Abstract
Diabetes is the leading cause and a common comorbidity of advanced chronic kidney disease. Glycaemic management in this population is challenging and characterised by frequent excursions of hypoglycaemia and hyperglycaemia. Current glucose monitoring tools, such as HbA1c, fructosamine and glycated albumin, have biases in this population and provide information only on mean glucose exposure. Revolutionary developments in glucose sensing and insulin delivery technology have occurred in the last decade. Newer factory-calibrated continuous glucose monitors provide real-time glucose data, with predictive alarms, allowing improved assessment of glucose excursions and preventive measures, particularly during and between dialysis sessions. Furthermore, integration of continuous glucose monitors and their predictive alerts with automated insulin delivery systems enables insulin administration to be decreased or stopped proactively, leading to improved glycaemic management and diminishing glycaemic fluctuations. While awaiting regulatory approval, emerging studies, expert real-world experience and clinical guidelines support the use of diabetes technology devices in people with diabetes and advanced chronic kidney disease.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is the most common cause of end-stage kidney disease (ESKD), and the most common comorbidity, present in up to 60% of people [1, 2]. Population-based studies show that individuals with ESKD have a disproportionately higher risk of developing severe hypoglycaemic events requiring emergency room visits and/or hospitalisations [3]. These events impose a significant burden of care, are costly to the health system and are associated with a higher risk of permanent sequalae and even death. Moreover, clinicians and individuals may try to avoid hypoglycaemia by allowing liberalisation of glycaemic status at the expense of developing hyperglycaemia. Recent studies using continuous glucose monitoring, which provides a more comprehensive assessment of glucose levels, have demonstrated that dysglycaemic events are very frequent in this population [4,5,6], with a tendency towards persistent hyperglycaemia. People with diabetes and advanced chronic kidney disease (CKD) also have a high incidence of diabetes-related comorbidities, such as CVD [2], diabetic retinopathy and visual impairment [7] and infections and amputations [8]. Clinicians should aim to optimise glycaemic management while minimising hypoglycaemia, which is paramount for preventing acute glycaemic crises and other diabetes-related complications.
Endogenous insulin clearance is first mediated by the liver and then by the kidneys. The kidneys are responsible for a larger proportion of exogenous insulin metabolism due to its bypass of first-pass metabolism in the liver [1]. Multiple factors in ESKD contribute to hypoglycaemia, including decreased gluconeogenesis, impaired insulin clearance, impaired counterregulatory hormone responses (cortisol), nutritional deficiencies and variable medication effects as a result of haemodialysis (see Text box: ‘Insulin and glucose metabolism in early and advanced CKD’). In addition, the accumulation of ‘uraemic toxins’ is believed to contribute to insulin resistance and postprandial hyperglycaemia [9].

There have been technological advances in diabetes care in the last decade, including the introduction of factory-calibrated continuous glucose monitors (CGMs) and their integration through computerised algorithms to insulin pumps, creating closed-loop systems or automated insulin delivery (AID) systems. However, many of these diabetes technologies have not been tested in validation studies nor approved for use in people with diabetes and ESKD treated with dialysis. In this review, we focus on recent developments in diabetes technology in this population, including glucose meters, CGMs and integrated AID systems.
Overview of technology developments in glucose sensing
In 1911, Stanley R. Benedict described the first method for assessing glucose levels in humans [10]. This qualitative method measured glucose in the urine and was based on the body’s capacity to reduce free carbonyl groups in glucose using different metals [11]. Since then, numerous scientific advancements have resulted in increasingly precise quantitative methods, culminating in modern techniques that are both highly accurate and relatively simple to use. Currently, three different methods are used for glucose measurement: electrochemical methods (enzymatic and non-enzymatic), optical methods (fluorophore-based and non-flourophore-based) and combinational or other methods (piezoelectric, electromagnetic impedance) [12].
Since the 1970s, enzyme-based electrodes have been widely used for amperometry detection of electrical current differences resulting from the glucose oxidase, hexokinase or glucose dehydrogenase reactions. Older generations of CGMs required people to perform self-monitoring of blood glucose (SMBG) using the finger-stick method, to calibrate the sensor and maintain sensor accuracy. In 2014, the first factory-calibrated CGM was introduced, which removed the need for finger-pricking and SMBG measurements. To estimate glucose levels, a microneedle-based sensor is placed subcutaneously [13, 14], thus creating a minimally invasive method that allows interstitial glucose measurements to be made every 1–5 min that correlate with and have a minimal lag time with blood glucose values [13]. The magnitude of the electrical current corresponds to the concentration of glucose in the tissue at the sensor site and is converted to glucose values through a calibration function. In the newer generation of CGMs, integration of miniaturised sensing electrodes into the lumen of a single hollow microneedle, with an opening facing the dermal space, allowing passive molecular diffusion over a short distance, have minimised the lag time between blood glucose and interstitial fluid glucose concentrations to 4–12 min [13, 15, 16]. These factory-calibrated CGMs contain calibration algorithms, developed by computer engineers, that help correct for sensor drift (gradual loss of accuracy in a CGM sensor's readings over time) by keeping track of the day since insertion and adjusting the calibration function based on the day [13, 15, 16]. Further developments have also been made in electrode materials, enzyme immobilisation techniques and materials used for covering membranes to minimise interference and improve accuracy [14, 17].
The development and refinement of predictive algorithms in factory-calibrated sensors has led to the development of predictive alarms. These alarms alert wearers or integrated devices (e.g. AID systems), providing an opportunity to adjust dietary intake and medications in anticipation of an impending extreme glucose level. A valuable development in this area has been the introduction of predictive low glucose alarms, which alert users or integrated devices of impending hypoglycaemia of ≤3.1 mmol/l (55 mg/dl) within 20 min of an impending hypoglycaemic event, allowing for interventions to prevent hypoglycaemia [18].
Glucose data from a CGM can also be shared wirelessly with a software-based algorithm that further integrates with an insulin infusion pump. Algorithms have been further developed for insulin pumps or insulin delivery systems to calculate more precise insulin doses based on real-time continuous glucose data, creating a closed-loop system. Subsequently, newer functionalities, including suspending insulin before hypoglycaemia occurs or providing small insulin boluses if there is an increasing glucose trend, have also been implemented. Most of these currently available AID systems need users to enter the amount of predicted carbohydrate intake into the pump/algorithm for an insulin bolus dose to be calculated; hence, these are called hybrid closed-loop insulin systems. In addition, some studies have assessed the use of insulin and glucagon together in ‘dual-hormone AID systems’ [e.g. 19]. The colloquially termed ‘artificial pancreas’ is also on the horizon, in which carbohydrate announcement is unnecessary and multiple hormones can be administered [20]. One currently available AID system does not require input of carbohydrate intake, although the user must enter the size of the meal to be consumed [21].
Notwithstanding the clinical impact of these advances, ESKD and/or dialysis treatment may cause abrupt fluid shifts that alter the volume equilibrium between the intravascular and interstitial compartments. Moreover, in the setting of haemodialysis, these changes occur two to four times per week for a few hours during the dialysis treatment sessions. Hence, this can potentially affect the relationship between interstitial glucose sensor readings and actual blood glucose levels. While there is a need for studies to investigate the impact that rapid fluid fluctuations during dialysis may have on interstitial fluid glucose levels, it is expected that machine-learning adjustments can be developed to adapt the algorithms and account for these fluctuations if any; however, to our knowledge this has not been adequately studied to date. Nevertheless, and as shown in previous studies (Tables 1 and 2), the use of continuous glucose monitoring in people with ESKD undergoing haemodialysis provides greater convenience for glycaemic monitoring, may potentially be more accurate than traditional metrics and should be considered for optimal glycaemic management, including avoidance of acute glycaemic complications. In our clinical practice, we have used CGMs and/or AID systems to prevent extreme hyper- and hypoglycaemic events in this population, with vast success. However, it is also important to consider if a sensor needs to be calibrated after rapid volume changes (e.g. during haemodialysis treatment sessions and/or immediately after haemodialysis), using a hybrid model of a CGM and glucose meter system [14].
Glycaemic monitoring in ESKD
The ADA and Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend individualised glycaemic targets in people with diabetes and CKD based on characteristics that could alter the risks and benefits of intensive glycaemic control [8, 22]. A meta-analysis including 83,684 participants found that both high HbA1c levels (≥69.4 mmol/mol [≥8.5%]) and very low HbA1c levels (≤35.5 mmol/mol [≤5.4%]) are associated with an increased mortality risk in people undergoing haemodialysis [8, 23]. In practice, we aim to prevent moderate to severe hyperglycaemia and minimise hypoglycaemia in people with diabetes and ESKD, to avoid acute and chronic complications of diabetes and reduce the risk of death.
Advantages and disadvantages of glycaemic biomarkers in advanced CKD
HbA1c is the most widely used test for monitoring long-term glycaemic management in people with diabetes. However, in those with ESKD, HbA1c can be falsely low owing to the presence of anaemia, use of erythropoietin-stimulating agents, reduced erythrocyte lifespan from uraemia, erythrocyte lysis during haemodialysis and/or frequent blood transfusions [1]. Fructosamine measurement, which reflects total serum proteins that undergo glycation, can also be inaccurate in ESKD as a result of hypoalbuminaemia, which is a common occurrence in ESKD. Glycated albumin, a ketoamine resulting from the non-enzymatic binding of glucose to albumin, has been proposed as a better indicator of glycaemic management in this population. Glycated albumin is less likely to be affected by confounding factors, although it can also be impacted by low albumin levels, and data suggest it can predict mortality and hospitalisation rates in people with diabetes mellitus and ESKD [8, 24]. However, the lack of availability of glycated albumin in the real-world setting has limited its widespread use. Fructosamine and glycated albumin reflect average blood glucose levels over the previous 2–3 weeks, which is a shorter time frame than is reflected by HbA1c. In addition, fructosamine and glycated albumin lack well-established glycaemic targets in populations with ESKD [8, 24]. The advantages and disadvantages of these biomarkers are detailed in Table 3.
Capillary glucose testing
Despite technological advances in blood glucose testing, capillary glucose testing is still widely used for assessing glycaemic management on a daily basis in people with ESKD treated with dialysis. However, beyond concerns specific to people with CKD and ESKD, currently available, US Food and Drug Administration (FDA)-approved, hand-held point-of-care capillary glucose testing meters have additional accuracy and technical limitations, even after post-marketing. A 2017 study of 17 commercially available blood glucose meters demonstrated that accuracy varied substantially, with mean absolute relative differences (MARDs) ranging from 5.6% to 20.8%, with less reliability in participants with anaemia [25], which is a very frequent complication in people with advanced stages of CKD and ESKD. A subsequent study also demonstrated that, among 18 commercially available blood glucose meters, less than half met the accuracy standards [26]. Specifically, among people with ESKD treated with peritoneal dialysis, there are additional concerns about the accuracy of specific blood glucose meters in the setting of icodextrin-based peritoneal dialysis solutions [1]. A previous study reported that icodextrin interference in meters using glucose dehydrogenase pyrroloquinoline quinone methods resulted in falsely high glucose readings and was associated with severe hypoglycaemic events and death [27]. Hence, clinicians should use caution when selecting blood glucose meters for individuals receiving peritoneal dialysis. Clinicians and people with ESKD are also encouraged to check for device safety updates from manufacturers. In addition to accuracy and technical constraints, the main limitation of capillary glucose testing is the infrequent and static information provided on glycaemic excursions compared with CGMs. The expanded use of CGMs has confirmed that glucose management is not a matter of assessing two to three capillary glucose measurements per day, but rather is a continuous and dynamic human process.
Continuous glucose monitoring
CGMs have the potential to provide many benefits in monitoring and managing diabetes in people with diabetes and ESKD undergoing dialysis and may overcome the limitations of more widely used traditional methods (Table 3). CGMs can provide a more comprehensive evaluation of glycaemic excursions over 24 h and can aid in achieving glycaemic targets in a population in which HbA1c measurement is known to be inaccurate, as well as help to prevent severe dysglycaemic events. In ESKD, the risk of hypoglycaemia is heightened owing to impaired kidney gluconeogenesis, decreased kidney degradation and clearance of insulin, increased erythrocyte glucose uptake during haemodialysis, and nutritional deprivation [1]. A recent study demonstrated that rates of hospitalisation for hyperglycaemia or hypoglycaemia decreased by 18.2% and 17.0%, respectively, in participants with CKD when continuous glucose monitoring was initiated [28]. People with type 2 diabetes and ESKD have been shown to have high levels of glycaemic variability and day-to-day variations in insulin requirements [29]. CGM data reveal that, compared with non-dialysis days, glucose levels are lower during haemodialysis and higher after haemodialysis (Table 2). The ability of CGMs to capture these fluctuations can aid in the adjustment of diet, time of dialysis, exercise, diabetes medications and especially insulin dosing. However, presently, most dialysis clinics check blood glucose levels among haemodialysis recipients before dialysis or less frequently. Hence, point-of-care glucose tests are typically used only if a person manifests symptoms suggestive of hypoglycaemia and many asymptomatic hypoglycaemic episodes may therefore not be detected during haemodialysis. Continuous glucose monitoring has the advantage of monitoring glucose levels before, during and after a haemodialysis session.
Continuous glucose monitoring is recommended for people with diabetes using insulin or at increased risk of hypoglycaemia. With the increased use of CGMs, international consensus groups have developed new glucose metrics, including mean glucose, the glucose management indicator (GMI), mean time in range (TIR; 3.9–10 mmol/l [70–180 mg/dl] glucose), mean time above range (TAR; level1 >10 mmol/l [180 mg/dl]; level 2 >13.9 mmol/l [>250 mg/dl] glucose) and mean time below range (TBR; level 1 <3.9 mmol/l [70 mg/dl]; level 2 <3.0 mmol/l [<54 mg/dl] glucose) [30]. These newer metrics are widely used and implemented in clinical practice; however, many of the goals proposed are based on expert opinion. Based on expert opinion, and given the higher risk of hypoglycaemia, the recommended TIR for individuals with advanced CKD or with ESKD on dialysis is >50%, with a TBR of <1% [30, 31].
Given the inherent bias in HbA1c measurements in ESKD, the KDIGO clinical practice guidelines recommend the use of the GMI, which is derived from CGM data to monitor glycaemic management in individuals whose HbA1c is discordant with directly measured blood glucose levels [8]. The GMI provides a measure of average blood glucose levels and is expressed in units of HbA1c (%) [32]. Some advocate for use of mean glucose levels over the GMI, citing the discordance between the GMI and HbA1c and the confusion it creates for people and providers, but there is a scarcity of studies focused specifically on this issue in ESKD [33, 34]. Regardless, it is expected that measures of average glucose exposure determined by CGMs, such as mean glucose or mean TIR, will overcome the limitations of HbA1c measurement in this group (Table 3). Several studies on the use of diabetes technology in people with CKD and ESKD are ongoing (Table 4).
For a more comprehensive assessment of glycaemic management, mean glucose, TIR and TBR can be used to define glycaemic targets [30, 31]. It should be noted that even individuals with a low TBR can still have a substantial number of hypoglycaemic events, particularly severe hypoglycaemia. Clinicians should also therefore monitor the frequency of hypoglycaemic events. The target for glycaemic variability metrics, such as %CV, is <36% (%CV = SD of sensor glucose/mean sensor glucose) [30]. This measure assesses glycaemic variability, which should be minimised to avoid glucose fluctuations and hypoglycaemia.
Use of CGMs in advanced CKD
Some studies have assessed the accuracy of CGMs in the population with advanced CKD; however, most have tested older technology—non-factory-calibrated CGMs—requiring SMBG using finger sticks for calibration, with a shorter time of use (e.g. 7–14 days) and only including one to two dialysis sessions (Table 1). Consequently, the current evidence on the accuracy of CGMs in individuals with advanced CKD derives mostly from sensors that are no longer available or used in clinical practice.
Some more recent studies have assessed the use of newer factory-calibrated CGMs in people treated with haemodialysis or peritoneal dialysis (Tables 1 and 2). Most of these studies have focused on accuracy comparisons, predominantly using capillary glucose testing as the comparator (Table 1), which may not be considered the gold standard by regulatory bodies. People with diabetes and ESKD undergoing dialysis commonly have a high comorbidity burden, frequent hospitalisations, chronic anaemia, difficulty achieving peripheral vein access, low functional status and poor quality of life. Given these unique challenges, it is anticipated that comparative accuracy studies using devices such as the Yellow Springs instruments or Nova Primary Glucose Analyzer System [35], which aim to meet regulatory standards, requiring frequent blood draws and for a prolonged time, will be difficult to perform. More importantly, people with diabetes and ESKD are not benefiting widely from these technological advances because of a lack of regulatory approval, which further widens health disparities. Nevertheless, emerging data on these sensors is encouraging, and helpful for clinical practice.
The literature on newer CGM metrics in people with ESKD is very limited. In a recent study, we compared the relationship between the CGM-derived GMI and laboratory-measured HbA1c in people treated by haemodialysis [4]. HbA1c and GMI (mean ± SD) were 7.1% ± 1.3% (54.1 ± 14.2 mmol/mol) and 7.8% ± 1.1% (61.7 ± 12.0 mmol/mol), respectively (difference 0.74% ± 0.95%). Up to 29% of participants had a discordance between HbA1c and GMI of <0.5%, 22% had a discordance between 0.5% and 1%, and 49% had a discordance of >1%. The GMI had a strong relationship with TIR, but HbA1c underestimated mean glucose levels and the GMI. In accordance with the KDIGO guidelines [8], this suggests that CGM metrics, such as mean glucose, GMI and/or TIR, may be better markers of glycaemic management [4].
While studies using factory-calibrated sensors are emerging, there is a need for research with sensors of longer duration (e.g. 10–14 days), assessed over a period of three to five haemodialysis sessions, as well as research on sensor performance on days when no dialysis sessions are performed. Current studies using older sensor technology demonstrate patterns of lower glucose levels during haemodialysis, reaching the lowest point at the end of dialysis treatment sessions, with a peak in glycaemic levels observed after haemodialysis (Table 2).
There is also a lack of large studies evaluating CGMs in individuals on peritoneal dialysis; however, it has been shown that continuous glucose monitoring can aid in the detection of asymptomatic glucose excursions related to hypertonic exchanges during peritoneal dialysis [36]. In peritoneal dialysis, the glucose concentration of the dialysate, dwell time and status of membrane transport all impact the glycaemic profile [37]. In a small observational study, Lee et al demonstrated that, within 1 h of exchange using glucose-containing dialysate, glucose levels increased [38]. The glycaemic excursion was similar with 1.25% and 2.25% glucose solutions, with more prominent increments observed with 3.86% glucose solutions. Icodextrin solution contains a mixture of glucose polymers that are slowly absorbed and are used as an alternative osmotic agent in peritoneal dialysis [37]. The use of icodextrin dialysate had no effect on, or reduced, glucose levels during peritoneal dialysis [38].
It has been widely established that up to 15–20% of people with diabetes and ESKD transitioning to haemodialysis will experience ‘burnt-out diabetes’. This condition is defined as normalisation of glycaemic levels based on HbA1c <47.5 mmol/mol (6.5%), without the need for medications for at least 6 months [5]. However, one of the major red flags of this definition is the use of HbA1c to determine glycaemic status, given the well-established body of literature demonstrating significant bias in this population. In a recent study using CGMs in individuals with ESKD, investigators from our group demonstrated that people with burnt-out diabetes treated with haemodialysis have higher mean glucose levels, lower TIR and higher TAR (>13.9 mmol/l glucose), and a prolonged duration of TAR (>10 mmol/l; ~4 h per day) compared with people without diabetes treated with haemodialysis [5]. This demonstrates that the term ‘burnt-out diabetes’ may be misleading and further challenges the widely accepted concept of ‘true normalisation of glycaemia’ in this group when determined using a non-reliable test: HbA1c.
Use of AID systems in advanced CKD
AID systems have revolutionised diabetes care, particularly in both children and adults with type 1 diabetes. Extensive evidence supports the benefits of current AID systems in improving glycaemic management, decreasing hypoglycaemia risk and fear of hypoglycaemia, and improving quality of life for people with diabetes. However, the evidence on the use of these devices in people with diabetes and ESKD is still limited.
In a post hoc analysis of an RCT, AID significantly improved glycaemic management in hospitalised participants with type 2 diabetes undergoing haemodialysis (n=17) compared with conventional insulin therapy [39]. The closed-loop group had a significantly higher TIR (69.0% in the AID group vs 31.5% in the conventional insulin therapy group) without increasing the risk of hypoglycaemia. The mean CGM glucose level was lower in the AID group (8.1 mmol/l) than in the control group (11.0 mmol/l). Boughton et al conducted a randomised crossover trial in adults with type 2 diabetes requiring dialysis (n=26). TIR was significantly higher with AID than with conventional insulin therapy (TIR 52.8% vs 37.7%, respectively) [40], and mean glucose was lower during the closed-loop period (10.1 ± 1.3 mmol/l vs 11.6 ± 2.8 mmol/l; p=0.003). There was also a reduction in TBR while using AID. These small studies demonstrate the glycaemic benefit of AID in adults with type 2 diabetes receiving haemodialysis, but further investigations are needed in this population, as well as in the type 1 diabetes population receiving haemodialysis and in individuals receiving peritoneal dialysis.
Clinical considerations
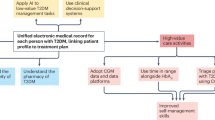
In clinical practice, if there is a need to use hypoglycaemic agents in people with diabetes and ESKD, such as insulin and/or sulfonylureas, including in combination with incretin therapy, it is recommended to use a CGM to monitor for glycaemic excursions in real time, especially given the proven benefits of CGMs in preventing hypoglycaemic events with the use of predictive low glucose alerts [18]. Similarly, using AID systems, which integrate the benefits of predictive glucose alerts with decreasing or stopping insulin administration by the insulin pump, is expected to prevent hypoglycaemic events and improve glycaemic management (Fig. 1). Our clinical experience and emerging studies support these recommendations.
Overview of the advantages and disadvantages of diabetes technology in individuals with diabetes and ESKD. This figure is available as a downloadable slide
CGMs can be a valuable tool for managing diabetes in people with ESKD, but it is important for clinicians to be aware of potential interferences and accuracy considerations. Except for the implantable sensor that uses fluorescence imaging (Eversense), current sensor technology is based on a glucose oxidase reaction. Hence, potential interferences include factors affecting the oxidase reaction, such as uraemic toxins, which can be present at higher concentrations in people with ESKD and have been shown to interfere with CGM readings [41, 42]. Low or high oxygen levels, high altitude, uraemia, icodextrin, low haematocrit and some exogenous substances (maltose, galactose and certain medications) may also pose challenges [37, 43, 44]. Studies have revealed that commonly used substances such as lisinopril, albuterol, acetaminophen, atenolol, ascorbic acid (vitamin C) and red wine, usually at higher than therapeutic doses, can interfere with the readings of electrochemical-based transcutaneous CGMs (those that use a glucose oxidase reaction) [43, 45]. In addition, tetracycline and mannitol have been found to interfere with the fully subcutaneously implantable Eversense glucose sensor [46]. There is also well-established literature reporting a lag time between blood glucose concentrations and interstitial glucose concentrations, which is what CGMs measure. However, predictive algorithms have been developed using glucose data from CGMs to predict the rate and direction of change, including algorithms to predict glucose variability during exercise [47, 48].
In lieu of no evidence in the population with diabetes and ESKD, we usually focus on glucose patterns and use confirmatory capillary glucose checks with validated glucose meters in cases of potential discordance or hypoglycaemic values without symptoms. Studies on interferences are limited and have been performed in non-dialysis populations. Previously, in silico (computer modelling) studies suggested a MARD of 10% for insulin dosing decisions [49]. Real-world experience has shown that a MARD of <15% can still be clinically beneficial for guiding diabetes management, and small differences in MARDs may not translate into clinically relevant changes within certain glucose thresholds [44, 50]. This highlights the importance of considering both ideal and practical accuracy levels when using CGMs in this specific population.
Placement of contemporary CGM sensors (e.g. typically on the arm or abdomen) requires careful consideration in the ESKD population and should be avoided in the ipsilateral (same) arm when arteriovenous fistulas (AVFs) and/or arteriovenous grafts (AVGs) are present. In general, any blood draws, infusions and/or blood pressure measurements are contraindicated in the AVF/AVG arm because of the risk of infection, clotting and/or damage to arteriovenous access, which is the ‘lifeline’ for individuals receiving dialysis, and this cautionary approach should also be extended to CGM sensors. Hence, subcutaneous sensors that are inserted just under the skin may be preferred in people with ESKD if placed in the non-AVF/AVG arm. It should also be noted that people with advanced CKD have a higher rate of coagulopathy and bleeding (e.g. due to uraemic platelet dysfunction), which may impact sensor functioning after insertion. Hence, providing training in the proper placement of CGMs is of paramount importance in this population. Compared with older sensors, newer factory-calibrated sensors are smaller, more user-friendly and less invasive, and lower the burden of diabetes care by not requiring frequent finger-prick glucose calibrations. They are also more accessible, although there are still cost constraints.
Future directions for diabetes technology in ESKD
While AID systems can effectively learn glucose patterns and insulin needs and deliver insulin based on current trends in people without ESKD, there is a need for validation studies of current AID algorithms in the population with ESKD. An ideal AID system for those with ESKD would learn individual patterns, including insulin clearance rates and sensitivity changes during and after haemodialysis or peritoneal dialysis. There is a need for more studies using CGM data in this cohort to enable personalised algorithms to be developed using machine learning. Table 4 provides details of selected ongoing studies that are assessing the use of diabetes technology in CKD and ESKD. It should also be underscored that ESKD and diabetes are conditions that disproportionately affect people with impaired social determinants of health and/or from racial and ethnic minority groups; hence, there is a compelling need to improve access to diabetes technologies in these vulnerable populations and to optimise dissemination of the benefits of CGMs to populations with broad health literacy.
Conclusion
Despite significant advances in diabetes technology and therapeutics, achieving optimal glycaemic management in people with diabetes and ESKD remains a challenge. CGMs and AID systems, although awaiting regulatory approval for people on dialysis, have already proved beneficial to both individuals with advanced CKD and clinicians in real-world settings (Fig. 1). They provide valuable insights into an individual’s glucose patterns and allow for more personalised treatment plans, preventing impending and asymptomatic hypoglycaemic and/or hyperglycaemic episodes, especially during dialysis sessions. CGM data can assist providers in tailoring insulin and diabetes medications and lifestyle changes for people with ESKD. We anticipate that machine-learning modelling using CGM data to predict glucose trends and fluctuations will reduce the impact of inter- and intra-dialysis variations on sensor performance. AID systems that can adjust target blood glucose levels based on the dialysis modality (in-centre haemodialysis, peritoneal dialysis and home haemodialysis) could significantly improve glycaemic management. We encourage clinicians, researchers and manufacturers to advocate for and pursue additional research and regulatory approval of these newer devices in this population to decrease disparities.
Abbreviations
- AID:
-
Automated insulin delivery
- AVF:
-
Arteriovenous fistula
- AVG:
-
Arteriovenous graft
- CGM:
-
Continuous glucose monitor
- CKD:
-
Chronic kidney disease
- ESKD:
-
End-stage kidney disease
- GMI:
-
Glucose management indicator
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- MARD:
-
Mean absolute relative difference
- SMBG:
-
Self-monitoring of blood glucose
- TAR:
-
Time above range
- TBR:
-
Time below range
- TIR:
-
Time in range
References
Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR (2020) Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev 41(5):756–774. https://doi.org/10.1210/endrev/bnaa017
United States Renal Data System (2023) USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA
Galindo RJ, Ali MK, Funni SA et al (2022) Hypoglycemic and hyperglycemic crises among U.S. adults with diabetes and end-stage kidney disease: population-based study, 2013–2017. Diabetes Care 45(1):100–107. https://doi.org/10.2337/dc21-1579
Galindo RJ, Moazzami B, Tuttle KR, Bergenstal RM, Peng L, Umpierrez GE (2024) Continuous glucose monitoring metrics and hemoglobin A1c relationship in patients with type 2 diabetes treated by hemodialysis. Diabetes Technol Ther. https://doi.org/10.1089/dia.2024.0145
Kaminski CY, Galindo RJ, Navarrete JE et al (2024) Assessment of glycemic control by continuous glucose monitoring, hemoglobin A1c, fructosamine, and glycated albumin in patients with end-stage kidney disease and burnt-out diabetes. Diabetes Care 47(2):267–271. https://doi.org/10.2337/dc23-1276
Galindo RJ, de Boer IH, Neumiller JJ, Tuttle KR (2023) Continuous glucose monitoring to optimize management of diabetes in patients with advanced CKD. Clin J Am Soc Nephrol 18(1):130–145. https://doi.org/10.2215/CJN.04510422
Egeolu M, Caleon RL, Manishimwe E et al (2023) Diabetic retinopathy in African-Americans with end-stage kidney disease: a cross-sectional study on prevalence and impact on quality of life. BMJ Open Diabetes Res Care 11(4):e003373. https://doi.org/10.1136/bmjdrc-2023-003373
de Boer IH, Khunti K, Sadusky T et al (2022) Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 45(12):3075–3090. https://doi.org/10.2337/dci22-0027
Siew ED, Ikizler TA (2010) Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial 23(4):378–382. https://doi.org/10.1111/j.1525-139X.2010.00763.x
Pollack H (1953) Stanley Rossiter Benedict creator of laboratory tests for glycosuria. Diabetes 2(5):420–421. https://doi.org/10.2337/diab.2.5.420
Benedict SR (2002) Benedict’s solution, a reagent for the detection of reducing sugars: the clinical chemistry of Stanley R. Benedict. J Biol Chem 277(16):e5–e6. https://doi.org/10.1016/S0021-9258(19)61050-1
Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH (2013) Biocompatible materials for continuous glucose monitoring devices. Chem Rev 113(4):2528–2549. https://doi.org/10.1021/cr300387j
Ribet F, Stemme G, Roxhed N (2018) Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed Microdevices 20(4):101. https://doi.org/10.1007/s10544-018-0349-6
Forlenza GP, Kushner T, Messer LH, Wadwa RP, Sankaranarayanan S (2019) Factory-calibrated continuous glucose monitoring: how and why it works, and the dangers of reuse beyond approved duration of wear. Diabetes Technol Ther 21(4):222–229. https://doi.org/10.1089/dia.2018.0401
Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G (2000) Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab 278(4):E716-728. https://doi.org/10.1152/ajpendo.2000.278.4.E716
Boyne MS, Silver DM, Kaplan J, Saudek CD (2003) Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 52(11):2790–2794. https://doi.org/10.2337/diabetes.52.11.2790
Galindo RJ, Aleppo G (2020) Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract 170:108502. https://doi.org/10.1016/j.diabres.2020.108502
Puhr S, Derdzinski M, Parker AS, Welsh JB, Price DA (2020) Real-world hypoglycemia avoidance with a predictive low glucose alert does not depend on frequent screen views. J Diabetes Sci Technol 14(1):83–86. https://doi.org/10.1177/1932296819840691
El-Khatib FH, Balliro C, Hillard MA et al (2017) Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 389(10067):369–380. https://doi.org/10.1016/S0140-6736(16)32567-3
US Food and Drug Administration (2018) The artificial pancreas device system. Available from: https://www.fda.gov/medical-devices/consumer-products/artificial-pancreas-device-system. Accessed 11 Jul 2024
Bionic Pancreas Research G, Russell SJ, Beck RW et al (2022) Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N Engl J Med 387(13):1161–1172. https://doi.org/10.1056/NEJMoa2205225
American Diabetes Association Professional Practice Committee (2024) 11. Chronic kidney disease and risk management: standards of care in diabetes-2024. Diabetes Care 47(Suppl 1):S219–S230. https://doi.org/10.2337/dc24-S011
Hill CJ, Maxwell AP, Cardwell CR et al (2014) Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis 63(1):84–94. https://doi.org/10.1053/j.ajkd.2013.06.020
Freedman BI, Andries L, Shihabi ZK et al (2011) Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin J Am Soc Nephrol 6(7):1635–1643. https://doi.org/10.2215/CJN.11491210
Ekhlaspour L, Mondesir D, Lautsch N et al (2017) Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol 11(3):558–566. https://doi.org/10.1177/1932296816672237
Klonoff DC, Parkes JL, Kovatchev BP et al (2018) Investigation of the accuracy of 18 marketed blood glucose monitors. Diabetes Care 41(8):1681–1688. https://doi.org/10.2337/dc17-1960
Frias JP, Lim CG, Ellison JM, Montandon CM (2010) Review of adverse events associated with false glucose readings measured by GDH-PQQ-based glucose test strips in the presence of interfering sugars. Diabetes Care 33(4):728–729. https://doi.org/10.2337/dc09-1822
Hannah KL, Nemlekar PM, Johnson ML, Chernavvsky DR, Norman GJ (2024) Continuous glucose monitors and reduced diabetes-related hospitalizations in patients with type 2 diabetes and chronic kidney disease. Kidney 360 5(4):515–521. https://doi.org/10.34067/KID.0000000000000396
Sobngwi E, Enoru S, Ashuntantang G et al (2010) Day-to-day variation of insulin requirements of patients with type 2 diabetes and end-stage renal disease undergoing maintenance hemodialysis. Diabetes Care 33(7):1409–1412. https://doi.org/10.2337/dc09-2176
Battelino T, Danne T, Bergenstal RM et al (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8):1593–1603. https://doi.org/10.2337/dci19-0028
Williams ME, Steenkamp D, Wolpert H (2022) Making sense of glucose sensors in end-stage kidney disease: a review. Front Clin Diabetes Healthc 3:1025328. https://doi.org/10.3389/fcdhc.2022.1025328
Bergenstal RM, Beck RW, Close KL et al (2018) Glucose Management Indicator (GMI): a new term for estimating a1c from continuous glucose monitoring. Diabetes Care 41(11):2275–2280. https://doi.org/10.2337/dc18-1581
Oriot P, Viry C, Vandelaer A et al (2023) Discordance between glycated hemoglobin A1c and the glucose management indicator in people with diabetes and chronic kidney disease. J Diabetes Sci Technol 17(6):1553–1562. https://doi.org/10.1177/19322968221092050
Selvin E (2024) The glucose management indicator: time to change course? Diabetes Care 47(6):906–914. https://doi.org/10.2337/dci23-0086
Klonoff DC, Yeung AM, Huang J, Nichols JH (2023) A newly FDA-cleared benchtop glucose analyzer heralds the dawn of the post-YSI 2300 Era. J Diabetes Sci Technol 17(2):269–273. https://doi.org/10.1177/19322968221139514
Ng JKC, Ling J, Luk AOY et al (2023) Evaluation of a fourth-generation subcutaneous real-time Continuous Glucose Monitor (CGM) in individuals with diabetes on peritoneal dialysis. Diabetes Care 46(6):1191–1195. https://doi.org/10.2337/dc22-2348
Ling J, Ng JKC, Chan JCN, Chow E (2022) Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol (Lausanne) 13:869899. https://doi.org/10.3389/fendo.2022.869899
Lee SY, Chen YC, Tsai IC et al (2013) Glycosylated hemoglobin and albumin-corrected fructosamine are good indicators for glycemic control in peritoneal dialysis patients. PLoS One 8(3):e57762. https://doi.org/10.1371/journal.pone.0057762
Bally L, Gubler P, Thabit H et al (2019) Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney Int 96(3):593–596. https://doi.org/10.1016/j.kint.2019.03.006
Boughton CK, Tripyla A, Hartnell S et al (2021) Fully automated closed-loop glucose control compared with standard insulin therapy in adults with type 2 diabetes requiring dialysis: an open-label, randomized crossover trial. Nat Med 27(8):1471–1476. https://doi.org/10.1038/s41591-021-01453-z
Zawada AM, Carrero JJ, Wolf M et al (2020) Serum Uric Acid And Mortality Risk Among Hemodialysis Patients. Kidney Int Rep 5(8):1196–1206. https://doi.org/10.1016/j.ekir.2020.05.021
Chinh NH (1974) Mechanism of interference by uric acid in the glucose oxidase/peroxidase method for serum glucose. Clini Chem 20(4):499–501. https://doi.org/10.1093/clinchem/20.4.499
Basu A, Slama MQ, Nicholson WT et al (2017) Continuous glucose monitor interference with commonly prescribed medications: a pilot study. J Diabetes Sci Technol 11(5):936–941. https://doi.org/10.1177/1932296817697329
Bellido V, Freckman G, Perez A, Galindo RJ (2023) Accuracy and Potential Interferences Of Continuous Glucose Monitoring Sensors In The Hospital. Endocr Pract 29(11):919–927. https://doi.org/10.1016/j.eprac.2023.06.007
Abbott. FreestyleLibre: important safety information. Available from: https://www.freestyleprovider.abbott/content/adc/freestyleprovider/countries/us-en/safety-information.html. Accessed 11 Jul 2024
Lorenz C, Sandoval W, Mortellaro M (2018) Interference assessment of various endogenous and exogenous substances on the performance of the eversense long-term implantable continuous glucose monitoring system. Diabetes Technol Ther 20(5):344–352. https://doi.org/10.1089/dia.2018.0028
Mosquera-Lopez C, Ramsey KL, Roquemen-Echeverri V, Jacobs PG (2023) Modeling risk of hypoglycemia during and following physical activity in people with type 1 diabetes using explainable mixed-effects machine learning. Comput Biol Med 155:106670. https://doi.org/10.1016/j.compbiomed.2023.106670
Tyler NS, Mosquera-Lopez C, Young GM, El Youssef J, Castle JR, Jacobs PG (2022) Quantifying the impact of physical activity on future glucose trends using machine learning. Science 25(3):103888. https://doi.org/10.1016/j.isci.2022.103888
Kovatchev BP, Patek SD, Ortiz EA, Breton MD (2015) Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther 17(3):177–186. https://doi.org/10.1089/dia.2014.0272
Vigersky RA, Shin J (2024) The myth of MARD (Mean Absolute Relative Difference): limitations of MARD in the clinical assessment of continuous glucose monitoring data. Diabetes Technol Ther 26(S3):38–44. https://doi.org/10.1089/dia.2023.0435
Matoba K, Hayashi A, Shimizu N, Moriguchi I, Kobayashi N, Shichiri M (2020) Comparison of accuracy between flash glucose monitoring and continuous glucose monitoring in patients with type 2 diabetes mellitus undergoing hemodialysis. J Diabetes Complications 34(11):107680. https://doi.org/10.1016/j.jdiacomp.2020.107680
Yajima T, Takahashi H, Yasuda K (2020) Comparison of interstitial fluid glucose levels obtained by continuous glucose monitoring and flash glucose monitoring in patients with type 2 diabetes mellitus undergoing hemodialysis. J Diabetes Sci Technol 14(6):1088–1094. https://doi.org/10.1177/1932296819882690
Hissa MRN, Hissa PNG, Guimaraes SB, Hissa MN (2021) Use of continuous glucose monitoring system in patients with type 2 mellitus diabetic during hemodialysis treatment. Diabetol Metab Syndr 13(1):104. https://doi.org/10.1186/s13098-021-00722-8
Mambelli E, Cristino S, Mosconi G, Gobl C, Tura A (2021) Flash glucose monitoring to assess glycemic control and variability in hemodialysis patients: the GIOTTO study. Front Med (Lausanne) 8:617891. https://doi.org/10.3389/fmed.2021.617891
Ólafsdóttir AF, Andelin M, Saeed A et al (2022) Performance of Dexcom G5 and FreeStyle Libre sensors tested simultaneously in people with type 1 or 2 diabetes and advanced chronic kidney disease. World J Clin Cases 10(22):7794–7807. https://doi.org/10.12998/wjcc.v10.i22.7794
Villard O, Breton MD, Rao S et al (2022) Accuracy of a factory-calibrated continuous glucose monitor in individuals with diabetes on hemodialysis. Diabetes Care 45(7):1666–1669. https://doi.org/10.2337/dc22-0073
Avari P, Tang W, Jugnee N et al (2023) The accuracy of continuous glucose sensors in people with diabetes undergoing hemodialysis (ALPHA Study). Diabetes Technol Ther 25(7):447–456. https://doi.org/10.1089/dia.2023.0013
Horne C, Cranston I, Amos M, Flowers K (2023) Accuracy of continuous glucose monitoring in an insulin-treated population requiring haemodialysis. J Diabetes Sci Technol 17(4):971–975. https://doi.org/10.1177/19322968231173447
Ling J, Ng JKC, Lau ESH et al (2024) Impact of body composition and anemia on accuracy of a real-time continuous glucose monitor in diabetes patients on continuous ambulatory peritoneal dialysis. Diabetes Technol Ther 26(1):70–75. https://doi.org/10.1089/dia.2023.0349
Kepenekian L, Smagala A, Meyer L et al (2014) Continuous glucose monitoring in hemodialyzed patients with type 2 diabetes: a multicenter pilot study. Clin Nephrol 82(4):240–246. https://doi.org/10.5414/CN108280
Gai M, Merlo I, Dellepiane S et al (2014) Glycemic pattern in diabetic patients on hemodialysis: continuous glucose monitoring (CGM) analysis. Blood Purif 38(1):68–73. https://doi.org/10.1159/000362863
Joubert M, Fourmy C, Henri P, Ficheux M, Lobbedez T, Reznik Y (2015) Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract 107(3):348–354. https://doi.org/10.1016/j.diabres.2015.01.026
Vos FE, Schollum JB, Coulter CV, Manning PJ, Duffull SB, Walker RJ (2012) Assessment of markers of glycaemic control in diabetic patients with chronic kidney disease using continuous glucose monitoring. Nephrology (Carlton) 17(2):182–188. https://doi.org/10.1111/j.1440-1797.2011.01517.x
Mirani M, Berra C, Finazzi S et al (2010) Inter-day glycemic variability assessed by continuous glucose monitoring in insulin-treated type 2 diabetes patients on hemodialysis. Diabetes Technol Ther 12(10):749–753. https://doi.org/10.1089/dia.2010.0052
Jung HS, Kim HI, Kim MJ et al (2010) Analysis of hemodialysis-associated hypoglycemia in patients with type 2 diabetes using a continuous glucose monitoring system. Diabetes Technol Ther 12(10):801–807. https://doi.org/10.1089/dia.2010.0067
Riveline JP, Teynie J, Belmouaz S et al (2009) Glycaemic control in type 2 diabetic patients on chronic haemodialysis: use of a continuous glucose monitoring system. Nephrol Dial Transplant 24(9):2866–2871. https://doi.org/10.1093/ndt/gfp181
Chantrel F, Sissoko H, Kepenekian L et al (2014) Influence of dialysis on the glucose profile in patients with diabetes: usefulness of continuous glucose monitoring. Horm Metab Res 46(11):810–813. https://doi.org/10.1055/s-0034-1370963
Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T et al (2009) Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care 32(7):1137–1142. https://doi.org/10.2337/dc08-1688
Sobngwi E, Ashuntantang G, Ndounia E et al (2010) Continuous interstitial glucose monitoring in non-diabetic subjects with end-stage renal disease undergoing maintenance haemodialysis. Diabetes Res Clin Pract 90(1):22–25. https://doi.org/10.1016/j.diabres.2010.06.001
Bergenstal RM, Gal RL, Connor CG et al (2017) Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med 167(2):95–102. https://doi.org/10.7326/M16-2596
Mayeda L, Zelnick L, Trikudanathan S, Hirsch IB, Watnick S, De Boer I (2023) Glycemic control assessed by continuous glucose monitoring among dialysis patients with and without diabetes mellitus. Diabetes 72(Suppl 1):430-P. https://doi.org/10.2337/db23-430-P
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
We would like to thank G. Arevalo, postdoctoral fellow in the Division of Endocrinology at the University of Miami, for her invaluable editorial support.
Funding
RJG is supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award numbers P30DK111024-8, 1K23DK123384-5, U2CDK137135 and 1R03DK138255-1.
Authors’ relationships and activities
RJG has received research support from Novo Nordisk, Eli Lilly and Dexcom, and consulting/advisory/honoraria fees from Abbott Diabetes, AsztraZeneca, Bayer, Boehringer, Dexcom, Eli Lilly, Novo Nordisk and Medtronic. DC is a former employee of Dexcom and holds stocks. CMR has received honoraria/funding from AstraZeneca, Dexcom, Fresenius and Vifor. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors were responsible for drafting the article and reviewing it critically for important intellectual content. All authors approved the version to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galindo, R.J., Soliman, D., Cherñasvvky, D. et al. Diabetes technology in people with diabetes and advanced chronic kidney disease. Diabetologia (2024). https://doi.org/10.1007/s00125-024-06244-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00125-024-06244-y