.org/10.1038/ngeo2942
Burnham A, Berry A, Halse H, Schofield P, Cibin G, Mosselmans J (2015) The oxidation state of europium in silicate melts as a function of oxygen fugacity, composition and temperature. Chem Geol 411:248–259. https://doi.org/10.1016/j.chemgeo.2015.07.002
Candela PA, Piccoli PM (2005) Magmatic Processes in the development of porphyry-type ore systems. In: Hedenquist JW, Thompson JFH, Goldfarb RJ, Richards JP (eds) Economic Geology 100th Anniversary Volume, pp 25–37
Cao M, Evans NJ, Hollings P, Cooke DR, McInnes BIA, Qin K (2021) Apatite texture, composition, and O-Sr-Nd isotope signatures record magmatic and hydrothermal fluid characteristics at the Black Mountain porphyry deposit, Philippines. Econ Geol 116:1189–1207. https://doi.org/10.5382/econgeo.4827
Cayley RA (2011) Exotic crustal block accretion to the eastern Gondwanaland margin in the Late Cambrian–Tasmania, the Selwyn Block, and implications for the Cambrian–Silurian evolution of the Ross, Delamerian, and Lachlan Orogens. Gondwana Res 19:628–649. https://doi.org/10.1016/j.gr.2010.11.013
Celis M, Bouzari F, Bissig T, Hart C, Ferbey T (2013) Petrographic characteristics of porphyry indicator minerals from alkalic porphyry copper-gold deposits in south-central British Columbia (NTS 092, 093). Geoscience BC Summary Activities 2013 Geoscience BC(Report 2014–1):53–62
Chelle-Michou C, Chiaradia M (2017) Amphibole and apatite insights into the evolution and mass balance of cl and S in magmas associated with porphyry copper deposits. Contrib Mineral Petrol 172. https://doi.org/10.1007/s00410-017-1417-2
Chelle-Michou C, Chiaradia M, Ovtcharova M, Ulianov A, Wotzlaw J-F (2014) Zircon petrochronology reveals the temporal link between porphyry systems and the magmatic evolution of their hidden plutonic roots (the Eocene Coroccohuayco deposit, Peru). Lithos 198:129–140. https://doi.org/10.1016/j.lithos.2014.03.017
Chu M-F, Wang K-L, Griffin WL, Chung S-L, O’Reilly SY, Pearson NJ, Iizuka Y (2009) Apatite composition: tracing petrogenetic processes in Transhimalayan granitoids. J Petrol 50:1829–1855. https://doi.org/10.1093/petrology/egp054
Claiborne LL, Miller CF, Wooden JL (2010) Trace element composition of igneous zircon: a thermal and compositional record of the accumulation and evolution of a large silicic batholith, Spirit Mountain, Nevada. Contrib Mineral Petrol 160:511–531. https://doi.org/10.1007/s00410-010-0491-5
Cooke DR, Hollings P, Wilkinson JJ, Tosdal RM (2014) Geochemistry of porphyry deposits. In Scott, S.D. (ed) Treatise on Geochemistry, 2nd edn, pp 357–381. https://doi.org/10.1016/b978-0-08-095975-7.01116-5
Cooke DR, Agnew P, Hollings P, Baker M, Chang Z, Wilkinson JJ, Ahmed A, White NC, Zhang L, Thompson J, Gemmell JB, Danyushevsky L, Chen H (2020) Recent advances in the application of mineral chemistry to exploration for porphyry copper–gold–molybdenum deposits: Detecting the geochemical fingerprints and footprints of hypogene mineralization and alteration. Geochem: Explor Env A 20:176–188. https://doi.org/10.1144/geochem2019-039
Dilles JH, Kent AJR, Wood JL, Tosdal RM, Koleszar AK, Lee RG, Farmer LP (2015) Zircon compositional evidence for sulfur-degassing from ore-forming arc magmas. Econ Geol 110:241–251. https://doi.org/10.2113/econgeo.110.1.241
Donovan JJ, Singer JW, Armstrong JT (2016) A new EPMA method for fast trace element analysis in simple matrices. Am Mineral 101:1839–1853. https://doi.org/10.2138/am-2016-5628
Duncan R, Huston D, Bastrakov E, Thomas M (2018) Known mineral occurrences in Stavely. In: Schofield A (ed) Regional geology and mineral systems of the Stavely Arc, western Victoria. Geoscience Australia, Canberra, pp 135–150
Dunn T, Sen C (1994) Mineral/matrix partition coefficients for orthopyroxene, plagioclase, and olivine in basaltic to andesitic systems: a combined analytical and experimental study. Geochim Cosmochim Acta 58:717–733
Ferry J, Watson E (2007) New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers. Contrib Mineral Petrol 154:429–437. https://doi.org/10.1007/s00410-007-0201-0
Foden J, Elburg MA, Turner S, Sandiford M, O’Callaghan J, Mitchell S (2002) Granite production in the delamerian orogen, South Australia. J Geol Soc Lond 159:557–575. https://doi.org/10.1144/0016-764901-099
Foden J, Elburg MA, Dougherty-Page J, Burtt A (2006) The timing and duration of the Delamerian Orogeny: correlation with the Ross Orogen and implications for Gondwana assembly. J Geol 114:189–210. https://doi.org/10.1086/499570
Foden J, Elburg M, Turner S, Clark C, Blades ML, Cox G, Collins AS, Wolff K, George C (2020) Cambro-Ordovician magmatism in the Delamerian orogeny: implications for tectonic development of the southern Gondwanan margin. Gondwana Res 81:490–521. https://doi.org/10.1016/j.gr.2019.12.006
Foster DA, Gray DR (2000) Evolution and structure of the Lachlan Fold Belt (Orogen) of eastern Australia. Annu Rev Earth Planet Sci 28:47–80. https://doi.org/10.1146/annurev.earth.28.1.47
Gagnevin D, Daly JS, Kronz A (2010) Zircon texture and chemical composition as a guide to magmatic processes and mixing in a granitic environment and coeval volcanic system. Contrib Mineral Petrol 159:579–596. https://doi.org/10.1007/s00410-009-0443-0
Gravestock DI, Alley NF, Benbow MC, Cowley WM, Farrand MG, Flint RB, Gatehouse CG, Krieg GW, Preiss WV (2012) Early and Middle Palaeozoic. In: Drexel JF, Preiss WV (eds) The geology of South Australia, Vol. 2, The Phanerozoic. Geological Survey of South Australia, Bulletin 54, Adelaide, pp 3–61
Greenfield JE, Musgrave RJ, Bruce MC, Gilmore PJ, Mills KJ (2011) The Mount Wright Arc: a Cambrian subduction system developed on the continental margin of East Gondwana, Koonenberry Belt, eastern Australia. Gondwana Res 19:650–669. https://doi.org/10.1016/j.gr.2010.11.017
Hong W, Fabris A, Wise T, Collins AS, Gilbert S, Selby D, Curtis S, Reid AJ (2023) Metallogenic setting and temporal evolution of porphyry Cu–Mo mineralization and alteration in the Delamerian Orogen, South Australia: insights from zircon U–Pb, molybdenite Re–Os, and white mica Rb–Sr geochronology. Econ Geol 118:1291–1318. https://doi.org/10.5382/econgeo.5012
Hosking AJ (1970) Open File Envelop No. 1088. Netley Hill. Progress and final reports to licence expiry/Surrender for the Period 1/12/1986 to 30/11/1969, pp 1-126
Huang W, Stock MJ, Guyett, PC, Xia X-P, Liang H, Sun W, Lai C-k (2024) Reconstructing volatile exsolution in a porphyry ore forming magma chamber: perspectives from apatite inclusions. Am Mineral in press. https://doi.org/10.2138/am-2023-8963
Jackson SE, Pearson NJ, Griffin WL, Belousova EA (2004) The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem Geol 211:47–69. https://doi.org/10.1016/j.chemgeo.2004.06.017
Johnson EL, Phillips G, Allen CM (2016) Ediacaran–Cambrian basin evolution in the Koonenberry Belt (eastern Australia): implications for the geodynamics of the Delamerian Orogen. Gondwana Res 37:266–284. https://doi.org/10.1016/j.gr.2016.04.010
Keith JD, WJA, Hattori K, Ballanyyne GH, Christiansen EH, Barr DL, Cannan TM, Hook CJ (1997) The role of magmatic sulfides and mafic alkaline magmas in the Binghan and Tinitic mining districts, Utah. J Petrol 38:1679–1690
Kemp AIS, Hawkesworth CJ, Collins WJ, Gray CM, Blevin PL (2009) Isotopic evidence for rapid continental growth in an extensional accretionary orogen: the tasmanides, eastern Australia. Earth Planet Sci Lett 284:455–466. https://doi.org/10.1016/j.epsl.2009.05.011
Kimura J-I, Yoshida T (2006) Contributions of slab fluid, mantle wedge and crust to the origin of quaternary lavas in the NE Japan arc. J Petrol 47:2185–2232. https://doi.org/10.1093/petrology/egl041
Klein M, Stosch H-G, Seck H (1997) Partitioning of high field-strength and rare-earth elements between amphibole and quartz-dioritic to tonalitic melts: an experimental study. Chem Geol 138:257–271. https://doi.org/10.1016/S0009-2541(97)00019-3
Konecke BA, Fiege A, Simon AC, Parat F, Stechern A (2017) Co-variability of S6+, S4+, and S2– in apatite as a function of oxidation state: implications for a new oxybarometer. Am Mineral 102:548–557. https://doi.org/10.2138/am-2017-5907
Konecke BA, Fiege A, Simon AC, Linsler S, Holtz F (2019) An experimental calibration of a sulfur-in-apatite oxybarometer for mafic systems. Geochim Cosmochim Acta 265:242–258. https://doi.org/10.1016/j.gca.2019.08.044
Langsford NR (1971) The investigation of the Bendigo copper and molybdenum prospect – report No. 1. Report Book 71/129. Department of Mines, South Australia., p 62
Langsford NR (1973) The investigation of the Bendigo copper and molybdenum prospect – report No. 4 – Diamond drilling. Report Book 73/63. Department of Mines, South Australia., p 33
Lee RG, Dilles JH, Tosdal RM, Wooden JL, Mazdab FK (2017) Magmatic evolution of granodiorite intrusions at the El Salvador Porphyry Cu deposit, Chile, based on trace element composition and U/Pb age of zircons. Econ Geol 112:245–273. https://doi.org/10.2113/econgeo.112.2.245
Lee RG, Byrne K, D’Angelo M, Hart CJR, Hollings P, Gleeson SA, Alfaro M (2020) Using zircon trace element composition to assess porphyry copper potential of the Guichon Creek batholith and Highland Valley Copper deposit, south-central British Columbia. https://doi.org/10.1007/s00126-020-00961-1. Mineral Deposita
Li H, Hermann J (2017) Chlorine and fluorine partitioning between apatite and sediment melt at 2.5 GPa, 800 C: a new experimentally derived thermodynamic model. Am Mineral 102:580–594. https://doi.org/10.2138/am-2017-5891
Li Q, Sun X, Lu Y, Wang F, Hao J (2021) Apatite and zircon compositions for Miocene mineralizing and barren intrusions in the Gangdese porphyry copper belt of southern Tibet: implication for ore control. Ore Geol Rev 39:104474. https://doi.org/10.1016/j.oregeorev.2021.104474
Lloyd JC, Blades ML, Counts JW, Collins AS, Amos KJ, Wade BP, Hall JW, Hore S, Ball AL, Shahin S, Drabsch M (2020) Neoproterozoic geochronology and provenance of the Adelaide Superbasin. Precambrian Res 350:105849. https://doi.org/10.1016/j.precamres.2020.105849
Lloyd JC, Collins AS, Blades ML, Gilbert SE, Amos KJ (2022) Early evolution of the Adelaide Superbasin. Geosciences 12:154. https://doi.org/10.3390/geosciences12040154
Loader MA, Wilkinson JJ, Armstrong RN (2017) The effect of titanite crystallisation on Eu and Ce anomalies in zircon and its implications for the assessment of porphyry Cu deposit fertility. Earth Planet Sci Lett 472:107–119. https://doi.org/10.1016/j.epsl.2017.05.010
Loader MA, Nathwani CL, Wilkinson JJ, Armstrong RN (2022) Controls on the magnitude of ce anomalies in zircon. Geochim Cosmochim Acta 328:242–257. https://doi.org/10.1016/j.gca.2022.03.024
Loucks RR (2014) Distinctive composition of copper-ore-forming arcmagmas. Aust J Earth Sci 61:5–16. https://doi.org/10.1080/08120099.2013.865676
Loucks RR, Fiorentini ML, Henríquez GJ (2020) New magmatic oxybarometer using trace elements in zircon. J Petrol 61:egaa034. https://doi.org/10.1093/petrology/egaa034
Lu Y-J, Loucks RR, Fiorentini M, McCuaig TC, Evans NJ, Yang Z-M, Hou Z-Q, Kirkland CL, Parra-Avila LA, Kobussen A (2016) Zircon compositions as a pathfinder for porphyry Cu ± Mo ± au deposits. SEG Special Publication 19:329–347
Lu Y-J, Smithies RH, Wingate MTD, Evans NJ, McCuaig TC, Champion D, Outhwaite M (2019) Zircon fingerprinting of magmatic–hydrothermal systems in the Archean Yilgarn Craton. Geological Survey of Western Australia, Report 197, 22 p
Mao M, Rukhlov AS, Rowins SM, Spence J, Coogan LA (2016) Apatite trace element compositions: a robust new tool for mineral exploration. Econ Geol 111:1187–1222. https://doi.org/10.2113/econgeo.111.5.1187
McDonald GD (1992) The petrology and timing of the Anabama Granite and associated igneous activity, Olary region, S.A. B.Sc. (Honours) thesis, Adelaide, The University of Adelaide, 40 p
McDonough WF, Sun S-s (1995) The compositions of the Earth. Chem Geol 120:223–253. https://doi.org/10.1016/0009-2541(94)00140-4
Meng X, Kleinsasser JM, Richards JP, Tapster SR, Jugo PJ, Simon AC, Kontak DJ, Robb L, Bybee GM, Marsh JH, Stern RA (2021) Oxidized sulfur-rich arc magmas formed porphyry Cu deposits by 1.88 Ga. Nat Commun 12:2189. https://doi.org/10.1038/s41467-021-22349-z
Miles A, Graham C, Hawkesworth C, Gillespie M, Hinton R, Bromiley G (2014) Apatite: a new redox proxy for silicic magmas? Geochim Cosmochim Acta 132:101–119. https://doi.org/10.1016/j.gca.2014.01.040
Miller JS, Wooden JL (2004) Residence, resorption and recycling of zircons in devils Kitchen rhyolite, Coso volcanic field, California. J Petrol 45:2155–2170. https://doi.org/10.1093/petrology/egh051
Morris BJ (1977) An investigation of copper and molybdenum mineralization at Anabama Hill and the Cronje Dam copper prospect. Department of Mines, South Australia, vol 03054. Open file Envelope, p 155
Nathwani CL, Loader MA, Wilkinson JJ, Buret Y, Sievwright RH, Hollings P (2020) Multi-stage arc magma evolution recorded by apatite in volcanic rocks. Geology 48:323–327. https://doi.org/10.1130/G46998.1
Nathwani CL, Simmons AT, Large SJE, Wilkinson JJ, Buret Y, Ihlenfeld C (2021) From long–lived batholith construction to giant porphyry copper deposit formation: petrological and zircon chemical evolution of the Quellaveco District, Southern Peru. Contrib Mineral Petrol 176:12. https://doi.org/10.1007/s00410-020-01766-1
Paolillo L, Chiaradia M, Ulianov A (2022) Zircon Petrochronology of the Kişladaǧ Porphyry au Deposit (Turkey). Econ Geol 117:401–422. https://doi.org/10.5382/econgeo.4864
Parat F, Holtz F (2004) Sulfur partitioning between apatite and melt and effect of sulfur on apatite solubility at oxidizing conditions. Contrib Mineral Petrol 147:201–212. https://doi.org/10.1007/s00410-021-01878-2
Parra-Avila L, Hammerli J, Kemp A, Rohrlach B, Loucks R, Lu Y, Williams I, Martin L, Roberts M, Fiorentini M (2022) The long-lived fertility signature of Cu–Au porphyry systems: insights from apatite and zircon at Tampakan, Philippines. Contrib Mineral Petrol 177:1–22
Pasero M, Kampf AR, Ferraris C, Pekov IV, Rakovan J, White TJ (2010) Nomenclature of the apatite supergroup minerals. Eur J Min 22:163–179. https://doi.org/10.1127/0935-1221/2010/0022-2022
Pearce NJ, Perkins WT, Westgate JA, Gorton MP, Jackson SE, Neal CR, Chenery SP (1997) A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostandard Newslett 21:115–144. https://doi.org/10.1111/j.1751-908X.1997.tb00538.x
Peng G, Luhr JF, McGee JJ (1997) Factors controlling sulfur concentrations in volcanic apatite. Am Mineral 82:1210–1224. https://doi.org/10.2138/am-1997-11-1217
Piccoli P, Candela P (1994) Apatite in felsic rocks: a model for the estimation of initial halogen concentrations in the Bishop Tuff (Long Valley) and Tuolumne Intrusive suite (Sierra Nevada Batholith) magmas. Am J Sci 294:92–135. https://doi.org/10.2475/ajs.294.1.92
Preiss W (2000) The Adelaide Geosyncline of South Australia and its significance in neoproterozoic continental reconstruction. Precambrian Res 100:21–63. https://doi.org/10.1016/S0301-9268(99)00068-6
Richards JP (2003) Tectono-magmatic precursors for porphyry Cu-(Mo-Au) deposit formation. Econ Geol 98:1515–1533. https://doi.org/10.2113/gsecongeo.98.8.1515
Richards JP (2009) Postsubduction porphyry Cu-Au and epithermal au deposits: products of remelting of subduction-modified lithosphere. Geology 37:247–250. https://doi.org/10.1130/g25451a.1
Richards JP (2011) High Sr/Y arc magmas and porphyry Cu ± Mo ± au deposits: just add water. Econ Geol 106:1075–1081. https://doi.org/10.2113/econgeo.106.7.1075
Richards JP (2015) The oxidation state, and sulfur and Cu contents of arc magmas: implications for metallogeny. Lithos 233:27–45. https://doi.org/10.1016/j.lithos.2014.12.011
Richards JP, Spell T, Rameh E, Razique A, Fletcher T (2012) High Sr/Y magmas reflect arc maturity, high magmatic water content, and porphyry Cu ± Mo ± au potential: examples from the Tethyan arcs of central and eastern Iran and western Pakistan. Econ Geol 107:295–332. https://doi.org/10.2113/econgeo.107.2.295
Richards JP, López GP, Zhu J-J, Creaser R, Locock A, Mumin AH (2017) Contrasting tectonic setting and sulfur contents of magmas associated with cretaceous porphyry Cu ± Mo ± au and intrusion-related iron oxide Cu-Au deposits in northern Chile. Econ Geol 112:295–318. https://doi.org/10.2113/econgeo.112.2.295
Robertson K, Taylor D, Thiel S, Heinson G (2015) Magnetotelluric evidence for serpentinisation in a Cambrian subduction zone beneath the Delamerian Orogen, southeast Australia. Gondwana Res 28:601–611. https://doi.org/10.1016/j.gr.2014.07.013
Rowe MC, Kent AJ, Nielsen RL (2009) Subduction influence on oxygen fugacity and trace and volatile elements in basalts across the Cascade Volcanic Arc. J Petrol 50:61–91. https://doi.org/10.1093/petrology/egn072
Sano Y, Terada K, Fukuoka T (2002) High mass resolution ion microprobe analysis of rare earth elements in silicate glass, apatite and zircon: lack of matrix dependency. Chem Geol 184:217–230. https://doi.org/10.1016/S0009-2541(01)00366-7
Schofield A (2018) Regional geology and mineral systems of the Stavely Arc, western Victoria. Geoscience Australia, Canberra, p 220. Record 2018/02
Seedorff E, Dilles JH, Proffett JM, Einaudi MT, Zurcher L, Stavast WJ, Johnson DA, Barton MD (2005) Porphyry deposits: Characteristics and origin of hypogene features. In: Hedenquist JW, Thompson JFH, Goldfarb RJ, Richards JP (eds) Economic Geology 100th Anniversary Volume, pp 251–298
Shen P, Hattori K, Pan H, Jackson S, Seitmuratova E (2015) Oxidation condition and metal fertility of granitic magmas: zircon trace-element data from porphyry Cu deposits in the central Asian Orogenic Belt. Econ Geol 110:1861–1878. https://doi.org/10.2113/econgeo.110.7.1861
Sillitoe RH (2010) Porphyry copper systems. Econ Geol 105:3–41. https://doi.org/10.2113/gsecongeo.105.1.3
Stavely Minerals (2022) The Stavely copper-gold project – Dawn of a new world-class copper-gold province in Western Victoria, MRE presentation, June 2022, p 31
Stokes TN, Bromiley GD, Potts NJ, Saunders KE, Miles AJ (2019) The effect of melt composition and oxygen fugacity on manganese partitioning between apatite and silicate melt. Chem Geol 506:162–174. https://doi.org/10.1016/j.chemgeo.2018.12.015
Sun X, Deng J, Lu Y, Si X, Hollings P, Santosh M, Li Q, Zheng X (2023) Two stages of porphyry Cu mineralization at Jiru in the tibetan collisional orogen: insights from zircon, apatite, and magmatic sulfides. GSA Bull 135:2971–2986. https://doi.org/10.1130/B36741.1
Thompson J, Meffre S, Maas R, Kamenetsky V, Kamenetsky M, Goemann K, Ehrig K, Danyushevsky L (2016) Matrix effects in Pb/U measurements during LA-ICP-MS analysis of the mineral apatite. J Anal Atom Spectrom 31:1206–1215. https://doi.org/10.1039/C6JA00048G
Timm C, de Ronde CE, Leybourne MI, Layton-Matthews D, Graham IJ (2012) Sources of chalcophile and siderophile elements in Kermadec arc lavas. Econ Geol 107:1527–1538. https://doi.org/10.2113/econgeo.107.8.1527
Trail D, Watson EB, Tailby ND (2012) Ce and Eu anomalies in zircon as proxies for the oxidation state of magmas. Geochim Cosmochim Acta 97:70–87. https://doi.org/10.1016/j.gca.2012.08.032
Turner S, Foden J, Morrison R (1992) Derivation of some A-type magmas by fractionation of basaltic magma: an example from the Padthaway Ridge, South Australia. Lithos 28:151–179. https://doi.org/10.1016/0024-4937(92)90029-X
Wallace P, Carmichael IS (1992) Sulfur in basaltic magmas. Geochim Cosmochim Acta 56:1863–1874. https://doi.org/10.1016/0016-7037(92)90316-B
Wallace PJ, Edmonds M (2011) The sulfur budget in magmas: evidence from melt inclusions, submarine glasses, and volcanic gas emissions. Rev Mineral Geochem 73:215–246. https://doi.org/10.2138/rmg.2011.73.8
Webster JD, Piccoli PM (2015) Magmatic apatite: a powerful, yet deceptive, mineral. Elements 11:177–182. https://doi.org/10.2113/gselements.11.3.177
Webster J, Goldoff B, Sintoni M, Shimizu N, De Vivo B (2014) C–O–H–Cl–S–F volatile solubilities, partitioning, and mixing in phonolitic–trachytic melts and aqueous–carbonic vapor ± saline liquid at 200 MPa. J Petrol 55:2217–2248. https://doi.org/10.1093/petrology/egu055
Wilkinson JJ (2013) Triggers for the formation of porphyry ore deposits in magmatic arcs. Nat Geosci 6:917–925. https://doi.org/10.1038/ngeo1940
Xie FW, Tang JX, Jia M, Lang XH, Sun Y, He YF, Liu JB (2023) Zircon and apatite fingerprints on the magma fertility for the jurassic igneous rocks in the southern Lhasa subterrane, Tibet. Ore Geol Rev 161:105662. https://doi.org/10.1016/j.oregeorev.2023.105662
Xing K, Shu Q, Lentz DR, Wang F (2020) Zircon and apatite geochemical constraints on the formation of the Huojihe porphyry Mo deposit in the lesser Xing’an Range, NE China. Am Mineral 105:382–396. https://doi.org/10.2138/am-2020-7226
Xu B, Hou Z-Q, Griffin WL, Lu Y, Belousova E, Xu J-F, O’Reilly SY (2021) Recycled volatiles determine fertility of porphyry deposits in collisional settings. Am Mineral 106:656–661. https://doi.org/10.2138/am-2021-7714
Zajacz Z, Candela PA, Piccoli PM, Wälle M, Sanchez-Valle C (2012) Gold and copper in volatile saturated mafic to intermediate magmas: solubilities, partitioning, and implications for ore deposit formation. Geochim Cosmochim Acta 91:140–159. https://doi.org/10.1016/j.gca.2012.05.033
Zhu J-J, Richards JP, Rees C, Creaser R, DuFrane SA, Locock A, Petrus JA, Lang J (2018) Elevated magmatic sulfur and chlorine contents in ore-forming magmas at the Red Chris porphyry Cu-Au deposit, Northern British Columbia, Canada. Econ Geol 113:1047–1075. https://doi.org/10.5382/econgeo.2018.4581
Zimmer MM, Plank T, Hauri EH, Yogodzinski GM, Stelling P, Larsen J, Singer B, Jicha B, Mandeville C, Nye CJ (2010) The role of water in generating the calc-alkaline trend: new volatile data for Aleutian magmas and a new tholeiitic index. J Petrol 51:2411–2444. https://doi.org/10.1093/petrology/egq062
Acknowledgements
This study is part of “Delamerian porphyry mineral systems” research project from June 2020 to September 2022, supported by the Mineral Exploration Cooperative Research Centre (MinEx CRC, https://minexcrc.com.au) whose activities are funded by the Australian Government’s Cooperative Research Centre Program. This is MinEx CRC Document 2022/73. David Groom and Keryn Groom from South Australia Drill Core Reference Library are thanked for their help during drill core inspection and samplings. Aoife McFadden is thanked for assisting with BSE-CL and SEM-MLA imaging and data processing at Adelaide Microscopy. Stacey Curtis and Rian Dutch are appreciated for insightful discussion to this manuscript. Inspiring comments from Yongjun Lu and two other reviewers that improved this paper are appreciated.
Funding
Financially supported by MinEx CRC and the Geological Survey of South Australia.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editorial handling: C. N. Mercer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, W., Fabris, A., Gilbert, S. et al. Using zircon and apatite chemistry to fingerprint porphyry Cu – Mo ± Au mineralization in the Delamerian Orogen, South Australia. Miner Deposita (2024). https://doi.org/10.1007/s00126-024-01287-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00126-024-01287-y