Abstract
New data on the multiple sulfur isotope signature of Archean sulfides from country rocks and magmatic mineralization at the Moran deposit (Kambalda, Western Australia) were combined with previously published geochemical data to constrain the various stages of the dynamic evolution of this magmatic system, unveiling new insights into the transport mechanisms of sulfide liquids in komatiite magmas. Sulfides in the Archean magmatic and sedimentary host rocks of the komatiites display a unique mass-independent sulfur isotope signature (Δ33S), which records a photochemical reaction of sulfur in an oxygen-poor atmosphere prior to the Great Oxidation Event.
Sedimentary rocks that are thought to be assimilated by komatiite show a distinctly positive Δ33S signature (+ 0.9 to + 2.4‰). Early ore sulfides situated above these sedimentary rocks contain relatively few valuable metals and display an overlapping Δ33S range (+ 0.6 to + 1.0‰). Subsequent but still early ore sulfides are situated above basalt, as the sedimentary rocks were thermo-mechanically eroded by the sulfide melt, displaying more mantle-like signatures (+ 0.2 to + 0.3‰) and valuable metal content - indistinguishable from the main ore deposit. This reflects a progressive dilution of the contaminant signature by the magmatic isotope signature of the komatiite liquid. Calculated volumes of the interaction of silicate melt and sulfide melt to explain the metal tenor of the ore and its Δ33S signature indicate a decoupling between chemical and isotopic signatures. This can be explained by upgrading the sulfide melt with valuable metals simultaneously with the dissolution of sulfur in the komatiite melt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Archean sedimentary rocks provide a unique record of the composition of the ocean and atmosphere in the early Earth (e.g., Ono et al. 2009). Their sulfur content and isotopic sulfur signature provide key insights into the volatile cycle on the early Earth and can give crucial information on a wide range of processes pertaining to controls on paleoclimate, primordial life, and the genesis of ore deposits enriched in chalcophile and highly siderophile metals (e.g., Kaufman et al. 2007; Kump 2012; LaFlamme et al. 2018). Key to this understanding is the recent developments in multiple sulfur isotope systematics, which have unveiled the complexity of the sulfur cycle in the Archean eon, whereas prior to 2.7 Ga, a methane-rich atmosphere provided partial protection of the Earth’s surface from ultraviolet radiation, after 2.7 Ga methane was consumed by aerobic methanotrophy, increasing the amount of ultraviolet radiation reaching the Earth’s surface and causing widespread sulfur mass-independent fractionation (MIF; Farquhar et al. 2000; Havig et al. 2017; Liu et al. 2019). This shift coincided with the late Archean emplacement of abundant Large Igneous Provinces (LIPs), which fluxed the hydrosphere and atmosphere with mantle-derived sulfur that acquired a distinctive MIF signature (Havig et al. 2017).
Sulfur isotope values that deviate from the mass-dependent fractionation (MDF) line δ33S ≈ 0.515 × δ34S (Δ33S; Farquhar et al. 2000) by more than ± 0.2‰ are the result of MIF due to photochemical reaction of sulfur in a methane-poor atmosphere after ca. 2.7 Ga, which was also oxygen-poor prior to the Great Oxidation Event at ca. 2.4 Ga (Farquhar et al. 2000; Farquhar and Wing 2003; Liu et al. 2019). LaFlamme et al. (2018) revised the definition of the threshold between MDF and MIF processes based on the assessment of kinetic reactions from microbial or thermochemical sulfate reduction. This deviation is a function of δ34S and varies between the slopes of 0.508 and 0.519. Data that fall outside this range are assumed to contain MIF sulfur originating from Archean sediments or exhalites. Data falling inside this range could be either explained by MDF sulfur only or by MIF sulfur diluted or mixed with a different sulfur source. Therefore, data inside this range cannot be uniquely interpreted. In this framework, it is thought that reduction of S8 aerosols produced positive Δ33S values, whereas microbial reduction of seawater sulfate produced negative Δ33S values (Philippot et al. 2007; Ono et al. 2009). It was suggested by Chen et al. (2022) that neutral Δ33S values originate from sulfide nanoparticles from seafloor vents.
Archean sedimentary sulfur, possibly carrying a MIF signature, can be mobilized during subsequent geological activities and incorporated into ore deposits, such as in the case of komatiite-hosted magmatic Ni–Cu–(PGE) mineralization, where sedimentary sulfur is thought to have been locally assimilated by the komatiite magma upon emplacement (Lesher and Groves 1986; Bekker et al. 2009; Fiorentini et al. 2012a; Godel et al. 2012). Accordingly, multiple sulfur isotopes in mineralized Archean magmatic rocks have been primarily used to identify sulfur sources (e.g., Lesher and Groves 1986; Ohmoto 1986; Ripley 1986; Ripley and Li 2003; Bekker et al. 2009; Ding et al. 2012; Fiorentini et al. 2012a; Ripley and Li 2017). In fact, assimilation of crustal sulfur is required to form magmatic sulfide deposits associated with komatiite lava flows, which are highly sulfide undersaturated upon emplacement at the seafloor. Thus, in most cases, sulfide saturation in such melts can only be reached by incorporation of external sulfur (MacLean 1969; Lesher and Campbell 1993; Mavrogenes and O’Neill 1999; Keays and Lightfoot 2010). External sulfur with a MIF signature can then be preserved in the ore sulfides, and only dilution with MDF sulfur can change this signature back toward mantle values (Ripley and Li 2017).
Although geochemical and textural evidence of sediment assimilation has been documented in Archean komatiite-hosted sulfide deposits at Kambalda (Lesher and Arndt 1995; Staude et al. 2017a), Western Australia, there is not yet any conclusive identification of the sulfur source that triggered sulfide saturation in the magmas using multiple sulfur isotopes. Bekker et al. (2009) highlighted a discrepancy between the signature of magmatic sulfide ore from the Victor deposit (Δ33S~0.0‰) and that of sulfide-bearing chert in adjacent sedimentary rocks, which were deposited between the komatiite lava flows and post-dated the magmatic mineralizing event (Δ33S of + 0.9‰ and + 1.1‰ both approximately 20 m above the basal basalt-komatiite contact and − 0.1‰ approximately 50 m above the basal basalt-komatiite contact). The sulfur isotope composition of the sulfides underlying the mineralized channels along the ore-bearing stratigraphic level, which could have been presumably thermo-mechanically eroded during the ore-forming event, is not known from this dataset. Furthermore, a multiple sulfur isotope study by Caruso et al. (2017) focused on a komatiite-hosted deposit in the Widgiemooltha Dome about 40 km southwest of Kambalda. There, the magmatic sulfides, which show a well-defined positive MIF signature, overlie sulfidic black shales that do not display any MIF signature. This discrepancy was explained by sulfur assimilation from sedimentary sources located 10–100 km upstream from the mineralized area, consistent with localities throughout the Eastern Goldfields Superterrane (Caruso et al. 2017). Interestingly, the authors also highlighted the significant role of sulfur dioxide degassing in shifting the sulfur isotope composition of komatiite-hosted mineralization to lighter values.
LaFlamme et al. (2016) analyzed a sample from another komatiite-hosted deposit at Kambalda, the Moran deposit, to test the suitability of magmatic pentlandite, pyrrhotite, and chalcopyrite Δ33S values to develop a standard for in situ multiple sulfur isotope analysis. This work showed that Δ33S is around + 0.2‰ in equilibrium between these minerals, but δ34S exhibits a slight fractionation between them. In addition, this work showed that a sample from the neighboring Victor South deposit was different, with Δ33S values around 0.0‰. LaFlamme et al. (2016) proposed that this difference was caused by the different R factors (silicate melt:sulfide liquid mass ratio) that characterize these deposits, whereas Victor South has a higher R factor, and hence, an apparently diluted contaminated signature, the Moran deposit, shows more evidence of sedimentary contamination due to an apparently lower R factor (Fiorentini et al. 2012b).
A recent study of the Moran deposit in Kambalda (Staude et al. 2022) revealed a multi-stage sulfide evolution of the orebody. All stages of this progressive evolution are preserved as snapshots of a highly dynamic lava flow system: from early stages in the flank of the deposit to the late stages in a steep-sided embayment. In the present study, well-characterized samples from various stages of ore formation in combination with sedimentary sulfides from different stratigraphic positions are used to decipher the source of sulfur for the magmatic sulfides. In addition, the sedimentary sulfide package underneath the ore-bearing lava flow has been sampled in more detail to investigate variations within one sedimentation cycle that got assimilated by the komatiite. In this contribution, we will integrate the new multiple sulfur isotope data with previously published information on the dynamic komatiite lava flow emplacement and the detailed geochemical data provided from the various stages of sulfide ore formation.
Geology of the Kambalda komatiite camp
The Kambalda Domain is part of the 2.7 Ga Kalgoorlie Terrane of the Yilgarn Craton in Western Australia (Gresham and Loftus-Hills 1981; Goscombe et al. 2009; Smithies et al. 2022). The preserved stratigraphy starts with the tholeiitic Lunnon Basalt at the base (Squire et al. 1998; Said et al. 2010). Rarely, the Lunnon Basalt contains interflow sedimentary rocks (Squire et al. 1998), which were intersected in a drill hole of the Long-Victor mine about 250 m underneath the contact with the komatiite. These sedimentary rocks comprise chlorite-rich layers and sulfide-bearing laminated chert (Figs. 1c and 2a, b). In the Long-Victor mine, an up to 30-m-thick layer of coarse-grained (grain size of ca. 0.5 cm) basalt was found containing widespread millimeter-sized magmatic sulfide globules (Fig. 2c). This layer is located about 100 m below the contact with the younger komatiite. The basalt is overlain by up to 10 m of sedimentary rocks with chlorite-rich layers and sulfide-bearing chert (Fig. 2d), referred to as “contact sediments,” as opposed to “interflow sediments” between basalt or komatiite lava flows. The chlorite-rich layers were interpreted to originate from the erosion of the basalt, whereas the chert was interpreted to be of chemical and volcaniclastic origin (Bavinton and Keays 1978; Bavinton 1981; Beresford and Cas 2001).
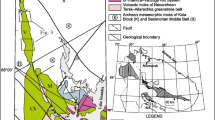
Geological maps and profiles of the sample locations for this study. a Map of the simplified Kambalda geology with highlighted Long and Victor channels and the Moran deposit, which is enlarged in b. The location of profiles A to C is shown in red and displayed in c to e. The inset shows the location of the Kambalda camp within the Yilgarn Craton (after Goscombe et al. 2009). b Sample locations of lithologies on the basalt-komatiite contact (sedimentary rocks and magmatic sulfides) as well as disseminated magmatic sulfides of the Lunnon Basalt, which are located around 100 m underneath the basalt-komatiite contact. Samples from the flank of Moran are partly situated on contact sedimentary rocks (blue circles) and partly on basalt (green circle). Samples from Moran are situated in an embayment about 40 m below the original basalt-komatiite surface. c Profile A-A′ along drill hole LG15-226 showing the location of the interflow sediment samples within the Lunnon Basalt. d Profile B-B′ along drill hole LG16-394 showing the location of the magmatic interflow sulfide samples. e Profile C-C′ along drill hole LSU-373A showing the location of interflow sediment samples within the komatiite. Sample colors and symbols are the same as in Figs. 3 and 4. Numbers are the same as in Figs. 3 and 4 and Table 1
Photos of representative samples for this study. a Drill core LG15-226 showing the interflow sulfide-bearing interflow sedimentary rock within the Lunnon Basalt. b Photomicrograph of the Lunnon Basalt interflow sediment. The main sulfide is pyrrhotite with minor chalcopyrite, pyrite, and sphalerite. c Photomicrograph of disseminated sulfides within the Lunnon Basalt. The main sulfide pyrrhotite is intergrown with chalcopyrite, pyrite, and ilmenite. d Sample of contact sediment overlain by magmatic sulfides of the flank at the Moran deposit
The contact sedimentary rock is overlain by the Silver Lake Komatiite, which has been dated between 2.71 and 2.70 Ga based on coeval felsic volcanism in other parts of the Kalgoorlie Terrane (Kositcin et al. 2008). Basal and channelized lava flows host the Ni sulfide deposits in thermo-mechanically eroded embayments within the channel. The thermo-mechanical erosion removed the contact sediments along the channel: where the embayments occur, the underlying upper portion of the Lunnon Basalt was also removed (up to 40 m in the embayment at the Moran deposit; Staude et al. 2016, 2017a). Next to the lava channels, the sheet flow facies occur (Gole and Barnes 2020), comprising thin (meters to tens of meters) lava flows underlain by contact sedimentary rocks, which are generally preserved.
Locally, a second lava flow on top of the basal one also contains magmatic sulfides (Groves et al. 1986; Barnes et al. 2016). One section containing these uneconomic interflow magmatic sulfides of the second lava flow was intersected in the Long-Victor mine, about 10 m above the basalt-komatiite contact (Fig. 1d). Between lava flows of the Silver Lake Komatiite, interflow sedimentary chlorite-bearing and sulfide-bearing cherty sedimentary rocks are ubiquitously deposited (Fig. 1e; Gresham and Loftus-Hills 1981). The Tripod Hill Komatiite above is barren of magmatic sulfides and does not contain interflow sedimentary rocks (Gresham and Loftus-Hills 1981). It is overlain by younger volcanic and sedimentary rocks and intruded by felsic to intermediate dykes, sills, and stocks. The whole stratigraphy was metamorphosed to lower amphibolite facies and folded to form the present dome structure (Gresham and Loftus-Hills 1981). Igneous textures are preserved in many magmatic rocks despite the metamorphic overprint, and hence, the prefix “meta” is omitted in literature.
Geology of the Moran orebody
The Moran orebody is part of the Long Channel in Kambalda and is situated south of the Long orebody (Fig. 1). Compared to other Kambalda orebodies, it is well preserved with little younger magmatic or deformational overprint (Staude et al. 2016, 2017a, 2022). Snapshots of various ore-forming stages in this highly dynamic magmatic system are preserved. Staude et al. (2022) interpreted massive sulfides on the flanks of the deposit as the remnants of an early concave embayment. Flank sulfides were cut off from further mineralization and frozen into place by the formation of a steep-sided embayment in the center of the komatiite lava channel by thermo-mechanical erosion. On the flank, sulfides farthest away from the channel center are found on contact sedimentary rocks and are thought to be the oldest preserved ore sulfides. Sulfides closer to the embayment, but still on the flanks, are found on basalt due to the erosion of the contact sedimentary rocks and represent the next stage in ore formation (Staude et al. 2022). The sulfide melt pool that formed the steep-sided embayment formed next. It is surrounded by an undercut feature, called pinchout, where magmatic sulfides occur above and underneath older basalt due to thermo-mechanical erosion that worked sideways (Staude et al. 2016). This sulfide melt pool crystallized into the main orebody and is overlain by net-textured ore, which is thought to represent the youngest ore-forming stage.
The solidified flank sulfides were interpreted to reflect their geochemical composition during freezing based on major and trace element values (Staude et al. 2022). Sulfides farther away from the embayment display a relatively low Ni tenor (Ni in 100% sulfide) of 6% and a higher Ni tenor of 12% closer to the embayment, which is similar to the calculated average of the main orebody (16%). This represents a gradual upgrade of the sulfide melt by the komatiite melt in the evolving ore-forming system. On the other hand, based on trace element data that are consistent with a fractional crystallization trend (Staude et al. 2022), it is inferred that the main orebody crystallized from the edge (the pinchout) toward the center of a homogenous melt pool. Elements compatible in the crystallizing sulfides (e.g., Ir, Os, Ru, Rh) are concentrated in the pinchout, whereas incompatible elements (e.g., Pt, Pd, Au) are concentrated in the center of the orebody.
Sample description
Samples have been collected underground and from drill holes to cover sedimentary and magmatic sulfides from (1) pre-ore stages, (2) various ore stages, and (3) post-ore stages.
-
(1)
Pre-ore sulfides include sulfide-bearing interflow chert within the Lunnon Basalt (Figs. 2a, b and 3), magmatic sulfides of the Lunnon Basalt (Figs. 2c and 3), and sulfide-bearing sedimentary rocks on the contact of the Lunnon Basalt with the overlying Silver Lake Komatiite unit (Figs. 2d and 3). One drill hole (LSU-413) intersected 11.9 m (2–2.1 m true widths due to the oblique angle of the drill hole) of contact sedimentary rocks. Two basal samples (sample LSU-413_1 and _2) were taken as representatives of the mafic volcaniclastic rocks, now rich in amphibole with variable amounts of biotite, chlorite, garnet, quartz, and feldspar. Two samples (sample LSU-413_3 and _4) were taken closer to the komatiite unit, representing carbonate-rich chert with minor biotite and amphibole. Sulfides hosted in sedimentary rocks are mostly pyrrhotite with minor amounts of chalcopyrite and sphalerite. They form either bands a few millimeters in thickness or millimeter-sized irregular aggregates. Magmatic sulfides of the Lunnon Basalt comprise millimeter-sized pyrrhotite globules with minor chalcopyrite and pentlandite exsolution flames.
-
(2)
Ore stage sulfides are massive sulfides of the Moran deposit (Figs. 1b and 3) and include early-stage sulfides from the flank situated on top of relic contact chert, early-to-intermediate-stage sulfides from the flank situated on basalt, and main-stage sulfides from the steep-sided embayment. Net-textured sulfides were sampled but could not be analyzed due to their small grain size. Massive sulfides comprise foliated pyrrhotite and pentlandite with minor interstitial chalcopyrite.
-
(3)
Post-ore stage sulfides include magmatic interflow sulfides (Figs. 1d and 3) and chert-hosted sulfides from about 20 m above the basalt-komatiite contact (Figs. 1e and 3). Magmatic sulfides comprise pyrrhotite and minor pentlandite. Sulfides in komatiite interflow chert are similar to older sulfides in Lunnon Basalt interflow and contact chert.
Methods
In situ triple sulfur isotope (32S, 33S, 34S) ratios were measured on a CAMECA IMS 1280 ion microprobe hosted by the Centre for Microscopy Characterisation and Analysis, at the University of Western Australia. Resin mounts with polished samples were trimmed at 1 cm of the side of the mount to accommodate a standard block, containing matrix-match standards within the SIMS sample holder. Regions of interest were located close to the standard block, within 5 mm from the center of the mount. After careful cleaning in ethanol and deionized water, both resin mount and standard block were given a ~ 20 nm gold coat to prevent charging during analysis.
The sample surface was sputtered over a 10 × 10 μm area with a 10 kV, Gaussian Cs+ beam with an intensity of ~ 2.5 nA, and a total impact energy of 20 keV. Secondary ions were admitted in the double-focusing mass spectrometer within a 75 μm entrance slit and focused within the center of a 3000 μm field aperture (× 130 magnification). Energy was filtered using a 30 eV bandpass with a 5 eV gap toward the high-energy side. All S isotopes (32S, 33S, and 34S) were collected simultaneously in Faraday cup detectors fitted with 1010 Ω (L’2, 32S) and 1011 Ω (L1, 33S and H1, 34S) resistors, all operating at a mass resolution of ~ 2500. The 32S1H and 33S peaks are not completely resolved under these conditions; thus, the magnetic field was offset slightly to the low-mass side to avoid interference from 32S1H on the 33S peak. The magnetic field was regulated using NMR control. Each analysis includes a pre-sputtering over a 15 × 15 μm area for 30 s, followed by the automatic centering of the secondary ions in the field aperture. Each analysis then consists of a 20 4-s cycles of acquisition. The analytical session was monitored in terms of drift using two bracketing standards every 5–6 sample analyses. Instrumental mass fractionation (IMF) was corrected using the matrix-matched reference material for pyrrhotite (Alexo pyrrhotite: Δ33S − 0.96 ± 0.04‰; LaFlamme et al. 2016). Results for SIMS measurements are reported as 34S relative to Vienna-Canyon Diablo troilite (VCDT). Data processing follows the procedure described by LaFlamme et al. (2016). Summarized results for each sample are given in Table 1, and individual analyses and standard analyses are given in electronic supplementary material (ESM) 1.
Results
There are large variations in pyrrhotite Δ33S values of both sedimentary rocks and magmatic sulfides (Table 1, Fig. 4, ESM 1). The oldest sulfides in the stratigraphy, which are hosted by the sedimentary interflow chert in the Lunnon Basalt, display a Δ33S value of + 0.5 ± 0.1‰. Higher in the stratigraphy, disseminated magmatic sulfides within the Lunnon Basalt display Δ33S values of + 0.2 ± 0.1‰. Contact sedimentary rocks, which are thought to be the source of sulfur to form the komatiite-hosted Ni deposits at Kambalda (Lesher et al. 1984; LaFlamme et al. 2016), are all well above 0‰ and show a large variation ranging from + 0.9 ± 0.1 to + 2.4 ± 0.1‰. The samples from drill hole LSU-413 display Δ33S values ranging from + 1.2 ± 0.1 to + 2.4 ± 0.1‰ within this one contact sedimentary unit.
Summary of the sulfur isotope data of pyrrhotite from different stratigraphic levels at Kambalda. Numbers on the contact sediment pyrrhotite represent the sample number of drill hole LSU-413 in Table 1. The uncertainty (2σ) is shown with error bars; however, in some instances, the error bars are smaller than the symbol size. Numbers are the same as in Figs. 1 and 3 and Table 1. The MDF sulfur range is adopted from LaFlamme et al. (2018) based on the assessment of kinetic reactions from microbial or thermochemical sulfate reduction. This deviation is a function of δ34S and varies between the slopes of 0.508 and 0.519. 1Bekker et al. (2009); 2LaFlamme et al. (2016); 3LaFlamme et al. (2018)
Flank massive sulfides farthest away from the main orebody are situated on contact sedimentary rocks and display Δ33S values varying between + 0.6 ± 0.1 and + 1.0 ± 0.1‰. Pyrrhotite from massive sulfides located closer to the main orebody, which is still on the flank but is directly overlying the basalt (i.e., the contact sediment had already been eroded at the time of crystallization of the sulfide liquid), exhibits a value of + 0.3 ± 0.1‰. This value is undistinguishable from the signature of massive sulfides of the main orebody within the steep-sided embayment, which only displays a small Δ33S range from + 0.2 ± 0.1 to + 0.3 ± 0.1‰.
Younger komatiite interflow magmatic sulfides (about 10 m above basal komatiite contact) show a Δ33S value of + 0.2 ± 0.1‰, which is also similar to the signature of the main Moran orebody. Post-ore komatiite interflow sedimentary rocks vary from 0.0 ± 0.1‰ (this work) to + 0.9–1.1‰ (data from Bekker et al. 2009; all about 20 m above the basal komatiite contact) and to − 0.1‰ (data from Bekker et al. 2009) about 50 m above the basal komatiite contact. The stratigraphic location within the drill hole was taken from the IGO Limited (now Wyloo Metals) database using sample numbers from Bekker et al. (2009).
The δ34S isotope signature is less variable than the Δ33S range, and it appears to be independent of stratigraphic position or relative age (Fig. 4). It is comprised between + 3.2 and + 4.5‰ for most samples. Only the disseminated sulfides within the Lunnon Basalt exhibit slightly lower values of + 1.1 ± 0.3‰.
Discussion
All investigated sedimentary rocks exhibit a relatively large variation in Δ33S, ranging from − 0.1 to + 2.4‰, whereas δ34S ranges from + 3.2 to + 4.5‰ (VCDT). Between the different types of sedimentary rocks, there is no systematic variation in Δ33S or δ34S in regard to their stratigraphic position or mineralogy. Within the sedimentary sequence on the contact of the Lunnon Basalt to the Silver Lake Komatiite, large variations in Δ33S of + 1.2 to + 2.4‰ are documented, a range that is in agreement with most pyrite data from contact sediments reported by Chen et al. (2022). These may reflect changes in sulfur source over short timescales, marking episodic eruptions of subaerial volcanoes that could generate distinctly positive Δ33S values if S8 aerosols came in contact with UV radiation (Ono et al. 2009). The anomalously positive signature of single layers within the sedimentary sequence would be superimposed on the varying negative Δ33S background value of seawater at the time of sedimentation. Subaerial volcaniclastic rocks (tuffs and lapilli) that are overlain by and intercalated with komatiite units are known from the Black Swan deposit located about 80 km to the north of Kambalda (Hill et al. 2004).
It is thought that ore sulfides at Kambalda were incorporated from the underlying sulfidic contact sedimentary rocks to form the magmatic ore (Huppert et al. 1984; Arndt and Jenner 1986; Lesher and Campbell 1993; Lesher 2017); however, clear evidence from multiple sulfur isotopes for that assimilation process is missing so far for the Kambalda ores because existing data are inconclusive and could be variably interpreted. To help solve this knowledge gap, early ore sulfides were investigated from the flanks of the lava channel, which are found above the still preserved contact sedimentary rocks. The flank has been shown to represent an initial lava flow of the sheet flow facies prior to evolving to a channelized lava flow (Barnes et al. 2013). The sulfides on this flank represent the initial ore sulfides prior to the formation of the sulfides situated inside the steep-sided erosional embayment (Staude et al. 2022). Early flank sulfides at Moran show clear evidence of contact sedimentary sulfide assimilation by the komatiite magma, with Δ33S values of the ore close to the ones observed in the contact sedimentary sulfides (Fig. 4). In the framework of the model of Lesher (2017), who suggested that sulfide incorporation occurred through melting of the sedimentary sulfides forming a xenomelt, rather than by sulfide saturation of the komatiite magma, the sulfide melt would have then formed a liquid pool at the base of the lava flow.
Mungall (2002) and Yao and Mungall (2021) pointed out that sulfide melt pools in lava flows should not reach chemical equilibrium and that a high ore metal content could not be attained during reasonable geological time scales associated with komatiite flow rates due to the kinetically slow partitioning between sulfide and silicate melt. Therefore, these authors suggested that upgrading the Fe-rich sulfide liquid in komatiites to form a Ni-rich sulfide melt must occur prior to the transport and settling of the sulfide droplets. It is argued that the dataset collected from the flank and the steep-sided embayment of the Moran deposit could reflect the process predicted by Mungall (2002) and Yao and Mungall (2021). With Δ33S values between + 0.6 and + 1.0‰, magmatic flank sulfides on contact sedimentary rocks are closer to the sediment values (+ 0.9 to + 2.4‰), whereas the main orebody within the steep-sided embayment displays values between + 0.2 and + 0.3‰ (Table 1, Fig. 4). In fact, on the flank close to the steep-sided embayment, sulfides are found on basalt, as it is thought that the contact sediments had already been eroded by the komatiite and/or sulfide melt prior to the formation of the steep-sided embayment (textural evidence and thermo-mechanical erosion model can be found in Staude et al. 2016, 2017a, 2022). These sulfides exhibit a Δ33S value of + 0.3‰, which is identical to sulfides within the steep-sided embayment.
These data show a dependency of Δ33S values with time and location during the ore-forming process. Prior to the formation of the steep-sided embayment, two contrasting Δ33S values are observed. Farther away from the center of the channel, the Δ33S signature of magmatic sulfides reflects that of contact sedimentary sulfides, whereas closer to the channel center, the Δ33S values of magmatic sulfides are more similar to the magmatic end-member of the Moran deposit (Δ33S~+ 0.2‰). This observation is in agreement with the chemistry of the sulfides (i.e., the Ni tenor; Staude et al. 2022). The difference between samples farther away and closer to the center of the channel also shows that the sulfide melt pool prior to the formation of the steep-sided embayment nearly reached the same metal content and Δ33S signature as the main orebody and that not much upgrading of the sulfide melt occurred afterward, as suggested by Mungall (2002) and Yao and Mungall (2021).
To evaluate the role of assimilation of sedimentary sulfur on the flank with a progressively increasing magmatic sulfur isotopic signature, mixing lines were quantified for various R factors using the known sulfur content of the involved lithologies as well as the sulfur tonnages of contact sedimentary rocks and orebody. At least part of the sulfur in the ore derives from the contact sediment, based on the observed Δ33S composition of the magmatic flank sulfides. Each orebody within any given komatiite lava channel is situated in a separate erosional embayment (Staude et al. 2017b), and the sulfide forms a xenomelt layer at the base of the lava flow (Lesher and Campbell 1993; Lesher 2017; Barnes and Robertson 2019). Therefore, the source area is most likely restricted to the area between two neighboring orebodies within one channel, as the heavy sulfide melt is trapped in the deep steep-sided embayment and cannot flow further downstream.
As the orebodies in the southern Kambalda area are generally situated on contact sedimentary rocks and display lower Ni tenors compared to orebodies in the north (Marston 1984), it can be assumed that lava flowed from north to south. Therefore, it is inferred that the sulfur source area for Moran is the area between Long and Moran, including the area of Moran itself (Fig. 1). The eroded contact sedimentary rocks covered approximately 1000 m north-south distance and 150 m width underneath the channel and were on average 4 m thick with an average sulfur content of 3.6 wt% based on the drill database, which is in agreement with data given for the whole of Kambalda sedimentary rocks by Bavinton (1981; 3.4 to 5.0 wt% S). Assuming an average density of the sediments of 3000 kg/m3, the amount of sulfur mobilized by the lava flow is approximately 65 kt. Consequently, it is estimated that the Moran massive sulfide pool would have been about 100,000 m3 based on the mine model. With an average density of 4700 kg/m3 and an average sulfur content of 33 wt%, the amount of sulfur in Moran is estimated to be around 155 kt.
Mixing calculations between komatiitic and sedimentary sulfur are shown in Fig. 5 for various sulfur contents and starting Δ33S compositions of the contact sedimentary rocks (+ 1.3 and + 2.0‰). These Δ33S compositions cover the observed range of the contact sediments in the vicinity of the orebody and are assumed to represent the Δ33S compositions of sedimentary sulfides that have been melted by the komatiite magma. The average sulfur content of the contact sedimentary rocks (3.6 wt%) was used as well as 6 wt% to represent sediments with a higher sulfide content. For the komatiite, Barnes et al. (2013) gave a background sulfur content of 0.2 wt%, which was used in addition to 1 wt% to allow for uncertainties. The amount of sulfur required to generate the magmatic sulfides that make up the Moran deposit compared with the amount of sulfur available (assuming all sulfur removed from the contact sedimentary rocks is now found in the ore) would result in a proportion of sedimentary sulfur in the ore of 40%. This translates to a Δ33S composition of + 0.5‰ or an apparent R factor of 25 (for the most realistic calculation based on actual data; red line in Fig. 5). This Δ33S composition is not observed in Moran sulfides of the main orebody, and the R factor is significantly lower than published factors of 100–500 based on major and trace elements of sulfides (Lesher and Campbell 1993) or 200 estimated for the Moran deposit based on the Ni content and trace elements of massive sulfides (e.g., PGE; Staude et al. 2022). To explain the observed range in Δ33S composition, about 20% sedimentary and 80% magmatic sulfur is required, which results in a R factor of about 75, which is still distinctly too low (Fig. 5). Therefore, it is suggested that the apparent R factors calculated from sulfur isotopes and geochemistry are most likely decoupled, as suggested in another study based on mineralized komatiites from the Agnew-Wiluna greenstone belt in the northern Kalgoorlie Terrane of the Yilgarn Craton (Virnes et al. 2023).
Calculated Δ33S values dependent on the R factor and the initial sulfur isotope value of the sedimentary rocks assuming complete sulfur partitioning into the sulfide melt. The magmatic flank sulfides on contact sedimentary rocks (blue bar) represent low R factors, whereas the magmatic flank sulfides on basalt (green bar) represent a higher R factor, which is similar to magmatic sulfides in the steep-sided embayment (red bar). 1Average of contact sedimentary rock, this work; 2average of drill hole LSU-413, this work; 3Barnes et al. (2013)
To explain the decoupling, different ore-forming processes need to be addressed. The upgrading of a sulfide melt to increase its Ni, Cu, and PGE content is based on high partition coefficients of these elements into an existing sulfide melt (Barnes and Ripley 2016). The higher the R factor, the higher the amount of these metals will be in the sulfide melt. Sulfur on the other hand is dependent on whether the komatiite is sulfur-saturated or not. A high proportion of magmatic sulfur is required (80%; see above) to explain the observed Δ33S value of the magmatic sulfides in the Moran deposit. Therefore, some of the komatiite melt had to be sulfur-saturated in the early stages of ore formation to explain the change from a sedimentary sulfur signature (~ ++ 1.3‰) toward mantle values (~ 0‰) during the evolution of the magmatic system. The discrepancy with the geochemical R factor on the other hand could indicate sulfide loss due to a subsequent sulfur-deficient komatiite melt assimilating the sulfide melt, as it was already proposed by Lesher et al. (2001) based on the geochemistry of several komatiite occurrences worldwide.
As mantle-derived silicate melts are thought to be sulfur-deficient after ascent through the lithosphere due to the known inverse relationship between sulfur capacity and pressure (Mavrogenes and O’Neill 1999), they are able to assimilate crustal sulfur upon emplacement. This assimilation has been proposed in the past as a process that can destroy sulfide deposits if a sulfur-deficient magma flows over an existing magmatic sulfide melt pool (Kerr and Leitch 2005). This assimilation was also reported to upgrade existing sulfide melts with PGE accompanied by sulfide dissolution of the silicate melt (Holwell et al. 2014). Taking into account that there was a sulfide melt pool at the base of the komatiite lava flow, which was formed by direct melting of sedimentary sulfides (Lesher 2017), the komatiite above would have still been sulfur-deficient due to slow diffusion of sulfur between the two melts (Barnes and Robertson 2019; Yao and Mungall 2021). This means that any mechanical disruption of the sulfide surface would have enhanced any diffusion mechanism; accordingly, Ni and other metals could more effectively partition into the sulfide melt. Simultaneously, part of the sulfide melt could have been assimilated more effectively and dissolved by the komatiite. Diffusion of metals into the sulfide melt must be faster than sulfide dissolution to explain the apparently higher R factor for geochemical data and the fact that sulfide is still preserved as an orebody.
This mechanical disruption could be caused by turbulently flowing komatiite (Yao and Mungall 2022) or by molten footwall rocks, where silicate plumes detached from the basal melt layer due to their relatively lower density compared with the sulfide melt (Staude et al. 2016, 2017a). The turbulently flowing komatiite is most likely more effective due to a larger melt velocity and larger volume and would be the dominant process in the early concave embayment. Once the steep-sided embayment formed, detached melt plumes from the thermo-mechanically eroded substrate would be the dominant process explaining the limited upgrading past this erosional step. Another factor that changes over time is the geometry of the channel and therefore the surface area where sulfide melt and komatiite melt are in direct contact. A wide concave embayment has a larger surface area between the two melts and hence a faster upgrading/sulfide loss potential compared to the final steep-sided embayment, resulting in another reason to explain that most of the upgrading/sulfide loss occurred in the initial ore stages.
Summary
Archean country rock sulfides and associated komatiite-hosted sulfide ore at Kambalda (Western Australia) have been systematically investigated with respect to their Δ33S signatures. The sedimentary sulfides exhibit a large variation from − 0.1 to + 2.4‰ independent of their stratigraphic position or type of host rock. The same is true for contact sediments underneath the komatiite lava channels, where large variations are observed in single sulfide–bearing layers across the sedimentary sequence, which can be linked to regional subaerial volcanic activity (Fig. 6a).
Schematic model of the evolving sulfur isotopic composition over time at Kambalda, shown through one of the lava flow channels and across one representative sulfide orebody. a Sedimentary sulfides incorporate sulfur that is dissolved in seawater displaying background Δ33S negative values. Following a nearby subaerial volcanic eruption, positive MIF sulfur is preserved within distinct thin layers within the sedimentary record. b The eruption of voluminous komatiite lava flows on the seafloor causing assimilation of the underlying contact sediments. The sedimentary sulfides melt and form a sulfide pool on the base of the lava flow in a wide concave embayment. If MIF sulfur was in the sediments, it is included in the sulfide melt pool and can only change back toward the MDF line by mixing with sulfur from the komatiite. This process requires that the komatiite is sulfur-saturated; otherwise, sulfide would be assimilated by the komatiite lava. c The continuous evolution of the lava flow deepens the embayment. This process stops further upgrading of the sulfide melt due to slow kinetic partitioning of chalcophile elements (like Ni, Cu, and PGE) into the sulfide melt (Mungall 2002; Yao and Mungall 2021). If subsequent komatiite lava is sulfur-deficient, sulfide assimilation into the komatiite could occur. In this case, the geochemical R factor will be decoupled from the R factor observed in Δ33S values. Changes in color of the sulfides reflect the evolving sulfide Δ33S composition. Not shown is the komatiite sheet flow facies next to the channelized flow
The earliest magmatic sulfides of the Moran deposit formed in a flat concave embayment overlying contact sedimentary rocks and display an overlapping signature with sedimentary sulfides, ranging in Δ33S from + 0.6 to + 1.0‰. Thus, it can be concluded that their MIF sulfur isotopic composition reflects the assimilation of contact sedimentary sulfides to form the magmatic orebodies (Fig. 6b). Subsequent but still early magmatic sulfides are situated on a concave embayment and overlie the Lunnon Basalt and display values that are not distinguishable from the main ore (Δ33S: + 0.2 to + 0.3‰) situated in a steep-sided embayment around 40 m underneath the original surface (due to thermo-mechanical erosion; Fig. 6c). It is argued that most of the metal enrichment of the sulfide melt occurred prior to the formation of the steep-sided embayment. This is in line with the recent investigation of Yao and Mungall (2021) proposing that a sulfide melt pool is less likely to upgrade the metal content compared with small dispersed sulfide droplets in a mafic/ultramafic magma.
The discrepancy in the calculation of the R factor using multiple sulfur isotopes and geochemical data is consistent with a decoupling of sulfur with other elements during the ore-forming process (cf. Virnes et al. 2023). Using multiple sulfur isotopes alone can show whether contamination occurred or not, but only the combination with R factor calculations using multiple sulfur isotopes and geochemical data is a powerful tool to test whether sulfide melt has been subsequently eroded by a younger sulfur-deficient magma.
References
Arndt NT, Jenner GA (1986) Crustally contaminated komatiites and basalts from Kambalda, Western Australia. Chem Geol 56:229–255
Barnes S-J, Ripley EM (2016) Highly siderophile and strongly chalcophile elements in magmatic ore deposits. Rev Miner Geochem 81:725–774
Barnes SJ, Heggie GJ, Fiorentini ML (2013) Spatial variation in platinum group element concentrations in ore-bearing komatiite at the Long-Victor deposit, Kambalda Dome, Western Australia: enlarging the footprint of nickel sulfide orebodies. Econ Geol 108:913–933
Barnes SJ, Beresford SW, Le Vaillant M (2016) Interspinifex Ni sulfide ore from the Coronet shoot, Kambalda: characterization using microbeam X-ray fluorescence mapping and 3-D X-ray computed tomography. Econ Geol 111:1509–1517
Barnes SJ, Robertson JC (2019) Time scales and length scales in magma flow pathways and the origin of magmatic Ni-Cu-PGE ore deposits. Geoscience Frontiers 10:77–87
Bavinton OA, Keays RR (1978) Precious metal values from interflow sedimentary rocks from the komatiite sequence at Kambalda, Western Australia. Geochim Cosmochim Acta 42:1151–1163
Bavinton OA (1981) The nature of sulfidic sediments at Kambalda and their broad relationships with associated ultramafic rocks and nickel ores. Econ Geol 76:1606–1628
Bekker A, Barley ME, Fiorentini ML, Rouxel OJ, Rumble D, Beresford SW (2009) Atmospheric sulfur in Archean komatiite-hosted nickel deposits. Sci 329:1086–1089
Beresford SW, Cas RAF (2001) Komatiitic invasive lava flows, Kambalda, Western Australia. Can Mineral 39:525–535
Caruso S, Fiorentini ML, Moroni M, Martin LAJ (2017) Evidence of magmatic degassing in Archean komatiites: insights from the Wannaway nickel-sulfide deposit, Western Australia. Earth Planet Sci Lett 479:252–262
Chen M, Campbell IH, Ávila JN, Tian W, Hayman PC, Cas RAF, Ireland TR (2022) Atmospheric and hydrothermal sulfur isotope signatures recorded in Neoarchean deep marine sedimentary pyrites from the Yilgarn Craton, Western Australia. Geochim Cosmochim Acta 322:170–193
Ding X, Ripley EM, Shirey SB, Li C (2012) Os, Nd, O and S isotope constraints on country rock contamination in the conduit-related Eagle Cu-Ni-(PGE) deposit, Midcontinent Rift System, Upper Michigan. Geochim Cosmochim Acta 89:10–30
Farquhar J, Bao H, Thiemens M (2000) Atmospheric influence of earth’s earliest sulfur cycle. Sci 28:756–758
Farquhar J, Wing BA (2003) Multiple sulfur isotopes and evolution of the atmosphere. Earth Planet Sci Lett 213:1–13
Fiorentini ML, Beresford SW, Barley M, Duuring P, Bekker A, Rosengren N, Cas RAF, Hronsky J (2012a) District to camp controls on the genesis of komatiite-hosted nickel sulfide deposits, Agnew-Wiluna Greenstone Belt, Western Australia: insights from the multiple sulfide isotopes. Econ Geol 107:781–796
Fiorentini ML, Bekker A, Rouxel O, Wing BA, Maier W, Rumble D (2012b) Multiple sulfur and iron isotope composition of magmatic Ni-Cu-(PGE) sulfide mineralization from eastern Botswana. Econ Geol 107:105–116
Godel B, González-Álvarez I, Barnes SJ, Barnes S-J, Parker P, Day J (2012) Sulfides and sulfarsenides from the Rosie nickel prospect, Duketon greenstone belt, Western Australia. Econ Geol 107:275–294
Gole MJ, Barnes SJ (2020) The association between Ni-Cu-PGE sulfide and Ni-Co lateritic ores and volcanic facies within the komatiites of the 2.7 Ga East Yilgarn Craton Large Igneous Province, Western Australia. Ore Geol Rev 116:103231
Goscombe B, Blewett RS, Czarnota K, Groenwald PB, Maas R (2009) Metamorphic evolution and integrated terrane analysis of the Eastern Yilgarn Craton: rationale, methods, outcomes and interpretation. Geosci Aust Rec 23:281
Gresham JJ, Loftus-Hills GD (1981) The geology of the Kambalda nickel field, Western Australia. Econ Geol 76:1373–1416
Groves DI, Korkiakoski EA, McNaughton NJ, Lesher CM, Cowden A (1986) Thermal erosion by komatiites at Kambalda, Western Australia and the genesis of nickel ores. Nature 319:136–139
Havig JR, Hamilton TL, Bachan A, Kump LR (2017) Sulfur and carbon isotopic evidence for metabolic pathway evolution and a four-stepped Earth system progression across the Archean and Paleoproterozoic. Earth Sci Rev 174:1–21
Hill RET, Barnes SJ, Dowling SE, Thodarson T (2004) Komatiites and nickel sulphide orebodies of the Black Swan area, Yilgarn Craton, Western Australia. 1. Petrology and volcanology of host rocks. Miner Deposita 39:684–706
Holwell DA, Keays RR, Firth EA, Findlay J (2014) Geochemistry and mineralogy of platinum group element mineralization in the River Valley Intrusion, Ontario, Canada: a model for early-stage sulfur saturation and multistage emplacement and the implications for “contact-type” Ni-Cu-PGE sulfide mineralization. Econ Geol 109:689–712
Huppert HE, Sparks RSJ, Turner JS, Arndt NT (1984) Emplacement and cooling of komatiite lavas. Nature 309:19–22
Kaufman AJ, Johnston DT, Farquhar J, Masterson AL, Lyons TW, Bates S, Anbar AD, Arnold GL, Garvin J, Buick R (2007) Late Archean biospheric oxygenation and atmospheric evolution. Sci 137:1900–1903
Keays RR, Lightfoot PC (2010) Crustal sulfur is required to form magmatic Ni-Cu sulfide deposits: evidence from chalcophile element signatures of Siberian and Decan Trap basalt. Mineral Deposita 45:241–257
Kerr A, Leitch AM (2005) Self-destructive sulfide segregation systems and the formation of high-grade magmatic ore deposits. Econ Geol 100:311–332
Kositcin N, Brown SJA, Barley ME, Krapež B, Cassidy KF, Champion DC (2008) SHRIMP U-Pb zircon age constraints on the late Archaean tectonostratigraphic architecture of the Eastern Goldfields Superterrane, Yilgarn Craton, Western Australia. Precambrian Res 161:5–33
Kump LR (2012) Sulfur isotopes and stepwise oxygenation of the biosphere. Elements 8:410–411
LaFlamme C, Martin L, Jeon H, Reddy SM, Selvaraja V, Caruso S, Bui TH, Roberts MP, Voute F, Hagemann S, Wacey D, Littman S, Wing B, Fiorentini ML, Kilburn MR (2016) In situ multiple sulfur isotope analysis by SIMS of pyrite, chalcopyrite, pyrrhotite, and pentlandite to refine magmatic ore genetic models. Chem Geol 444:1–15
LaFlamme C, Jamieson JW, Fiorentini ML, Thébaud N, Caruso S, Selvaraja V (2018) Investigating sulfur pathways through the lithosphere by tracing mass independent fractionation of sulfur to the Lady Bountiful orogenic gold deposit, Yilgarn Craton. Gondwana Res 58:27–38
Lesher CM, Arndt NA (1995) REE and Nd isotope geochemistry, petrogenesis and volcanic evolution of contaminated komatiites at Kambalda, Western Australia. Lithos 34:127–157
Lesher CM, Arndt NT, Groves DI (1984) Genesis of komatiite-associated nickel sulphide deposits at Kambalda, Western Australia: a distal volcanic model. In: Buchanan DL, Jones MJ (eds) Sulphide deposits in mafic and ultramafic rocks. Inst. Min. and Metal, London, pp 70–80
Lesher CM, Groves DI (1986) Controls on the formation of komatiite-associated nickel-copper sulfide deposits. In: Friedrich GH, Genkin AD, Naldrett AJ, Ridge JD, Sillitoe RH, Vokes FM (eds) Geology and metallogeny of copper deposits. Special Publication No. 4 of the Society for Geology Applied to Mineral Deposits, vol 4. Springer, Berlin, Heidelberg
Lesher CM, Campbell IH (1993) Geochemical and fluid dynamic modelling of compositional variations in Archean komatiite-hosted nickel sulfide ores in Western Australia. Econ Geol 88:804–816
Lesher CM, Burnham OM, Keays RR, Barnes SJ, Hulbert L (2001) Trace-element geochemistry and petrogenesis of barren and ore-associated komatiites. Can Mineral 39:673–696
Lesher CM (2017) Roles of xenomelts, xenoliths, xenocrysts, xenovolatiles, residues, and skarns in the genesis, transport, and localization of magmatic Fe-Ni-Cu-PGE sulfides and chromite. Ore Geol Rev 90:465–484
Liu P, Harman CE, Kasting JF, Hu Y, Wang J (2019) Can organic haze and O2 plumes explain patterns of sulfur mass-independent fractionation during the Archean? Earth Planet Sci Lett 526:115767
MacLean WH (1969) Liquidus phase relations in the FeS-FeO-Fe3O4-SiO2 system, and their application in geology. Econ Geol 64:865–884
Marston RJ (1984) Nickel mineralization in Western Australia. Geol Surv West Aust Mineral Resour Bull 14:271
Mavrogenes JA, O’Neill HS (1999) The relative effects of pressure, temperature and oxygen fugacity on the solubility of sulfide in mafic magmas. Geochim Cosmochim Acta 63:1173–1180
Mungall JE (2002) Kinetic controls on the partitioning of trace elements between silicate and sulfide liquids. J Geol 43:749–768
Ohmoto H (1986) Stable isotope geochemistry of ore deposits. In: Valley JW, Taylor HP, O’Neil JR (eds) Stable isotopes in high temperature geological processes. De Gruyter, Berlin, Boston, pp 491–560
Ono S, Beukes NJ, Rumble D (2009) Origin of two distinct multiple-sulfur isotope compositions of pyrite in the 2.5 Ga Klein Naute Formation, Griqualand West basin, South Africa. Precamb Res 169:48–57
Philippot P, Van Zuilen M, Lepot K, Thomazo C, Farquhar J, Van Kranendonk MJ (2007) Early Archaean microorganisms preferred elemental sulfur, not sulfate. Sci 317:1534–1537
Ripley EM (1986) Application of stable isotopic studies to problems of magmatic sulfide ore genesis with special reference to the Duluth Complex, Minnesota. In: Friedrich GH, Genkin AD, Naldrett AJ, Ridge JD, Sillitoe RH, Vokes FM (eds) Geology and metallogeny of copper deposits. Special Publication No. 4 of the Society for Geology Applied to Mineral Deposits, vol 4. Springer, Berlin, Heidelberg
Ripley EM, Li C (2003) Sulfur isotope exchange and metal enrichment in the formation of magmatic Cu-Ni-(PGE) deposits. Econ Geol 98:635–641
Ripley EM, Li C (2017) A review of the application of multiple S isotopes to magmatic Ni-Cu-PGE deposits and the significance of spatial variable Δ33S values. Econ Geol 112:983–991
Said N, Kerrich R, Groves D (2010) Geochemical systematics of basalts of the lower basalt unit, 2.7 Ga Kambalda sequence, Yilgarn craton, Australia: plume impingement at a rifted craton margin. Lithos 115:82–100
Smithies RH, Lowrey JR, Sapkota J, De Paoli MC, Hayman P, Barnes SJ, Champion DC, Masurel Q, Thebaud N, Grech LL, Drummond M, Maas R (2022) Geochemical characterization of the magmatic stratigraphy of the Kalgoorlie and Black Flag Groups – Ora Banda to Kambalda Region. Geological Survey of Western Australia Report 226:101
Squire RJ, Cas RAF, Clout JMF, Behets R (1998) Volcanology of the Archaean Lunnon Basalt and its relevance to nickel sulfide-bearing trough structures at Kambalda, Western Australia. Aust J Earth Sci 45:695–715
Staude S, Barnes SJ, Le Vaillant M (2016) Evidence of lateral thermomechanical erosion of basalt by Fe-Ni-Cu sulfide melt at Kambalda, Western Australia. Geol 44:1047–1050
Staude S, Barnes SJ, Le Vaillant M (2017a) Thermomechanical erosion of ore-hosting embayments beneath komatiite lava channels: textural evidence from Kambalda, Western Australia. Ore Geol Rev 90:446–464
Staude S, Sheppard S, Parker P, Paggi J (2017b) Long-Victor nickel sulfide deposits, Kambalda. In: Philips N (ed) Australian ore deposits monograph 32. Australian Institute of Mining and Metallurgy, Melbourne, pp 107–112
Staude S, Oelze M, Markl G (2022) Multi-stage sulfide evolution of the Moran Ni sulfide ore, Kambalda, Western Australia: insights into the dynamics of ore forming processes of komatiite-hosted deposits. Mineral Deposita 57:889–909
Virnes AB, Fiorentini ML, Barnes SJ, Caruso S, Martin LAJ, Aleshin M, Schoneveld LE, Roberts MP, Masurel Q, Thebaud N (2023) Decoupling of sulfur isotope signatures from platinum group elements in komatiite-hosted ore systems: Evidence from the Mount Keith MKD5 Ni-(Co-Cu) deposit, Western Australia. Econ Geol. https://doi.org/10.5382/econgeo.5030
Yao ZS, Mungall JE (2021) Kinetic controls on the sulfide mineralization of komatiite-associated Ni-Cu-(PGE) deposits. Geochim Cosmochim Acta 305:185–211
Yao ZS, Mungall JE (2022) Transport and deposition of immiscible sulfide liquid during lateral magma flow. Earth-Sci Rev 227:103964
Acknowledgements
We are grateful to IGO Limited (i.e., Susan Leeson, Mike Whitford, and Karly Ding) and Mincor Resources NL (now Wyloo Metals) for providing samples. We are grateful to an anonymous reviewer.
Funding
Open Access funding enabled and organized by Projekt DEAL. The research was funded by the German Research Foundation (grant number: 407352165) to SS. Drill hole LG15-226 was co-funded by the Western Australian Government under grant DAG2013/00212545.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editorial handling: B. Lehmann
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Staude, S., Martin, L.A., Aleshin, M. et al. The multiple sulfur isotope architecture of the Kambalda nickel camp, Western Australia. Miner Deposita 59, 505–518 (2024). https://doi.org/10.1007/s00126-023-01223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-023-01223-6