Abstract
Many sedimentary-exhalative (SEDEX) sulfide deposits have been subject to regional metamorphism and, if the metamorphic grade was high enough, this could have resulted in sulfide anatexis. Although experiments and textures indeed showed that some deposits were partially molten, there is an ongoing debate as to the extent to which metamorphosed ore deposits were molten. Since some SEDEX deposits underwent amphibolite to granulite facies metamorphism, not only sulfides but also the host silicate rocks should have reached anatectic conditions. Due to the two immiscible silicate and sulfide melts, the formation of typical mingling and emulsion textures, as already known from magmatic sulfide deposits, should form. To test this hypothesis, we investigate sulfide-silicate textures from the granulite-facies Bodenmais SEDEX deposit (Germany). Textures from Bodenmais are similar to magmatic sulfide deposits including sulfide-matrix breccia, emulsion textures, pegmatitic leucosomes, and massive sulfides overlain by net-textured intergrowths of refractory quartz, which is interpreted to be a relic of silicate anatexis. Minerals crystallized during the interaction of both immiscible melts differ in their chemistry compared to the same minerals found in the adjacent migmatitic host rocks: for example, garnet in sulfides is Mn-rich (spessartine), but Fe-rich (almandine) in the migmatites and sulfide-enclosed cordierite is more enriched in Mg (Mg/(Mg + Fe): 0.84) than migmatitic cordierite (Mg/(Mg + Fe): 0.54). The textures themselves, their spatial arrangement within the deposit, the differences in mineral chemistry, and the observed crystallization sequence provide unequivocal evidence that the sulfides at Bodenmais were molten to a large extent under granulite facies conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that base-metal sulfide deposits can melt during high-grade metamorphism (amphibolite facies or higher), although the extent of this anatexis is controversial (Lawrence 1967; Bailie and Reid 2005; Frost et al. 2005; 2011; Spry et al. 2008; Spry and Teale 2021). Although experiments and textural observations suggest that some Pb-Zn-Ag deposits underwent anatexis to at least 20% of the ore volume (for Broken Hill, Australia; Frost et al. 2011), there are still studies arguing against it or leave the topic out altogether when discussing “Broken Hill Type” deposits (Spry et al. 2008; Sangster 2020; Spry and Teale 2021; Beeson and Webster 2023). Höhn et al. (2021) interpreted symplectic intergrowth of sulfides from the Zn-Pb-Ag deposit Gamsberg in South Africa, commonly found in high-grade metamorphic sulfide deposits, as sulfidation of oxidic ore during metamorphism, rather than resulting from crystallization of an anatectic melt.
Frost et al. (2002) suggested the following characteristics to identify solidified sulfide melt in metamorphic rocks: (1) localized concentrations of Ag and Au in the presence of low-melting-point chalcophile elements (LMCE) such as Sb and As, (2) multiphase sulfide inclusions in high-temperature gangue minerals, (3) low interface angles between sulfides or sulfosalts with restites, (4) sulfide and sulfosalt filling in fractures, and (5) Ca- and Mn-rich selvages around massive sulfides. Besides from Broken Hill (Brett and Kullerud 1967; Lawrence 1967; Mavrogenes et al. 2001), the occurrence of anatectic sulfide melts during metamorphism has been suggested also for Aggeneys (South Africa; Bailie and Reid 2005) as well as for Rajpura-Dariba and Rampura-Agucha (both India; Mishra and Bernhardt 2009; Pruseth et al. 2016; Govindarao et al. 2020a). An example of a partially molten volcanic-hosted massive sulfide (VHMS) deposit (Hongtoushan, China) in an upper amphibolite facies setting was reported recently by Li et al. (2022) based on pyrrhotite-chalcopyrite ore with minor pyrite and sphalerite containing granular rounded quartz and intergrowths of euhedral gahnite, hornblende, and anhydrite inclusions, without foliation or recrystallization textures that are usually found in parts of the orebody that has not undergone melting. On top of that, Frost et al. (2002) present 23 additional ore deposits, including Zn-Pb-(Ag)-, Cu-Fe-Zn-, Au-, and Au-base metal deposits, that could have melted.
Experiments have shown that sulfide anatexis is mainly depending on the composition of the sulfides (Tomkins et al. 2007). The system PbS-FeS-ZnS melts at 800 °C at ambient pressure with a slight increase of 6 °C/GPa (Mavrogenes et al. 2001); the addition of CuFeS2 lowers the melting point by approximately 70–100 °C (Stevens et al. 2005). Frost et al. (2002) used the term LMCE for Ag, As, Au, Bi, Cd, Ga, Hg, In, Se, Sb, Sn, Tl, and Te which lower the melting point of base metal sulfides. Many sedimentary exhalative (SEDEX) sulfide deposits or VHMS deposits contain these metals as minor or trace elements. Additionally, H2O (Wykes and Mavrogenes 2005), oxygen (Naldrett 1969), halogens (Mungall and Brenan 2003; Millsteed 2011), and excess S (in the form of pyrite; Stevens et al. 2005) lower the melting point of base metal sulfides.
Experiments at 820 °C and 0.5 GPa in the system galena, sphalerite, and pyrrhotite with added LMCE (Sb, Cu, Ag, As, Au) identified three immiscible melts at this temperature (Mavrogenes et al. 2013). These are as follows: (1) a sulfide melt crystallizing pyrite, pyrrhotite, chalcopyrite, galena, and sphalerite; (2) a sulfosalt melt containing Sb and As and crystallizing sulfosalts or a mixture of sulfosalts and sulfides; and (3) a metallic melt crystallizing alloys of Sb, As, Bi, Te, and/or Au. Thus, Mavrogenes et al. (2013) showed that polymetallic sulfide deposits can melt, and if containing LMCE can form multiple immiscible melts and therefore fractionate the metals during crystallization. Their experiments showed that a sulfide melt crystallizing galena causing increased concentrations of Cu and Ag in the remaining melt. Alloys that formed during the experiment by Mavrogenes et al. (2013) contained up to 15.4% Au.
Experimental work by Pruseth et al. (2014, 2016) showed that even at 600 °C, partial sulfide melting takes place in the system Cu-Fe-Zn-Pb-S with pyrite and sphalerite representing the first sulfides to crystallize from this melt. Pruseth et al. (2016) also showed that two immiscible melts form due to fractional crystallization of the sulfide melt; (1) a melt crystallizing sphalerite, chalcopyrite, pyrrhotite, and galena, and (2) a melt crystallizing galena and Pb-Ag-As-Sb-bearing sulfosalts. Govindarao et al. (2020a, b) conducted similar experiments at 500 °C and reported significant amounts of sulfide melt containing Pb, Cu, Sb, and Ag. They also determined the eutectic composition for the system galena-tetrahedrite at this temperature.
In metamorphosed silicate rocks, textural evidence for anatexis has long been known (i.e., migmatites with mesosomes, leucosomes, and melanosomes; Mehnert 1968). If sulfides and silicates are molten simultaneously, the immiscible melts should form typical textures similar to the ones known from magmatic sulfide deposits, such as emulsions or sulfide-matrix breccias (Barnes et al. 2018). However, despite clear textural evidence of partial sulfide anatexis, there is no study dealing with textural evidence at hand-specimen or orebody-scale for sulfide melting.
In this contribution, we examine whether sulfide melting had occurred in the metamorphosed SEDEX-type, migmatite-hosted Silberberg deposit (Bodenmais, Germany; Troll et al. 1987), and if so, to what extent it happened. Kalt et al. (1999) showed peak metamorphic conditions of 0.5–0.7 GPa and at least 800–850 °C, which, as noted above, is high enough to induce anatexis of both sulfides and silicates. We will investigate on thin-section, hand-specimen, and ore body scales typical textures of anatectically molten sulfides and their interaction with migmatitic host rocks.
Geology of the Silberberg
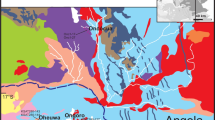
The Silberberg deposit is situated in the monotonous group of the Bohemian Massif (Dill 1990) which is part of the Moldanubian Zone of the Variscan orogeny (Fig. 1). The Moldanubian monotonous group consists of migmatites which originate from psammopelitic sediments of the upper Proterozoic (Stettner 1981). During sedimentation, hydrothermal vents caused syn-sedimentary sulfides to accumulate on the seafloor, forming sulfide lenses that belong to the SEDEX type of deposits (Carne and Cathro 1982; Vornehm et al. 2002). Both the host rocks and the sulfides later experienced high temperature–low pressure metamorphism during the Variscan orogeny with peak metamorphic conditions estimated to be at least 800–850 °C and 0.5–0.7 GPa (Kalt et al. 1999). The migmatites can be divided into a cordierite-sillimanite-garnet migmatite to the west and a biotite-feldspar migmatite to the east of the sulfide bodies (Weinschenk 1901). Minor sulfide lenses are found within the biotite-feldspar migmatites about 40 m to the east of the main sulfides.
a Geological map of the southern Bavarian Forest with metamorphic isogrades modified from Pfeufer (1976) to show the location of the Silberberg and other minor sulfide deposits. An increase in metamorphic conditions is observed from north to south with the Silberberg deposit found between cordierite-biotite gneiss (biotite-feldspar migmatite in this study) and cordierite gneiss (cordierite-sillimanite-garnet migmatite in this study). b Schematic profile through the Silberberg modified from Pfeufer (1976) that show the historic mine development as well as the lens-like orebodies and the studied gossan outcrops. Afs, alkali feldspar; And, andalusite; Bt, biotite; Crd, cordierite; Grt, garnet; Ms, muscovite; Sil, sillimanite
The sulfides form orebodies which occur as several lenses that are up to 16 m thick (Linhardt 2015; Weinschenk 1901). Between these lenses, sulfides appear as fine, disseminated, decimeter-thick bands (Figs. 2, 3; Pfeufer 1976). Ore minerals are dominated by pyrrhotite followed by pyrite, sphalerite, chalcopyrite, galena, with minor marcasite, magnetite, molybdenite, native Ag, and native Bi (Schreyer et al. 1964).
Schematic illustration of an orebody amended from Weinschenk (1901) showing the relative occurrences of the textures described in the text
An up to 1-m thick pegmatite is located next to massive and semi-massive sulfides (Fig. 3). Where observed, the pegmatite exclusively occurs at the eastern contact between the sulfides and the biotite-feldspar gneiss. A gahnite-dominated rock always contains quartz and is found on both sides of the orebody but only at the direct contact between sulfides with either the migmatite or with the pegmatite (Fig. 3; Pfeufer 1976).
The spatial occurrences of the different textures within the orebodies were studied in a gossan outcrop on the southern side of the Silberberg mountain shown in the electronic supplementary material (ESM 1). Although sulfides have been completely replaced by goethite, their textural relationships are well preserved. To the west, the cordierite-sillimanite-garnet migmatite starts the succession with the breccia east of it. The next horizon is formed by pure limonite where the original grain boundaries and cleavage of some sulfides are still preserved. This approximately 1.5-m thick interval represents the massive sulfides. The next horizon is the semi-massive sulfide for 1.5 to 2 m in thickness, which is represented by numerous quartz grains within the goethite, which is then bound to the pegmatite to the east of the sulfides. The pegmatite at the contact to the sulfides (now goethite) is brecciated and cemented by the semi-massive sulfides (now goethite). The pegmatite is approximately 1 m thick and is on contact to the biotite-feldspar migmatite to the east.
Methods
About 40 hand samples have been taken underground in the visitor mine, from mine dumps, the gossan outcrops, and the mineralogical collection of the University of Tübingen to investigate the petrography, mineral chemistry, and textures of the deposit.
Electron microprobe analysis (EMPA)
The mineral major element chemistry was determined using a JEOL JXA-8230 electron microprobe at the University of Tübingen. Analyses were carried out in wavelength-dispersive (WD) mode using an acceleration voltage of 15–25 kV, a probe current of 20 nA, and focused beam for all minerals with the exception of feldspar, where a spot size of 10 µm was used. Counting times of 16/8 s (peak/background) for main elements and 30/15 s for trace elements were used. Data processing was carried out using PRZ correction for all sulfides (chalcopyrite, galena, pyrite, pyrrhotite, sphalerite) and silicates (feldspar, cordierite, garnet). Detailed analytic conditions for every minerals are found in the ESM 5. Representative results are presented in Table 1 and all results in wt% and atoms per formula unit are provided in ESM 5.
Microbeam X-ray fluorescence element mapping
Large 2D chemical element maps were created on polished hand specimens using a Bruker Tornado M4 desktop microbeam X-ray fluorescence spectrometer at the University of Tübingen (Germany). The unit is equipped with a rhodium target X-ray tube operating at 50 kV and 500 nA without filters and with two silicon drift detectors. The beam has a spot size of 20 µm with a pixel size chosen between 20 and 40 µm and a dwell time per pixel at 10 to 20 ms/pixel. Results are displayed in unquantified false-color maps for Kα peaks of the respective elements. It is important to note that the color and color intensity do not reflect a true composition and only displays relative differences in the element concentration.
Petrography
Host rocks
On outcrop scale, the cordierite-sillimanite-garnet migmatite displays a foliation which is not visible at thin section scale. The mesosomes consist to a large extent of cordierite overgrowing quartz, K-feldspar, and sillimanite. Locally, large poiciloblastic garnet and minor euhedral biotite occur. Accessory phases are ilmenite, hematite, and hercynite as well as local globular aggregates of pyrrhotite, galena, and pyrite and minor sphalerite. The leucosomes are dominated by quartz and K-feldspar with minor amounts of cordierite and biotite.
The biotite-feldspar migmatite is characterized by a distinct foliation macroscopically and in thin section. The melanosome contains a high amount of biotite, large garnet grains, quartz, K-feldspar, sillimanite, and as accessories ilmenite, hematite, and hercynite. The leucosome is composed of quartz, K-feldspar, muscovite, and minor amounts of biotite.
The quartz-gahnite rock is almost entirely composed of equant euhedral gahnite crystals, containing magnetite exsolutions, within a quartz matrix. Other accessory phases are monazite-(Ce), ilmenite, hematite, cassiterite, and undetermined Nb-phases.
The pegmatite consists of quartz, microcline and orthoclase, muscovite, and biotite which in most samples shows a symplectic intergrowth with quartz at the contact to K-feldspar. In hand specimen, the microcline displays a weak to partly strong green color (Fig. 4a). Close to and at the contact, patches of quartz and gahnite occur (Figs. 4b, c). In contrast to the quartz-gahnite rock, the pegmatite-hosted gahnite shows skeletal textures. The pegmatite also contains roundish sulfide inclusions of galena, pyrite, chalcopyrite, and minor pyrrhotite and sphalerite (Fig. 4d, e) and accessory cordierite, sillimanite, and cassiterite. These round sulfide inclusions most likely represent trapped sulfide melt in crystallizing anatectic silicates as suggested by Frost et al. (2002). Round pegmatitic inclusions in sulfides next to the sulfide-pegmatite contact contain symplectic intergrowths of K-feldspar and quartz (Fig. 4f).
Hand specimens, a 2D elemental map, and photomicrographs of characteristic samples of the pegmatite that accompanies the sulfide orebody. a Polished slab of green feldspar containing sphalerite that is surrounded by gahnite, as well as roundish pyrrhotite inclusions. b Polished slab of the sulfide-pegmatite contact showing clasts of the pegmatite within sulfides and patchy occurences of gahnite within the pegmatite. c 2D elemental map of a sulfide-pegmatite contact. At the bottom visible is the semi-massive sulfides followed by an irregular thick band of gahnite and pyrrhotite followed by the pegmatite. d Hand specimen of the pegmatite displaying inclusions of round single-crystalline galena. e Photomicrograph (reflected light in oil) of the pegmatite with the typical sulfide assemblage pyrite and sphalerite overgrown by galena. f Photomicrograph (transmitted light, crossed polarizers) of pegmatite clast that is hosted in adjacent sulfides displaying symplectic quartz-feldspar intergrowth
Sulfide-bearing rocks
Textures of sulfide-bearing rocks can be subdivided based on their appearance: (1) massive sulfides, (2) semi-massive sulfides, and (3) host rock breccias cemented by sulfides. All sulfides have in common that they contain millimeter-sized roundish voids within pyrrhotite, from which a phase or several phases have been dissolved. In some voids, younger euhedral crystals of pyrite have been observed. Ramdohr (1950) described similar voids in pyrrhotite from the similar Broken Hill deposit (Australia) and suggested that the dissolved phase could have been alabandite. Millsteed (2011) reported micrometer-sized voids within galena and explained them to have been originally filled with Pb-halides. In this petrographic description, we add interpretation of the observed mineralization and its intergrowths to focus in the “Discussion” section on hitherto undescribed hand specimen and outcrop scale textures and compare them to known magmatic sulfide-silicate textures.
(1) Massive sulfides (Figs. 5, 6) are mostly composed of coarse-grained (up to 3 cm) pyrrhotite with 120° contact angles intergrown with medium-grained (up to 1 cm) sphalerite and coarse-grained (up to 5 cm) euhedral pyrite (Fig. 5a–c). Pyrite forms often symplectic intergrowths with sphalerite (Fig. 5d). Chalcopyrite is common as centimeter-sized aggregates containing abundant inclusions of Ag-Hg alloys. Common is also micrometer-sized galena displaying symplectic intergrowths with pyrrhotite and showing abundant platy exsolution lamellae of native Bi (Fig. 5e). Exsolution lamellae of matildite were described by Pfaffl (2015), but have not been observed in the investigated samples. Massive sulfides often contain macroscopically visible, irregular schlieren-like inclusions of silicates and quartz (Fig. 5a, b, f). They are composed of euhedral spessartine, growing exclusively on the sulfide-silicate contact, euhedral K-feldspar overgrown by quartz, and cordierite and quartz that often display a symplectic intergrowth. Another type of K-feldspar is anhedral and is replaced and surrounded by a symplectic intergrowth of cordierite with quartz followed by biotite with quartz inclusions on the contact to the sulfides (Fig. 6). Spessartine often contains sulfide inclusions with galena being the most abundant. If other sulfides are present, the spatial arrangement at the sulfide-spessartine contact usually is pyrrhotite, followed by chalcopyrite and then galena. Spessartine also has been observed in symplectic intergrows with pyrrhotite (Fig. 5g).
Hand specimen, 2D elemental maps, and photomicrographs of characteristic samples of massive sulfides. a Polished slab of massive sulfides that contain silicate inclusions (euhedral K-feldspar, round quartz grains, schlieren-like mixed silicates). The inset highlights a round silicate inclusion that contains numerous round or lobate sulfide inclusions at its margin. b 2D elemental map of the left side of a. Note that round quartz grains are concentrated with sphalerite. c 2D elemental map of polished slab showing an inhomogeneous and patchy distribution of silicates and quartz within the sulfides. Pyrite is euhedral and overgrown by chalcopyrite. d Photomicrograph (reflected light in air) showing a symplectic intergrowth of sphalerite with pyrite. Photomicrograph (reflected light in oil) showing an interegrowth of chalcopyrite, sphalerite, pyrrhotite, and galena. Galena contains several inclusions of native Bi. f Photomicrograph (transmitted light) of a schlieren-like texture. On this scale, sulfides and silicates display a lobate intergrowth with spessartine growing on the contact of both. g Back-scattered image of a schlieren-like texture, where spessartine and pyrrhotite form a symplectic intergrowth. Spessartine contains several inclusions of galena
Photomicrograph and 2D elemental map of silicate inclusions within massive sulfides (similar to the inset in Fig. 5a). a Photomicrograph (transmitted light with crossed polarizers) showing an anhedral K-feldspar grain in the center of the inclusions. It is surrounded by a symplectic quartz-cordierite intergrowth that is followed by symplectic quartz-cordierite-biotite intergrowth on the contact to the sulfides. b 2D elemental map of a highlighting the anhedral nature of the K-feldspar and the symplectites as well as a patchy area of spessartine. The highlighted areas represent biotite within the symplectite that is altered to undetermined Fe-rich minerals
The abundant symplectites between various minerals (Figs. 4, 5, and 6) represent eutectic textures (Mavrogenes et al. 2001). The symplectic-like intergrowth of spessartine and pyrrhotite (Fig. 5g) could represent a skeletal spessartine crystal that trapped sulfide melt during growth. Schlieren-like intergrowth of sulides and silicates (Figs. 5, 7) resembling emulsions or mingling of immiscible melts in magmatic systems. Due to their similarities to the magmatic textures presented in Barnes et al. (2018), this schlieren-like intergrowth is interpreted to form by the same process, although the melts are anatectic in origin. Spessartine on lobate sulfide-silicate contacts (Fig. 5) can be explained as reaction between sulfides and silicates to form garnet. Garnet is also known from magmatic sulfide deposits, situated between sulfides and silicates (Barnes et al. 2020). Neither of the involved minerals at Bodenmais contains large amounts of Mn, but Ramdohr (1950) suggested that the voids observed at Broken Hill (which look identical to those at Bodenmais) were Mn-sulfides now dissolved away due to their low stability under oxidizing alteration or weathering conditions. If large amounts of Mn were dissolved in the anatectic sulfide melt, this Mn could have formed spessartine at the lobate contact to the silicate melt. Anhedral feldspar surrounded by symplectic quartz-cordierite-biotite hosted as inclusions in massive sulfides (Figs. 5a, b, 6) can best be explained by an anatectic silicate melt that still contains refractory feldspar. This feldspar-silicate melt mush could then be trapped in the sulfide melt and crystallized to the observed texture.
Polished slabs and 2D elemental maps of characteristic samples of the semi-massive sulfides. a and b Hand specimen that contains a network of euhedral elongate silicate crystals in a pyrrhotite matrix. The inset in b highlights the random orientation of the crystal network. Also highlighted in b are centimeter-sized silicate crystals. The boundary to the adjacent massive sulfides is undulating. Note the larger grain size of massive sulfides and that they are free of silicates. c and d Hand specimen that contains several round to elongate schlieren-like sulfide-silicate intergrowths as well as homogeneously distributed round quartz grains within the sulfides. Note the preferential overgrowth of sphalerite on quartz. e and f Hand specimen that is similar to c but where the silicate inclusions are aligned in bands. In addition, a euhedral K-feldspar crystal overgrows round quartz grains and subhedral cordierite contains sulfide inclusions. Sphalerite that preferentially overgrows round quartz is overgrown by chalcopyrite in some places
(2) Semi-massive sulfides (Fig. 7) form the largest proportion of the ore (Weinschenk 1901) and are composed of the same minerals and grain sizes as massive sulfides. Two different types of semi-massive sulfides occur. The rare first type forms lenses within massive sulfides. These lenses are composed of an intergrown network of euhedral actinolite or rarely biotite with interstitial pyrrhotite. Massive sulfides surrounding this texture display a larger grain size and are free of silicates (Fig. 7a, b). The most common second type contains numerous anhedral quartz grains and, less abundant, subhedral cordierite and K-feldspar crystals (Fig. 7c–f). Sphalerite in this texture preferentially surrounds the quartz grains or round symplectic intergrowths of quartz and cordierite which are only found in schlieren-like textures. Chalcopyrite then preferentially surrounds sphalerite.
Centimeter-sized silicate needles with interstitial sulfides (Fig. 7a) are interpreted as a relic emulsion where the silicate portion of it crystallized forming an amphibole crystal network. This stops any movement of the two melts, which is required to stabilize emulsions (Chen and Tao 2005), and hence separating the remaining silicate from the sulfide melt. A similar explanation was given by Staude et al. (2021) to explain the formation of olivine plates hosted in magmatic sulfides. Euhedral feldspar overgrowing quartz and being completely enclosed in sulfides (Fig. 7f) can be explained by a similar process to the silicate needles. When the temperature decreases, feldspar starts crystallizing, preferentially around the quartz grains. Due to a decreasing flowing velocity of both melts, the emulsion brakes down leaving behind a feldspar in the sulfide melt. The sulfide melt could also be partially solid at this time, preventing the feldspar to float upwards. Sphalerite preferentially overgrowing quartz (Figs. 5, 7) can be explained by sphalerite-crystallizing sulfide melt wetting quartz grains and therefore crystallizing around it. Similar effects are known from magmatic systems (Rose and Brenan 2001).
(3) Breccias (Fig. 8) are composed of host rock clasts cemented by sulfides that contain a high amount of rounded quartz grains and minor anhedral gahnite, biotite, garnet, cordierite, sillimanite, actinolite, and K-feldspar. All sulfides described before are found in the breccia cement. One sample contains pyrite that shows a zonation where pyrite contains roundish galena, chalcopyrite, and sphalerite inclusions (Fig. 8c), resembling trapped melt during pyrite growth. This aggregate is surrounded by galena that contains intergrowth of acanthite with an undetermined Sb-Hg-Ag-S phase (Fig. 8d) as well as round droplets of a graphical intergrowth of galena and an undetermined Ag-S-Te phase (Fig. 8e). In vein-like, centimeter-long joints of the host rock, the spatial sulfide succession is pyrrhotite and sphalerite at the start of the joint, followed by chalcopyrite, galena, and Ag-Au-Bi mineralization farther away. That can be interpreted as a crystallization sequence with the most refractory phases (pyrrhotite and sphalerite) crystallizing first followed by less refractory chalcopyrite and galena and then by immiscible polymetallic melts of Au-Ag-Bi (Mavrogenes et al. 2013).
Polished slab, 2D elemental map, and SEM pictures of characteristic samples of the breccia. a and b The lower part of the slab is dominated by the cordierite-sillimanite garnet migmatite that displays a central zone where sulfides are found along grain boundaries. Further up, the sulfides are dominating with host rock clasts and quartz grains. A larger foliated host rock clast (center) disintegrates into smaller fragments, especially to the left of the clast. Note, quartz is mostly angular in the host rock but rounded within the sulfides. c Subhedral pyrite grain surrounded by galena. Note the growth-zone with numerous roundish inclusions of galena, sphalerite, and chalcopyrite. d Enlargement of c to highlight an intergrowth of galena and pyrite with acanthite and an Sb-Hg-Ag-S phase. e Spherical inclusion in galena containing a graphic intergrowth of galena and an Ag-S-Te phase
Mineral chemistry
Silicate minerals
The feldspar group is dominated by K-feldspar, containing between 0.8 and 0.9 atoms per formula unit (apfu; normalized to 8 apfu O) K with minor plagioclase (0.45–0.65 apfu Na) limited only to one sample of the host rock breccia. K-feldspar in the pegmatite adjacent to sulfide lenses contains up to 2.8 wt% PbO (ESM 1). Similarly, K-feldspar in one leucosome sample of the cordierite-sillimanite-garnet migmatite contains up to 1.6 wt% PbO (containing abundant disseminated sulfides; mostly pyrrhotite, chalcopyrite, and galena), while in other country rock K-feldspar, the PbO is under the detection limit of the microprobe (750 ppm). K-feldspar of the schlieren-like texture only reaches 0.9 wt% PbO. K-feldspar at the direct contact between the sulfides and the pegmatite, showing symplectic intergrowths with quartz, exhibits a BaO content of up to 4.3 wt% with PbO below the detection limit of the microprobe.
Two groups of cordierite are observed in the samples (Fig. 9a): cordierite in the migmatites displays an XMg (Mg/(Mg + Fe)) between 0.5 and 0.6 and a low Mn content (below 0.04 apfu, normalized to 18 apfu O), whereas cordierite in contact with sulfides mostly displays an XMg of 0.8 to 0.9. Sulfide-hosted cordierite forms two groups distinguished by their Mn content (Fig. 9a): the Mn-poor group (0.04–0.06 apfu) usually forms sub- to euhedral crystals within sulfides and is free of sillimanite (i.e., Fig. 7e). The second group displays higher Mn concentrations (0.1 to 0.17 apfu) and is found as irregular aggregates within sulfides containing numerous sillimanite inclusions.
Mineral chemical composition of cordierite and garnet from migmatite and the ore zone. a Mn versus XMg of cordierite showing that the host rock cordierite displays significantly lower Mn and XMg values than the sulfide-hosted one. Sulfide-hosted cordierite forms two clusters distinguished by their Mn content. b Mn versus XMg of garnet; the host rock garnet displays significantly lower Mn and XMg values than the sulfide-hosted one. See text for discussion
Garnet formulae, normalized to 12 apfu O, also display two compositional groups (Fig. 9b). Both types of migmatite contain garnet as almandine-pyrope solid-solution with XMg-values between 0.1 and 0.2, whereas sulfide-hosted garnet has XMg values between 0.2 and 0.3. The Mn content varies even stronger: migmatite-hosted garnet has Mn contents below 0.3 apfu, whereas garnet within sulfides contains between 1.5 and 2.0 apfu Mn (Fig. 9b).
Sulfide minerals and native elements
Pyrrhotite, normalized to 1 apfu S, exhibits Fe contents of 0.8–0.9 apfu and contains some minor trace elements detectable with EMPA. Of these trace elements, As is the most abundant ranging mostly from 300 to 500 ppm with the upper limit being 5000 ppm in symplectite together with galena.
Pyrite shows two groups with regard to its Co-content, but these groups do not show any correlation with their host rocks or any specific textures. Cobalt in the first group is below the detection limit of the microprobe of 220 ppm, whereas the other group exhibits an average Co content of 300–800 ppm.
Sphalerite, normalized to 1 apfu S, contains 0.12 to 0.17 apfu Fe as well as an average Cd content of 0.3 wt%; some sphalerite reaches Cd contents of up to 3 wt%. In addition, sphalerite contains unusually high Bi contents (on average 430 ppm).
Chalcopyrite contains on average 7000 ppm Ag, but up to 5.2 wt% Ag was observed in some samples. Typically, the Ag-rich portions of chalcopyrite exhibit a fast tarnishing (Fig. 10). The Ag concentration is not homogeneous within one grain and the highest Ag concentration appears to be located at one corner only (see Fig. 10). In some cases, where the highest Ag content was analyzed, native Ag inclusions were observed in chalcopyrite. Hence, the high Ag contents may in general be related to nano-inclusions of native Ag or other Ag minerals not visible in a microscope and even not visible in the SEM.
Galena contains on average 0.7 wt% Ag and 1.2 wt% Bi, but can contain up to 3 wt% Ag and 2 wt% Bi. Normalized to 1 apfu S, the Ag and Bi concentration nicely correlates indicating the matildite substitution (see, e.g., Staude et al. 2010) (ESM 1).
Silver minerals occur exclusively as alloys, except for rare acanthite. These undetermined alloys are either Au-free and then span the range from native Ag to up to 30 wt% Hg, or they contain 11–15 wt% Au and 12–14 wt% Hg.
Discussion
Sulfide anatexis at Bodenmais
From experimental work, it is known that sulfides can melt during high-grade metamorphism (e.g., Mavrogenes et al. 2001; Stevens et al. 2005; Tomkins et al. 2007; Pruseth et al. 2014, 2016). Especially, the combination of sulfides found in SEDEX deposits is believed to melt easily (because of their trace element contents, see in the “Introduction” section), but the extent of partial melting is debated (e.g., Spry et al. 2008; Tomkins 2007). The main sulfides from Bodenmais (pyrrhotite, chalcopyrite, pyrite, sphalerite, galena) also contain trace elements (i.e., As, Ag, Cd) or accessory minerals (i.e., native Bismuth, the undetermined Sb-Hg-Ag-S and Ag-S-Te phases) that lower the melting temperature of sulfides considerably (Tomkins et al. 2007). Based on this major and trace element chemistry, the petrographic descriptions above, the known peak-metamorphic conditions (800–850 °C, 0.5–0.7 GPa; Kalt et al. 1999), and the experimental data (see “Introduction” section), it is most likely that the sulfides at Bodenmais were molten.
Comparison with textures known from magmatic sulfide deposits
At peak metamorphic conditions at Bodenmais, a sulfide melt formed in addition to migmatitic anatexites in the surrounding host rocks. Hence, the two immiscible melts were in immediate contact (Fig. 11). Accordingly, textures between sulfide and silicate melt could form that are similar to textures known from magmatic sulfides deposits such as emulsions (Barnes et al. 2018). The specific texture formed depends on several factors, such as viscosity and density of both melts (Dobson et al. 2000), the chemical composition, and therefore the liquidus and solidus temperatures, which dictate the temperature range in which two melts, with or without solids, can interact (Barnes et al. 2016). Another important factor is the wetting angle between a melt and various minerals as this determines whether a melt can migrate or is immobile (Rose and Brenan 2001). Whatever the cause for a certain texture in magmatic sulfide deposits, it is important that the preserved texture is only the last process frozen in and textural evidence of processes prior to it may not be preserved. All this should also be true for textures formed during silicate and sulfide anatexis.
Simplified cartoon illustrating the formation of the observed textures around peak metamorphic conditions. The silicate country rock contains a leusosome melt (1), and where in contact with the sulfide melt (2), it is buoyant due to its lower density and therefore forms plumes, which can detach from the silicate melt layer and float through the sulfide melt (3). Where silicate melts along fractures larger clasts of the silicate rock can detach forming sulfide matrix breccias (4). On the contact to the biotite-feldspar migmatite, the silicate melt is trapped underneath (possibly mixing with anatectic melt from this migmatite) forming a silicate melt layer on top of the sulfide melt (5). During retrograde cooling, the pegmatite starts crystallizing while the sulfides are still partially molten (6). Part of the sulfides also crystallized and fractionated causing the pegmatite to trap fractionated sulfides forming now globular sulfides within the pegmatite (7). The constant detachment of basal silicate melt leaves refractory quartz behind (8) which floats through the sulfide melt when completely detached from the host rock forming a layer of floating quartz with interstitial sulfides underneath the solidified pegmatite (9). Where sulfide melt and silicate melt are on direct contact, gahnite can form (10). Where there was sulfide melt silicate melt mingling while crystallizing, emulsion textures are preserved within massive sulfides (11) or within the quartz-rich semi-massive sulfides (12)
In magmatic sulfide deposits, sulfide melts are able to melt the country rock and the sulfide melt can migrate away from the magmatic mafic or ultramafic host melt for several hundreds of meters (Barnes et al. 2020). The typical texture that is produced by this process is the so-called sulfide-matrix breccia (Barnes et al. 2018, 2020), where clasts of the country rock are surrounded by a sulfide matrix. Some of these clasts can be surrounded by multiple mineral grains of the disintegrated silicate rock or, when the silicates were molten, they form an emulsion layer around the silicate clast. A similar texture is found at Bodenmais on the western contact of the sulfides to the cordierite-sillimanite-garnet migmatite. Clasts of the host rock are fractured and filled by sulfides. The clasts themselves are surrounded by fragmented silicates with interstitial sulfides, similar to the magmatic sulfide-matrix breccia (Fig. 8).
A typical texture in magmatic systems is the net-texture, where euhedral magmatic silicates, forming a cumulate, are surrounded by interstitial sulfides (Barnes et al. 2017). This is thought to form either by crystallizing silicates sinking into the sulfide melt underneath due to an increasing weight of the increasing crystal pile (Naldrett 1973), or by sulfide melt infiltrating from above into an existing cumulate pile and replacing the interstitial silicate melt (Barnes et al. 2017). Anhedral quartz grains surrounded by sulfides, as in the semi-massive sulfides at Bodenmais, are very similar to such net-textured sulfides. This texture is found above the massive sulfides and underneath the pegmatite (Fig. 11, ESM 1). The pegmatite must have been crystallizing prior to sulfide solidification as it is brecciated and filled by semi-massive sulfides. Often, clasts of the pegmatite are also found within semi-massive sulfides. The semi-massive sulfides could form in a similar way as net-textured sulfides in magmatic systems. Instead of magmatic silicates sinking into the sulfide melt, the underlying silicates underwent anatexis, as evident by the migmatite. The eutectic silicate melt floated through the sulfide melt leaving a more refractory mineral assemblage (in this case mostly quartz) behind. When detached from the host silicate rock, these grains could also float through the sulfide melt (Fig. 11). The silicate melt then forms a silicate melt layer above the sulfides; refractory minerals floating in the silicate melt can get dissolved in it; however, once the silicate melt crystallizes to form the pegmatite, the refractory minerals, as well as silicate melt droplets, are trapped underneath the pegmatite, forming a texture very similar to the magmatic net-texture. The silicate melt droplets then crystallize to coarse cordierite, K-feldspar, and garnet (Fig. 7). Due to the interaction with the sulfide melt, the crystallizing silicates then exhibit a different chemistry compared to the counterpart in the migmatite, as evident with cordierite and garnet.
The pegmatite itself has similarities in magmatic sulfide deposits. Magmatic sulfide and silicate melts have a higher temperature then the country rocks they intrude into and, therefore, can melt them. Especially, sulfide melts are able to infiltrate and melt the country rock due to their high density, low viscosity, and high thermal conductivity (Staude et al. 2017). At Nova-Bollinger (Australia), sulfide melt melted and migrated into meta-sedimentary rocks away from the host intrusion (Barnes et al. 2020). In these parts, felsic leucosomes are found above sulfides and are interpreted as partially molten meta-sediments, molten by the sulfides, and trapped above them (Barnes et al. 2020), resembling the pegmatite found above the Bodenmais ore.
Magmatic sulfide deposits show a chemical zonation due to fractional crystallization (Li et al. 1992; Staude et al. 2022). The same has been suggested for Fe-Zn-Pb- and LMCE-bearing sulfide melts based on experiments (see “Introduction” section; Mavrogenes et al. 2013; Pruseth et al. 2016). Similar to these experiments, the earliest minerals to grow from the sulfide melt at Bodenmais are pyrite and sphalerite which preferentially overgrow quartz grains in the semi-massive ore. Other signs of fractional crystallization are the preferential overgrowth of sphalerite by chalcopyrite, the zonation of sphalerite/pyrrhotite to chalcopyrite to galenite to Ag-Au-Bi minerals in fractures, and the overall chemical zonation of pyrrhotite-rich, sphalerite-rich, and galena-rich portions of the orebody (Weinschenk 1901). The zoned chalcopyrite, where one part of the grain is enriched in Ag, may also be explained this way by assuming that the Ag-poor chalcopyrite crystallizes first leaving Ag in the remaining sulfide melt which then crystallizes to the Ag-rich chalcopyrite.
In some magmatic sulfide deposits, spinel (chromite) has been observed on contacts between sulfide melt and silicate melt and is thought to form due to the interaction between the two melts (Staude et al. 2016). This texture resembles the spinel (gahnite) found at Bodenmais at the sulfide-silicate contacts such as the pegmatite sulfide contact and at emulsion-like intergrowths of sulfides and silicates (Fig. 4). After Hobbs (1975), gahnite can form from sphalerite and sillimanite according to the following reaction:
As the anatectic melt of the migmatites was peraluminous (because it crystallized cordierite; Kalt et al. 1999), the gahnite could form on sulfide-silicate contacts by the reaction of the sulfide melt or relic sphalerite with the peraluminous melt.
Extent of sulfide anatexis and comparison to similar deposits
The proportion of sulfide melt formation during metamorphism in other deposits which were overprinted by upper amphibolite or granulite-facies metamorphism is unclear. Most studies assume only minor amounts of sulfide melt (Tomkins et al. 2007; Spry et al. 2008). Frost et al. (2011) suggested based on samples from Broken Hill (Australia) that the sulfide melt portion was between 14 and 21% for this deposit; however, they state that this is probably an underestimation. At Bodenmais, the textural observations presented above suggest that the sulfides were completely molten and that they crystallized to enclose quartz grains in the semi-massive ores. To explain the profile with its characteristic textures in each layer (Figs. 3, 4, 5, 6, 7, 8, 11), the sulfides also must have been molten to a large degree (if not completely), as anatectic silicate melts and refractory quartz grains needed to migrate through the sulfide melt to form the silicate melt layer above the sulfide melt and the net-textured layer of semi-massive ores. The stratigraphy was approximately horizontal during anatexis, as originally deposited, to explain that the silicate melt is found always on the eastern side of the orebody which is consistent with the regional geology, where the metamorphic grade decreases towards the east and therefore represent rocks higher in the stratigraphy. The steepening of the stratigraphy in its modern position had to occur after peak metamorphism. The footwall is composed of the cordierite-sillimanite-garnet migmatite and the hanging wall of the biotite-feldspar migmatite.
Similar lithologies or large-scale textures in other metamorphosed sulfide deposits have only rarely been described, as most publications deal with the chemistry of minerals, with micropetrographic observations or with the pre-metamorphic origin of the deposits. However, at least some indications for high-volume anatectic sulfide melting, as suggested at Bodenmais, have been published. These include pegmatites containing green Pb-bearing K-feldspar next to sulfide lenses at Broken Hill in Australia and at Broken Hill in South Africa (Stevenson and Martin 1986; Bailie and Reid 2005). Bailie and Reid (2005) also described large, meter-sized single chalcopyrite and galena grains in the metasedimentary country rocks from Broken Hill (South Africa) and interpreted them as crystallized from a sulfide melt. DeLorraine (2001) reported a similar texture to the semi-massive sulfides of Bodenmais from Balmat (New York, USA) where quartz grains are enclosed in sphalerite and pyrite, but interpreted this as a tectonic mobilization texture, although no foliation or rotation of clasts is visible. The Hongtoushan VHMS deposit in China contains a texture similar to the semi-massive sulfides at Bodenmais. The multiple quartz grains are enclosed in pyrrhotite-chalcopyrite ore which was interpreted by Li et al. (2022) as the product of sulfide anatexis.
Conclusion
Textural observations and outcrop-scale zonation of sulfide-silicate textures suggest that the metamorphosed SEDEX-type Bodenmais deposit was largely molten during peak-metamorphic conditions of at least 800–850 °C and 0.5–0.7 GPa (Kalt et al. 1999). Typical textures - comparable to textures found in magmatic sulfide deposits - formed mostly due to interaction of the sulfide melt with anatectic silicate melt of the country rocks. This includes a sulfide-matrix breccia, net-textured intergrowths of refractory quartz “swimming” in sulfides, lobate contacts between silicates and sulfides resembling emulsions, and spinel formation on sulfide-silicate contacts. The succession of these textures within the orebody is also similar to magmatic sulfide deposits: at the base, the sulfide-matrix breccia reflects the interaction of sulfide melt with the country rock and is followed by massive sulfides above which in turn is followed by the net-textured quartz-sulfide intergrowths and, finally, a pegmatite, which represents the floating and trapped silicate melt originally formed at the base of the sulfide deposit. Similar to magmatic sulfides, the molten SEDEX sulfides fractionate during crystallization, which is best visible in fractures whit a typical sulfide succession. The crystallization starts with symplectic pyrite-sphalerite intergrowths overgrown by pyrrhotite and then followed by chalcopyrite, galena, and finally Ag-Au-Bi minerals.
References
Brett R, Kullerud G (1967) The Fe-Pb-S system. Econ Geol 62:354–369
deLorraine WF (2001) Metamorphism, polydefomration, and extensive remobilization of the Balmat Zinc orebodies, Northwest Adirondacks, New York. Econ Geol 35:25–54
Mehnert KR (1968) Migmatites and the origin of granitic rocks. Elsevier, Amsterdam
Staude S, Barnes SJ, Le Vaillant M (2016) Evidence of lateral thermomechanical erosion of basalt by Fe-Ni-Cu sulfide melt at Kambalda, Western Australia. Geology 44:1047–1050
Bailie RH, Reid DL (2005) Ore textures and possible sulphide partial melting at Broken Hill, Aggeneys, South Africa I: Petrography. S Afr J Geol 108:51–70
Barnes SJ, Cruden AR, Arndt N, Saumur BM (2016) The mineral system approach applied to magmatic Ni-Cu-PGE sulphide deposits. Ore Geol Rev 76:296–316
Barnes SJ, Mungall JE, Le Vaillant M, Godel B, Lesher CM, Holwell D, Lightfoot PC, Krivolutskaya N, Wei B (2017) Sulfide-silicate textures in magmatic Ni-Cu-PGE sulfide ore deposits: disseminated and net-textured ores. Am Min 102:473–506
Barnes SJ, Staude S, Le Vaillant M, Piña R, Lightfoot PC (2018) Sulfide-silicate textures in magmatic Ni-Cu-PGE sulfide ore deposits: massive, semi-massive and sulfide-matrix breccia ores. Ore Geol Rev 101:629–651
Barnes SJ, Taranovic V, Miller JM, Boyce G, Beresford S (2020) Sulfide emplacement and migration in the Nova-Bollinger Ni-Cu-Co deposit, Albany-Fraser Orogen, Western Australia. Econ Geol 115:1749–1776
Beeson R, Webster AE (2023) A classification of Broken Hill Type deposits a reply. Ore Geol Rev. https://doi.org/10.1016/j.oregeorev.2023.105331
Carne RC, Cathro RJ (1982) Sedimentary exhalative (sedex) zinc-lead-silver deposits, northern Canadian Cordillera. Canadian Institute of Mining Bulletin 75:66–78
Chen G, Tao D (2005) An experimental study of stability of oil-water emulsion. Fuel Process Technol 86:499–508
Dill HG (1990) Chemical basin analysis of metalliferous “viariegated metamorphics” of the Bodenmais Ore district (F.R. of Germany). Ore Geol Rev 5:151–173
Dobson DP, Crichton WA, Vočadlo L, Jones AP, Wang Y, Uchida T, Rivers M, Sutton S, Brodholt JP (2000) In situ measurements of viscosity of liquids in the Fe-FeS system at high pressures and temperatures. Am Mineral 85:1838–1842
Frost BR, Mavrogenes JA, Tomkins AG (2002) Partial melting of sulfide ore deposits during medium- and high-grade metamorphism. Can Mineral 40:1–18
Frost BR, Swapp SM, Gregory RW (2005) Prolonged existence of sulfide melt in the Broken Hill orebody, New South Wales, Australia. Can Mineral 43:479–493
Frost BR, Swapp SM, Mavrogenes J (2011) Textural evidence for extensive melting of the Broken Hill orebody. Econ Geol 106:869–882
Govindarao B, Pruseth KL, Mishra B (2020a) Experimentally produced Cu-Pb-Ag-Sb-S melts at 500 °C: implications to partial melting of massive sulfide ores. Ore Geol Rev 121:103560
Govindarao B, Pruseth KL, Mishra B (2020b) Sulfide partial melting and galena-tetrahedrite intergrowth texture: an experimental study. Mineral Mag 84:859–868
Hobbs BE (1975) The Broken Hill lode horizon project: Broken Hill, South Africa. Broken Hill Mining Managers’ Association, unpublished report, 98 p
Höhn S, Frimmel HE, Price W (2021) Syn-metamorphic sulfidation of the Gamsberg zinc deposit, South Africa. Miner Petrol 115:709–728
Kalt A, Berger A, Blümel P (1999) Metamorphic evolution of cordierite-bearing migmatites from the Bayerische Wald (Variscan Belt, Germany). J Petrol 40:601–627
Lawrence LJ (1967) Sulphide neomagmas and highly metamorphosed sulphide deposits. Miner Deposita 2:5–10
Li C, Naldrett AJ, Coats CJA, Johannessen P (1992) Platinum, palladium, gold, and copper-rich stringers at the Strathcona Mine, Sudbury: their enrichment by fractionation of a sulfide liquid. Econ Geol 87:1584–1598
Li LH, Fan HR, Qui ZJ, Yang KF, Han Y, Zhao G (2022) Sulfide texture and geochemistry of the Neoarchean Hongtoushan Cu-Zn deposit (NE China): implication for mixed-state metamorphic remobilization. Ore Geol Rev 145:104885
Linhardt E (2015) SEE-Potenzial der Sulfiderz-Lagerstätte Silberberg / Bodenmais. Bayerisches Landesamt für Umwelt 1 – 58
Mavrogenes JA, MacIntosh IW, Ellis DJ (2001) Partial melting of the Broken Hill galena-sphalerite ore: experimental studies in the system PbS-FeS-ZnS-(Ag2S). Econ Geol 96:205–210
Mavrogenes JA, Frost BR, Sparks HA (2013) Experimental evidence of sulfide melt evolution via immiscibility and fractional crystallization. Can Mineral 51:841–850
Millsteed PW (2011) The role of halogens with sulfide melting at Broken Hill, New South Wales, Australia. PhD Thesis, The Australian National University, Canberra, 310 p. https://doi.org/10.25911/5d51413b17446
Mishra B, Bernhardt HJ (2009) Metamorphism, graphite crystallinity, and sulfide anatexis of the Rampura-Agucha massive sulfide deposit, northwestern India. Miner Deposita 44:183–204
Mungall JE, Brenan JM (2003) Experimental evidence for the chalcophile behaviour of the halogens. Can Mineral 41:207–220
Naldrett AJ (1969) A portion of the system Fe-S-O between 900 and 1080 °C and its application to sulfide ore magmas. J Petrol 10:171–201
Naldrett AJ (1973) Nickel sulphide deposits – their classification and genesis, with special emphasis on deposits of volcanic association. CIM Bull 66:45–63
Pfaffl F (2015) Das Sulfiderz-Bergbaurevier von Bodenmais im Bayerischen Wald. Der Bayerische Wald 28:88–95
Pfeufer J (1976) Das Schwefelerzvorkommen am Silberberg bei Bodenmais (Bayerischer Wald). Erzmetall 29:340–355
Pruseth KL, Jehan N, Sahu P, Mishra B (2014) The possibility of a ZnS-bearing sulfide melt at 600 °C: evidence from the Rajpura-Dariba deposit, India, supported by laboratory melting experiment. Ore Geol Rev 60:50–59
Pruseth KL, Mishra B, Jehan N, Kumar B (2016) Evidence of sulfide melting and melt fractionation during amphibolite facies metamorphism of the Rajpura-Dariba polymetallic sulfide ores. Ore Geol Rev 72:1213–1223
Ramdohr P (1950) Die Lagerstätte von Broken Hill in New South Wales. Heidelberger Beiträge Zur Mineralogie Und Petrographie 2:291–333
Rose LA, Brenan JM (2001) Wetting properties of Fe-Ni-Co-Cu-O-S melts against olivine: implications for sulfide melt mobility. Econ Geol 96:145–157
Sangster DF (2020) Evidence that Broken Hill-type Pb-Zn deposits are metamorphosed SEDEX deposits. Miner Deposita 55:1263–1270
Schreyer W, Kullerud G, Ramdohr P (1964) Metamorphic conditions of ore and country rock of the Bodenmais, Bavaria, sulfide deposit. Neues Jahrb Mineral Abh 101:1–26
Spry PG, Teale GS (2021) A classification of Broken Hill-type deposits: a critical review. Ore Geol Rev 130:103935
Spry PG, Plimer IR, Teale GS (2008) Did the giant Broken Hill (Australia) Zn–Pb–Ag deposit melt? Ore Geol Rev 34:223–241
Staude S, Dorn A, Pfaff K, Markl G (2010) Assemblages of Ag-Bi sulfosalts and conditions of their formation: The type locality of schapbachite (Ag0.4Pb0.2Bi0.4S) and neighboring mines in the Schwarzwald ore district, Southern Germany. Can Mineral 48:441–466
Staude S, Barnes SJ, Le Vaillant M (2017) Thermomechanical erosion of ore-hosting embayments beneath komatiite lava channels: textural evidence from Kambalda, Western Australia. Ore Geol Rev 90:446–464
Staude S, Barnes SJ, Markl G (2021) Interspinifex Ni sulfide ore from Victor South-McLeay, Kambalda, Western Australia. Miner Deposita 56:125–142
Staude S, Oelze M, Makl G (2022) Multi-stage sulfide evolution of the Moran Ni sulfide ore, Kambalda, Western Australia: insights into the dynamics of ore forming processes of komatiite-hosted deposits. Miner Deposita 57:889–909
Stettner G (1981) Grundgebirge. Erläuterungen Zur Geologischen Karte Von Bayern 1(500):000
Stevens G, Prinz S, Rozendaal A (2005) Partial melting of the assemblage sphalerite + galena + pyrrhotite + chalcopyrite + sulfur: implications for high-grade metamorphosed massive sulfide deposits. Econ Geol 100:781–786
Stevenson RK, Martin RF (1986) Implications of the presence of amazonite in the Broken Hill and Geco metamorphosed sulfide deposits. Can Mineral 24:729–745
Tomkins AG (2007) Three mechanisms of ore re-mobilisation during amphibolite facies metamorphism at the Montauban Zn–Pb–Au–Ag deposit. Miner Deposita 42:627–637
Tomkins AG, Pattison DRM, Frost BR (2007) On the initiation of metamorphic sulfide anatexis. J Petrol 48:511–535
Troll G, Linhardt E, Skeries R (1987) Petrographic and geochemical studies on country rock of the Bodenmais (Bavaria) sulphide deposit. Neues Jahrbuch für Geologie und Paläontologie - Monatshefte 726–752
Vornehm C, Bender S, Lehrberger G, Wohnlich S (2002) Impact of ore deposits and anthropogenic activities on local hydrochemistry at “Silberberg”, S-Germany. In: Merkel BJ, Planer-Friedrich B, Wolkersdorfer C (eds) Uranium Mining and Hydrology, pp 1065–1074
Weinschenk E (1901) Die Kieslagerstätte im Silberberg bei Bodenmais. Abh Bay Akad Wiss 21Bd II Cl II Abt 352–410
Wykes JL, Mavrogenes JA (2005) Hydrous sulfide melting: experimental evidence for the solubility of H2O in sulfide melts. Econ Geol 100:157–164
Acknowledgements
We are grateful to the Silberberg visitors mine for samples. Simone Schafflick and Per Jeisecke are thanked for sample preparation. The mineral chemistry was performed using a microprobe which was partly funded by the German Research Foundation (DFG; grant INST 37/1026-1 FUGG). We are particularly grateful to B. Ronald Frost about a critical review and his helpful comments as well as Bernd Lehmann for his editorial handling of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editorial handling: B. Lehmann
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

ESM 1_1
Merged photo of a gossan outcrop in the south of the Silberberg. Sulfides are replaced by limonite, but primary textures are preserved and described in the petrography section. To the west, the cordierite-sillimanite-garnet migmatite is brecciated at the contact to limonitized sulfides and cemented by them. This is followed by massive limonite with grain boundaries and cleavage planes of the original sulfides preserved. Above massive limonite is limonite with frequent quartz grains reflecting the semi-massive sulfide texture which is in contact to the pegmatite to the east. The contact to the pegmatite is not a clear contact and rather forms a breccia, where the pegmatite is brecciated and cemented by the semi-massive sulfide texture. There are also veins of semi-massive sulfides cutting through the pegmatite. Not on the picture is the straight contact of the pegmatite to the biotite-feldspar migmatite to the east (PNG 4975 kb)

ESM 1_2
Histogram of the PbO content of K-feldspar showing that the pegmatite-hosted feldspar displays the highest PbO content (probably microcline). The low PbO content of pegmatite-bearing feldspar is most likely orthoclase. The inset shows a PbO versus BaO digram of the pegmatite-hosted feldspar only to discriminate between orthoclase (high BaO, low PbO) versus microcline (high PbO, low BaO). Apfu: atoms per formula unit (PNG 50 kb)

ESM 1_3
Bi versus Ag concentration in galena which follow the 1:1 line due to coupled substitution of Ag+ and Bi3+ with Pb2+ (PNG 34 kb)
ESM 2
(DOCX 14 kb)
ESM 3
(XLSX 299 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Staude, S., Raisch, D. & Markl, G. Sulfide anatexis during high-grade metamorphism: a case study from the Bodenmais SEDEX deposit, Germany. Miner Deposita 58, 987–1003 (2023). https://doi.org/10.1007/s00126-023-01166-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-023-01166-y