Summary
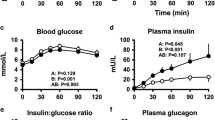
It is suggested that amylin (islet associated polypeptide), co-secreted with insulin from the pancreatic beta cells acts as a circulating hormone which opposes the action of insulin on muscle and increases hepatic glucose production. We have tested the effect of amylin in human subjects on postabsorptive glucose homeostasis and on insulin sensitivity using the euglycaemic hyperinsulinaemic clamp. The amylin used opposed insulin-mediated glucose disposal in rat soleus muscle at concentrations of 10 nmol/1. Seven subjects were studied on two occasions and infused with either amylin or placebo for 6 h, initially when postabsorptive and then during a euglycaemic hyperinsulinaemic clamp. Mean plasma amylin concentrations during the first 3 h were 2006 ±327 pmol/1 during amylin infusion and 20 ±9 pmol/1 during the control infusion. Amylin infusion had no effect on postabsorptive plasma concentrations of insulin (control: 32 ±16 vs amylin: 25 ±8 pmol/1) or glucose (5.1 ±0.1 vs 5.3 ±0.1 mmol/1). During the clamp, amylin concentrations were 1636 ±422 pmol/1 when it was infused and 24 ±6 during control infusions. Plasma glucose and insulin concentrations were well matched during the control and amylin infusions (glucose: 4.7 ±0.1 vs 4.8 ±0.1 mmol/1; insulin: 198 ±37 vs 195 ±22 pmol/1). Exogenous glucose infusion rates were a mean of 13 % lower than control values during the amylin infusion but were not statistically different (p =0.17). Therefore, an approximately 100-fold elevation of plasma amylin concentration failed to consistently alter glucose metabolism. Our data suggest that amylin does not act as a circulating hormone to influence glucose metabolism in humans.
Similar content being viewed by others
References
Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH (1987) Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA 84: 3881–3885
Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB (1987) Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA 84: 8628–8632
Johnson KH, O’Brien TD, Hayden DW et al. (1988) Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol 130: 1–8
Ohsawa H, Kanatsuka A, Yamaguchi T, Makino H, Yoshida S (1989) Islet amyloid polypeptide inhibits glucose-stimulated insulin secretion from isolated rat pancreatic islets. Biochem Biophys Res Commun 160: 961–967
Wang ZL, Bennet WM, Ghatei MA, Byfield PGH, Smith DM, Bloom SR (1993) Influence of islet amyloid polypeptide and the 8–37 fragment of islet amyloid polypeptide on insulin release from perifused rat islets. Diabetes 42: 330–335
Roden M, Liener K, Fürnsinn C et al. (1992) Effects of islet amyloid polypeptide on hepatic insulin resistance and glucose production in the isolated perfused rat liver. Diabetologia 35: 116–120
Morishita T, Yamaguchi A, Fujita T, Chiba T (1990) Activation of adenylate cyclase by islet amyloid polypeptide with COOH-terminal amide via calcitonin gene-related peptide receptors on rat liver plasma membranes. Diabetes 39: 875–877
Leighton B, Cooper GJ (1988) Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature 335: 632–635
Young AA, Gedulin B, Wolfe Lopez D, Greene HE, Rink TJ, Cooper GJ (1992) Amylin and insulin in rat soleus muscle: dose responses for cosecreted noncompetitive antagonists. Am J Physiol 263: E274-E281
Koopmans SJ, Van Mansfeld AD, Jansz HS et al. (1991) Amylin-induced in vivo insulin resistance in conscious rats: the liver is more sensitive to amylin than peripheral tissues. Diabetologia 34: 218–224
Frontoni S, Choi SB, Banduch D, Rossetti L (1991) In vivo insulin resistance induced by amylin primarily through inhibition of insulin-stimulated glycogen synthesis in skeletal muscle. Diabetes 40: 568–573
Sowa R, Sanke T, Hirayama J et al. (1990) Islet amyloid polypeptide amide causes peripheral insulin resistance in vivo in dogs. Diabetologia 33: 118–120
Bretherton Watt D, Gilbey SG, Ghatei MA, Beacham J, Macrae AD, Bloom SR (1992) Very high concentrations of islet amyloid polypeptide are necessary to alter the insulin response to intravenous glucose in man. J Clin Endocrinol Metab 74: 1032–1035
Eriksson J, Nakazato M, Miyazato M, Shiomi K, Matsukura S, Groop L (1992) Islet amyloid polypeptide plasma concentrations in individuals at increased risk of developing type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35: 291–293
Bevan SJ, Parry-Billings M, Opara E, Liu CT, Dunger DB, Newsholme EA (1992) The effect of insulin-like growth factor II on glucose uptake and metabolism in rat skeletal muscle in vitro. Biochem J 286: 561–565
Shiomi K, Nakazato M, Miyazato M, Kangawa K, Matsuo H, Matsukura S (1992) Establishment of hypersensitive radioimmunoassay for islet amyloid polypeptide using antiserum specific for its N-terminal region. Biochem Biophys Res Commun 186: 1065–1073
Hartter E, Svoboda T, Ludvik B et al. (1991) Basal and stimulated plasma levels of pancreatic amylin indicate its cosecretion with insulin in humans. Diabetologia 34: 52–54
Kassir AA, Upadhyay AK, Lim TJ, Moossa AR, Olefsky JM (1991) Lack of effect of islet amyloid polypeptide in causing insulin resistance in conscious dogs during euglycemic clamp studies. Diabetes 40: 998–1004
Bhogal R, Smith DM, Bloom SR (1992) Investigation and characterization of binding sites for islet amyloid polypeptide in rat membranes. Endocrinology 130: 906–913
Young AA, Carlo P, Rink TJ, Wang MW (1992) 8-37 hCGRP, an amylin receptor antagonist, enhances the insulin response and perturbs the glucose response to infused arginine in anesthetized rats. Mol Cell Endocrinol 84: R1-R5
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilding, J.P.H., Khandan-Nia, N., Bennet, W.M. et al. Lack of acute effect of amylin (islet associated polypeptide) on insulin sensitivity during hyperinsulinaemic euglycaemic clamp in humans. Diabetologia 37, 166–169 (1994). https://doi.org/10.1007/s001250050088
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s001250050088