Abstract
Aims/hypothesis
We conducted a systematic review and network meta-analysis to compare the efficacy and safety of s.c. administered tirzepatide vs s.c. administered semaglutide for adults of both sexes with type 2 diabetes mellitus.
Methods
We searched PubMed and Cochrane up to 11 November 2023 for RCTs with an intervention duration of at least 12 weeks assessing s.c. tirzepatide at maintenance doses of 5 mg, 10 mg or 15 mg once weekly, or s.c. semaglutide at maintenance doses of 0.5 mg, 1.0 mg or 2.0 mg once weekly, in adults with type 2 diabetes, regardless of background glucose-lowering treatment. Eligible trials compared any of the specified doses of tirzepatide and semaglutide against each other, placebo or other glucose-lowering drugs. Primary outcomes were changes in HbA1c and body weight from baseline. Secondary outcomes were achievement of HbA1c target of ≤48 mmol/mol (≤6.5%) or <53 mmol/mol (<7.0%), body weight loss of at least 10%, and safety outcomes including gastrointestinal adverse events and severe hypoglycaemia. We used version 2 of the Cochrane risk-of-bias tool (ROB 2) to assess the risk of bias, conducted frequentist random-effects network meta-analyses and evaluated confidence in effect estimates utilising the Confidence In Network Meta-Analysis (CINeMA) framework.
Results
A total of 28 trials with 23,622 participants (44.2% female) were included. Compared with placebo, tirzepatide 15 mg was the most efficacious treatment in reducing HbA1c (mean difference −21.61 mmol/mol [−1.96%]) followed by tirzepatide 10 mg (−20.19 mmol/mol [−1.84%]), semaglutide 2.0 mg (−17.74 mmol/mol [−1.59%]), tirzepatide 5 mg (−17.60 mmol/mol [−1.60%]), semaglutide 1.0 mg (−15.25 mmol/mol [−1.39%]) and semaglutide 0.5 mg (−12.00 mmol/mol [−1.09%]). In between-drug comparisons, all tirzepatide doses were comparable with semaglutide 2.0 mg and superior to semaglutide 1.0 mg and 0.5 mg. Compared with placebo, tirzepatide was more efficacious than semaglutide for reducing body weight, with reductions ranging from 9.57 kg (tirzepatide 15 mg) to 5.27 kg (tirzepatide 5 mg). Semaglutide had a less pronounced effect, with reductions ranging from 4.97 kg (semaglutide 2.0 mg) to 2.52 kg (semaglutide 0.5 mg). In between-drug comparisons, tirzepatide 15 mg, 10 mg and 5 mg demonstrated greater efficacy than semaglutide 2.0 mg, 1.0 mg and 0.5 mg, respectively. Both drugs increased incidence of gastrointestinal adverse events compared with placebo, while neither tirzepatide nor semaglutide increased the risk of serious adverse events or severe hypoglycaemia.
Conclusions/interpretation
Our data show that s.c. tirzepatide had a more pronounced effect on HbA1c and weight reduction compared with s.c. semaglutide in people with type 2 diabetes. Both drugs, particularly higher doses of tirzepatide, increased gastrointestinal adverse events.
Registration
PROSPERO registration no. CRD42022382594
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Semaglutide, administered s.c., has shown superior efficacy compared with other glucose-lowering agents, including its oral formulation, in reducing HbA1c and in facilitating weight loss in individuals with type 2 diabetes [1, 2]. Initially approved at doses of 0.5 mg and 1.0 mg once weekly, it has subsequently received authorisation for a 2.0 mg once-weekly dose for the management of type 2 diabetes. Tirzepatide, a novel agent belonging to the glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 receptor agonist (GLP-1 RA) class (dual GIP/GLP-1 RA), has also been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of type 2 diabetes. Data from RCTs have consistently shown the efficacy of tirzepatide in reducing HbA1c and body weight in people with type 2 diabetes [3].
The ADA Standards of Care and the ADA/EASD consensus report recommend s.c. administered semaglutide and tirzepatide as the most efficacious medications for glycaemic control (alongside dulaglutide) and weight reduction [4, 5]. However, direct comparison between s.c. tirzepatide and s.c. semaglutide in RCTs is scarce [6, 7], presenting a challenge in drawing robust and precise conclusions regarding their comparative efficacy. To address this research gap, we conducted a network meta-analysis utilising both direct and indirect comparative data between the two medications [8].
The aim of our systematic review and network meta-analysis was to compare the efficacy (in terms of glycaemic control and weight management) and safety (in terms of adverse events) of s.c. tirzepatide and s.c. semaglutide in people with type 2 diabetes based on data from RCTs.
Methods
The protocol of this systematic review and meta-analysis is registered in PROSPERO (registration no. CRD42022382594) [9]. We report our methods and results in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement for network meta-analyses [10].
Eligibility criteria
We included RCTs published in English that assessed s.c. tirzepatide at maintenance doses of 5 mg, 10 mg or 15 mg once weekly, or s.c. semaglutide at maintenance doses of 0.5 mg, 1.0 mg or 2.0 mg once weekly for a minimum duration of 12 weeks. Eligible trials compared any of the specified doses of tirzepatide and semaglutide against each other, placebo or other glucose-lowering drugs. For a glucose-lowering drug to be included as a comparator, it was required to have been evaluated in at least one trial comparison against tirzepatide and one trial comparison against semaglutide. This approach was adopted to prevent unconnected networks, ensuring that each comparator served as a link for indirect comparisons between tirzepatide and semaglutide. We included trials recruiting adults with type 2 diabetes regardless of their background glucose-lowering treatment, defined as the glucose-lowering therapy used both in the intervention and control arms after the randomisation.
Information sources and searches
We searched PubMed and Cochrane databases from inception until 11 November 2023. Our search strategy included both free-text and Medical Subject Headings (MeSH) terms, utilising the keywords ‘tirzepatide,’ ‘ly3298176,’ ‘semaglutide’ and ‘nn9535’ (electronic supplementary material [ESM] Table 1).
Study selection
After deduplication, search results were screened at title and abstract level, and potentially eligible records were examined in full text with reasons for exclusion being recorded. Two independent reviewers performed the study selection process and any disagreements were resolved by a third reviewer. For the deduplication and the screening process we used the Systematic Review Accelerator (SRA) web application [11].
Data collection
Using predesigned forms, we extracted information on study characteristics, participants’ baseline characteristics and outcome data. Given the aggregated data format of the included RCTs in our meta-analysis, direct information on how sex or gender was determined in the individual studies was beyond the scope of our analysis. Our two primary outcomes were the change from baseline in HbA1c and in body weight. Secondary efficacy outcomes were the proportion of participants attaining an HbA1c target of ≤48 mmol/mol (≤6.5%) or <53 mmol/mol (<7.0%), and those achieving a minimum of 10% body weight loss. Safety outcomes included the incidence (no. of participants with at least one outcome event) of nausea, vomiting, diarrhoea, treatment discontinuation due to gastrointestinal events, severe adverse events and severe hypoglycaemia (a hypoglycaemic event requiring assistance). Data were extracted from the intention-to-treat population, which included all randomly assigned participants who received at least one dose of the study medication. For eligible trials identified through our database searches, we utilised ClinicalTrials.gov, using their respective National Clinical Trial (NCT) identifiers, to retrieve additional information when outcome data were absent or incomplete in the published articles. Data extraction was conducted by two independent reviewers, with discrepancies resolved by a third reviewer.
Risk-of-bias assessment
We used version 2 of the Cochrane risk-of-bias tool for randomised trials (ROB 2) to assess the risk of bias for the two primary outcomes [12]. Following the tool’s algorithms, each trial’s overall risk of bias was classified as low if all domains were at low risk, and high if any domain was at high risk. If none of the domains were classified as high risk but one or more were deemed to have some concerns, the overall risk of bias for that trial was categorised as ‘of some concern’. This assessment was conducted independently by two reviewers, with a third reviewer resolving any disagreements. We evaluated the presence of small-study effect (publication bias) by means of comparison-adjusted funnel plots [13].
Data analysis
We explored the transitivity assumption by comparing the distribution of potential effect modifiers (baseline HbA1c and body weight) across treatment comparisons [14]. We conducted frequentist random-effects network meta-analyses and calculated mean differences (MDs) for the two primary outcomes and risk ratios for dichotomous outcomes, alongside 95% CIs [15]. We evaluated heterogeneity for the primary outcomes based on the agreement between CIs and prediction intervals in relation to the null effect and the clinically important effect on the opposite direction to the point estimate [16, 17]. We assumed a minimum reduction in HbA1c of 5.5 mmol/mol (0.5%) and in body weight of 4.5 kg (5% of mean body weight value at baseline across all trials) as clinically important [18]. We addressed incoherence (inconsistency) both locally by comparing directly with indirect evidence using the Separating Indirect from Direct Evidence (SIDE) method [19] and globally using the design-by-treatment interaction model [20]. Moreover, we used P-scores, ranging from 0 to 1, to rank treatments; these can be interpreted as the average degree of certainty for a treatment to be better than the other treatments in the network [21]. Statistical analyses were performed in R (R Core Team 2019, R Foundation for Statistical Computing, Vienna, Austria) using the R packages ‘meta’ and ‘netmeta’ [22], and in NMAstudio (version 2.0) web application [23, 24].
Evaluation of confidence in findings
We evaluated Confidence In Network Meta-Analysis (CINeMA) effect estimates for the primary outcomes utilising the CINeMA methodological framework and application [17, 25]. The six domains evaluated were within-study bias (risk of bias), across-study bias (small-study effect/publication bias), indirectness, imprecision, heterogeneity and incoherence (inconsistency). We assigned judgements at three levels (no concerns, some concerns and major concerns) to each domain and summarised judgements across domains to an overall assessment ranging across very low, low, moderate or high level of confidence [17, 25].
Results
Search results and study characteristics
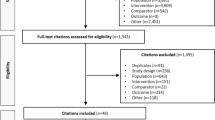
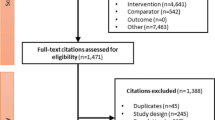
The search retrieved 2798 records, of which 28 RCTs [6, 7, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] with 23,622 participants were included in the systematic review and network meta-analysis (ESM Fig. 1). Study and participant characteristics are presented in Table 1. Only two trials directly compared tirzepatide with semaglutide, with one of these also including a placebo arm [6, 7]. Sixteen trials compared semaglutide with placebo, other GLP-1 RAs, basal insulin, prandial insulin or varying doses of semaglutide. The remaining ten trials compared tirzepatide with placebo, GLP-1 RA (other than semaglutide), basal insulin, prandial insulin or varying doses of tirzepatide. All trials had a parallel-group design and 15 were open-label (Table 1). Most trials were multinational, except for five that recruited exclusively Japanese participants [39,40,41, 48, 49]. The intervention duration ranged from 24 to 28 weeks in five trials and from 30 to 56 weeks in 21 trials. The remaining two trials, a trial with tirzepatide in people with obesity and type 2 diabetes (SURMOUNT-2) [50] and a cardiovascular outcomes trial with semaglutide (SUSTAIN 6) [31], had a duration of 72 and 104 weeks, respectively. The background glucose-lowering therapy, referring to the common treatment received by all trial groups post-randomisation, varied across the trials. However, the predominant background treatment was metformin, used either as monotherapy or in combination with other medications. Across all trials, 10,442 participants (44.2%) were female, participants’ mean HbA1c at baseline was 66.6 mmol/mol (8.3%), mean body weight was 88.8 kg and mean age was 57.8 years (Table 1). The distribution of potential effect modifiers (HbA1c and body weight at baseline) was deemed sufficiently similar across all treatment comparisons to assume that a network meta-analysis was appropriate (ESM Figs 2 and 3).
Overview of network
Figure 1 shows the network of comparisons used in the meta-analysis. Risk of bias for the change in HbA1c was assessed as low in all trials except for one that was at high risk of bias and one with some concerns (ESM Table 2). For the change in body weight, seven trials were at high risk of bias and one trial had some concerns; all other trials were at low risk of bias (ESM Table 3). Comparison-adjusted funnel plots did not suggest the presence of small-study effect (ESM Figs 4 and 5). There was presence of heterogeneity in some comparisons, particularly those involving semaglutide 2.0 mg (ESM Tables 4 and 5). In terms of incoherence, the design-by-treatment interaction model did not identify global inconsistency in the analyses for both primary outcomes (ESM Tables 4 and 5), while local inconsistency was also low.
Network plot for change in HbA1c. Each circle indicates a treatment node. Lines connecting two nodes represent direct comparisons between two treatments. The size of the nodes is proportional to the number of trials evaluating each treatment; the thickness of the lines is proportional to the number of trials directly comparing the two connected treatments
Glycaemic efficacy
Compared with placebo, tirzepatide 15 mg was the most efficacious treatment in reducing HbA1c (MD [95% CI]: −21.61 mmol/mol [−23.26 to −19.97] [−1.96% (−2.11 to −1.82)]), followed by tirzepatide 10 mg (−20.19 mmol/mol [−21.89 to −18.48] [−1.84% (−1.99 to −1.69)]), semaglutide 2.0 mg (−17.74 mmol/mol [−22.03 to −13.45] [−1.59% (−1.95 to −1.22)]), tirzepatide 5 mg (−17.60 mmol/mol [−19.36 to −15.84] [−1.60% (−1.75 to −1.44)]), semaglutide 1.0 mg (−15.25 mmol/mol [−16.73 to −13.77] [−1.39% (−1.52 to −1.26)]) and semaglutide 0.5 mg (−12.00 mmol/mol [−13.74 to −10.26] [−1.09% (−1.24 to −0.94)]) (Fig. 2 and ESM Fig. 6). In comparisons between tirzepatide and semaglutide, when HbA1c was measured in mmol/mol, all tirzepatide doses were comparable with semaglutide 2.0 mg and superior to semaglutide 1.0 mg and 0.5 mg (ESM Table 6). Specifically, effect estimates (MD [95% CI]) for tirzepatide 15 mg vs semaglutide 2.0 mg, tirzepatide 10 mg vs semaglutide 1.0 mg, and tirzepatide 5 mg vs semaglutide 0.5 mg were, respectively, as follows: −3.87 mmol/mol (−8.22 to 0.48); −4.94 (−6.65 to −3.23); and −5.60 mmol/mol (−7.60 to −3.60) (ESM Table 6). When HbA1c was measured in %, tirzepatide at doses of 15 mg, 10 mg and 5 mg demonstrated greater efficacy than semaglutide at doses of 2.0 mg (MD = −0.38% [95% CI −0.75% to −0.01%]), 1.0 mg (MD = −0.45% [95% CI −0.60% to −0.31%]) and 0.5 mg (MD = −0.51% [95% CI −0.68% to −0.33%]), respectively (ESM Table 7). The confidence in estimates for comparisons between tirzepatide and semaglutide was high to moderate, except for comparisons vs semaglutide 2.0 mg, where the confidence was generally low (ESM Table 8). Consistently with meta-analysis findings, tirzepatide 15 mg held the highest probability (P-score = 0.99) of being the most efficacious treatment in reducing HbA1c (ESM Fig. 7).
Compared with placebo, semaglutide 2.0 mg (risk ratio = 7.73 [95% CI 5.62, 10.63]) and tirzepatide 15 mg (risk ratio = 7.01 [95% CI 5.73, 8.57]) were the most efficacious in achieving an HbA1c target of ≤48 mmol/mol (≤6.5%) (ESM Table 9). In between-drug comparisons, tirzepatide 15 mg and 10 mg outperformed semaglutide 1.0 mg and 0.5 mg and tirzepatide 5 mg was superior to semaglutide 0.5 mg, while no differences were found between semaglutide 2.0 mg and any of the tirzepatide doses (ESM Table 9). Similarly, semaglutide 2.0 mg (risk ratio = 4.01 [95% CI 3.24, 4.95]) and tirzepatide 15 mg (risk ratio = 3.70 [95% CI 3.26, 4.20]) were the most efficacious in achieving an HbA1c target of <53 mmol/mol (<7%) as compared with placebo (ESM Table 10). No differences were found when any of the tirzepatide doses were compared with semaglutide 2.0 mg or 1.0 mg, while all tirzepatide doses were superior to semaglutide 0.5 mg (ESM Table 10).
Body weight
In comparisons vs placebo, tirzepatide was the most efficacious medication for lowering body weight, resulting in reductions ranging from 9.57 kg (95% CI 8.36, 10.78) with tirzepatide 15 mg to 5.27 kg (95% CI 3.98, 6.56) with tirzepatide 5 mg (Fig. 3). Semaglutide showed a less pronounced effect, with reductions ranging from 4.97 kg (95% CI 1.68, 8.26) with semaglutide 2.0 mg to 2.52 kg (95% CI 1.26, 3.78) with semaglutide 0.5 mg (Fig. 3). In between-drug comparisons, tirzepatide at doses of 15 mg, 10 mg and 5 mg demonstrated greater efficacy than semaglutide at doses of 2.0 mg (MD = −4.60 kg [95% CI −7.94, −1.26]), 1.0 mg (MD = −3.53 kg [95% CI −4.80, −2.25]) and 0.5 mg (MD = −2.75 kg [95% CI −4.23, −1.28]), respectively (ESM Table 11). The confidence in estimates for comparisons between tirzepatide and semaglutide was high to moderate, except for comparisons vs semaglutide 2.0 mg, where the confidence was low (ESM Table 12). Tirzepatide 15 mg was ranked highest (P-score = 1.00) among all treatments in terms of weight reduction (ESM Fig. 8).
All doses of tirzepatide and semaglutide were superior to placebo in achieving at least a 10% body weight reduction, with tirzepatide 15 mg (risk ratio = 10.51 [95% CI 7.55, 14.64]) and tirzepatide 10 mg (risk ratio = 8.84 [95% CI 6.35, 12.32]) being the most efficacious treatments (ESM Table 13). In between-drug comparisons, tirzepatide at both the 15 mg and 10 mg doses outperformed all doses of semaglutide, while tirzepatide at the 5 mg dose was more efficacious than semaglutide 0.5 mg (ESM Table 13).
Gastrointestinal adverse events
Compared with placebo, all doses of tirzepatide and semaglutide demonstrated an increase in the risk for nausea (ESM Fig. 9), vomiting (ESM Fig. 10) and diarrhoea (ESM Fig. 11). Specifically, the risk ratios for nausea ranged from 2.07 to 3.51 across different doses of tirzepatide, and from 2.45 to 2.84 for semaglutide (ESM Table 14). For vomiting, the risk ratios ranged from 2.39 to 4.36 with tirzepatide, and from 2.33 to 3.62 with semaglutide (ESM Table 15). For diarrhoea, the risk ratios ranged from 1.81 to 2.18 with tirzepatide, and from 1.66 to 1.80 with semaglutide (ESM Table 16). In comparisons between tirzepatide and semaglutide, all doses of tirzepatide had similar risk profiles for gastrointestinal adverse events when compared with semaglutide 2.0 mg. However, tirzepatide 15 mg and 10 mg generally exhibited an increased risk compared with semaglutide 1.0 mg and 0.5 mg (ESM Tables 14–16). Discontinuation of treatment due to gastrointestinal adverse events was more frequent with any dose of tirzepatide (risk ratios ranging from 6.39 to 10.65) or semaglutide (risk ratios ranging from 4.99 to 8.91) compared with placebo (Fig. 4). No differences were observed when comparing tirzepatide with semaglutide, except for tirzepatide 15 mg vs semaglutide 0.5 mg (ESM Table 17).
Serious adverse events and severe hypoglycaemia
Neither tirzepatide nor semaglutide were associated with an increased risk for serious adverse events when compared with placebo (ESM Fig. 12), and no differences were observed in the comparisons between tirzepatide and semaglutide (ESM Table 18). We did not conduct a meta-analysis for severe hypoglycaemia due to the absence of events in most treatment arms across all trials. In particular, in the overall population, 107 participants experienced an episode of severe hypoglycaemia, with 30 of the cases occurring in a single trial arm wherein participants were randomised to prandial insulin [46].
Discussion
Our systematic review and network meta-analysis provides an up-to-date evidence synthesis on the comparative efficacy of the FDA- and EMA-approved doses of s.c. semaglutide and tirzepatide for type 2 diabetes. All tirzepatide doses were comparable with semaglutide 2.0 mg and superior to semaglutide 1.0 mg and 0.5 mg in reducing HbA1c. In terms of body weight reduction, tirzepatide at doses of 15 mg, 10 mg and 5 mg demonstrated greater efficacy than semaglutide at doses of 2.0 mg, 1.0 mg and 0.5 mg, respectively. All doses of both drugs, particularly tirzepatide 15 mg, increased the occurrence of gastrointestinal adverse events vs placebo. Neither tirzepatide nor semaglutide increased the risk for serious adverse events or severe hypoglycaemia.
Unlike a previous network meta-analysis, which was limited to eight RCTs featuring only tirzepatide [52], and another focusing exclusively on semaglutide [53], we compared the two medications by including RCTs that either directly compared s.c. tirzepatide with s.c. semaglutide or used any common comparator such as placebo, basal insulin, prandial insulin or another GLP-1 RA. Moreover, our systematic review extends beyond the scope of another recent network meta-analysis, which, while including s.c. tirzepatide and high-dose GLP-1 RA, did not account for lower, yet clinically relevant, s.c. doses of semaglutide (1.0 mg and 0.5 mg) [54]. Conversely, we included these doses and incorporated data from two additional recent RCTs with tirzepatide [46, 50]. Furthermore, our systematic review builds upon the findings of a network meta-analysis that found s.c. tirzepatide to be more effective than s.c. semaglutide in weight management [55]. However, the authors included only six RCTs involving tirzepatide and, as opposed to our meta-analysis, did not provide information on comparative effects across different doses of the two medications [55]. Our network meta-analysis, while reaching similar conclusions to another recent network meta-analysis regarding the more pronounced effect of s.c. tirzepatide over s.c. semaglutide in reducing HbA1c and body weight [56], differs in methodology and scope. In particular, as opposed to Ding et al’s Bayesian approach [56], we employed a frequentist method and were more selective in our inclusion criteria, focusing on the s.c. formulation of semaglutide due to its demonstrated efficacy over other glucose-lowering agents, including orally administered semaglutide [1, 2]. Furthermore, our analysis incorporated six additional RCTs, including a trial with s.c. semaglutide 2.0 mg and two recently published trials with s.c. tirzepatide [7, 37, 41, 46, 48, 50]. In addition, we focused on comparisons of clinically approved doses for both drugs, omitting lower doses that are not used in clinical practice, and formally evaluated the confidence in meta-analysis findings [25]. Finally, our findings offer a more comprehensive assessment compared with another analysis that produced indirect estimates between tirzepatide and semaglutide 2.0 mg using data solely from two RCTs [57]. As opposed to this study, we did not find a difference between tirzepatide 10 mg and semaglutide 2.0 mg in terms of HbA1c or body weight reduction. These differences likely arise from our much larger dataset encompassing 28 RCTs, allowing for more accurate comparative estimates between treatments.
Specific limitations should be acknowledged. Given that our systematic review was designed to assess the comparative efficacy and safety between s.c. tirzepatide and s.c. semaglutide, eligible RCTs focused on either direct comparisons between these two medications or vs common comparators (comparators that have been assessed in at least one trial comparison against s.c. tirzepatide and in one trial comparison against s.c. semaglutide). This focused approach, while providing insights into comparisons between tirzepatide and semaglutide, is not as well suited for an evaluation of the two medications vs the common comparators included in the analysis. Moreover, we observed low confidence in meta-analysis results in comparisons involving semaglutide 2.0 mg, attributable to the inclusion of only one RCT assessing this dose. As such, interpretations concerning the comparative efficacy and safety of semaglutide 2.0 mg vs tirzepatide doses should be approached with caution. In addition, the treatment response observed in our analysis may also be influenced by ethnic differences, given that five RCTs recruited exclusively Japanese participants [39,40,41, 48, 49]. In particular, it has been shown that East Asian people with type 2 diabetes typically present with less severe obesity and are characterised by lower beta cell function and lesser insulin resistance compared with White populations [58]. Furthermore, evidence suggests that sex differences may influence the efficacy and safety profiles of GLP-1 RAs [59]. Specifically, women treated with GLP-1 RAs may experience greater glycaemic control and weight-reduction benefits, as well as a higher incidence of gastrointestinal adverse events, compared with men [59]. However, we did not perform subgroup analyses based on sex. This limitation reflects the broader issue of inconsistent reporting of sex-disaggregated outcomes in diabetes-treatment research and underscores the need for future research to systematically explore and report the effects of diabetes treatments according to sex. Another limitation is the a priori exclusion of long-term cardiovascular or mortality outcomes from our analysis, a decision based on the fact that the dedicated cardiovascular outcomes trial for tirzepatide (SURPASS-CVOT) is still ongoing [60]. Finally, in our analysis of the change in HbA1c, we noted a variation in the results based on the measurement units used. Specifically, when HbA1c was measured in mmol/mol, semaglutide 2.0 mg showed a marginally more pronounced effect compared with placebo than tirzepatide 5 mg, whereas this trend was reversed when analysing HbA1c in percentage units. Of note, the percentage-based results are potentially more precise, as they did not require imputations or borrowing of variance values from other studies. This aspect was necessary in the mmol/mol analysis due to some studies not reporting complete measures of variance, highlighting the need for future trials to report results for HbA1c in both mmol/mol and percentage units to aid in comprehensive analysis and interpretation of findings.
There were some protocol deviations in our analysis. We did not include achievement of at least a 5% reduction in body weight as an outcome because all doses of both drugs were superior to placebo in achieving at least a 10% weight reduction. As such, including an additional outcome with less clinically meaningful threshold would overload the study results with redundant information rather than providing added clinical insights. Our plan to conduct sensitivity or subgroup analyses based on risk-of-bias assessment, trial duration and background glucose-lowering treatment was also not implemented. The sensitivity analysis based on risk of bias was not performed because only a few trials were at high risk of bias, while the average risk of bias across treatment comparisons was incorporated into our assessment of confidence in the findings using the CINeMA framework [25]. Regarding trial duration, this ranged between 26 weeks and 56 weeks in all trials except for two, making a subgroup analysis based on duration unwarranted. Furthermore, performing subgroup analyses based on background glucose-lowering therapy was not feasible due to the varied treatments across trials. However, in most trials, participants received metformin, either as monotherapy or in combination with other agents, except for three trials where participants received no background glucose-lowering therapy after randomisation [26, 40, 49].
Clinical practice recommendations by the ADA Standards of Care and the ADA/EASD consensus report place s.c. tirzepatide and s.c. semaglutide among the most efficacious treatment for lowering glucose (alongside dulaglutide) and reducing weight in people with type 2 diabetes [4, 5]. In line with these recommendations, our meta-analysis corroborates the clinical benefits of both medications compared with placebo. Our findings also suggest that s.c. tirzepatide could be a preferable option over s.c. semaglutide for individuals who prioritise glycaemic and weight management due to its more pronounced effect in both outcomes. However, the goal of glucose-lowering therapy extends beyond controlling blood glucose levels and body weight, encompassing the reduction of long-term cardiovascular complications. For example, s.c. semaglutide has demonstrated cardiovascular benefits in the SUSTAIN-6 trial, which was designed to assess the non-inferiority of s.c. semaglutide as compared with placebo in terms of cardiovascular safety in people with type 2 diabetes at increased cardiovascular risk [31]. While pooled data from the SURPASS clinical trial programme indicate that tirzepatide does not increase the risk of major cardiovascular events [61], definitive conclusions regarding its cardiovascular profile should await the results of SURPASS-CVOT, with its completion anticipated in late 2024 [60]. Furthermore, our analysis suggests that the increased gastrointestinal adverse events associated with both s.c. tirzepatide and s.c. semaglutide can lead to treatment discontinuation in some patients, particularly with the higher dose of tirzepatide. In older and frail individuals, where vomiting and diarrhoea could result in dehydration, these medications might need to be prescribed with caution.
Complementing our meta-analysis findings, a recent preprint of a large observational study comparing s.c. tirzepatide with s.c. semaglutide in the USA provides valuable real-world evidence [62]. This study found that s.c. tirzepatide was more effective than s.c. semaglutide in reducing body weight among obese or overweight individuals, a benefit that was apparent regardless of the presence of type 2 diabetes, while the rates of gastrointestinal adverse events were similar between the two drugs [62]. These real-world findings provide insights into the effectiveness and tolerability of these medications outside the controlled environment of RCTs, reinforcing the potential of tirzepatide as a highly effective option for weight management in routine clinical practice. However, it is essential to consider the broader implications of adopting these therapies in real-world settings, particularly concerning their cost. Observational data suggest a notable under-utilisation of GLP-1 RAs among individuals in lower socioeconomic groups, primarily due to the high cost of these medications, highlighting the disparity in access to effective diabetes treatments based on socioeconomic factors [63]. From a broader societal perspective, even though s.c. tirzepatide has been suggested to be cost-effective compared with s.c. semaglutide in the USA [64], cost-effectiveness analyses conducted in low-, middle- and high-income countries have demonstrated that GLP-1 RAs are not cost-effective compared with other glucose-lowering drugs [63].
Conclusions
Our network meta-analysis of 28 RCTs found that s.c. tirzepatide generally had a more pronounced effect than s.c. semaglutide in reducing HbA1c and body weight in people with type 2 diabetes. Notably, both drugs, particularly the higher doses of tirzepatide, were associated with an increased incidence of gastrointestinal adverse events. These findings can inform clinical decisions and optimising treatment strategies in the management of type 2 diabetes.
Abbreviations
- CINeMA:
-
Confidence In Network Meta-Analysis
- EMA:
-
European Medicines Agency
- FDA:
-
US Food and Drug Administration
- GIP:
-
Glucose-dependent insulinotropic peptide
- GLP-1 RA:
-
Glucagon-like peptide-1 receptor agonist
- MD:
-
Mean difference
References
Tsapas A, Avgerinos I, Karagiannis T et al (2020) Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med 173(4):278–287. https://doi.org/10.7326/M20-0864
Tsapas A, Karagiannis T, Kakotrichi P et al (2021) Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab 23(9):2116–2124. https://doi.org/10.1111/DOM.14451
Karagiannis T, Avgerinos I, Liakos A et al (2022) Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia 65(8):1251–1261. https://doi.org/10.1007/S00125-022-05715-4
Davies MJ, Aroda VR, Collins BS et al (2022) Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 65(12):1925–1966. https://doi.org/10.1007/S00125-022-05787-2
American Diabetes Association Professional Practice Committee (2024) 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2024. Diabetes Care 47(Suppl 1):S158–S178. https://doi.org/10.2337/DC24-S009
Frías JP, Davies MJ, Rosenstock J et al (2021) Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 385:503–515. https://doi.org/10.1056/NEJMoa2107519
Heise T, Mari A, DeVries JH et al (2022) Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol 10(6):418–429. https://doi.org/10.1016/S2213-8587(22)00085-7
Rouse B, Chaimani A, Li T (2017) Network meta-analysis: an introduction for clinicians. Intern Emerg Med 12(1):103–111. https://doi.org/10.1007/S11739-016-1583-7
Page MJ, Shamseer L, Tricco AC (2018) Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev 7(1):32. https://doi.org/10.1186/S13643-018-0699-4
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784. https://doi.org/10.7326/M14-2385
Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM (2020) A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol 121:81–90. https://doi.org/10.1016/J.JCLINEPI.2020.01.008
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/BMJ.L4898
Chaimani A, Salanti G (2012) Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 3(2):161–176. https://doi.org/10.1002/JRSM.57
Cipriani A, Higgins JPT, Geddes JR, Salanti G (2013) Conceptual and technical challenges in network meta-analysis. Ann Intern Med 159(2):130–137. https://doi.org/10.7326/0003-4819-159-2-201307160-00008
Rücker G (2012) Network meta-analysis, electrical networks and graph theory. Res Synth Methods 3(4):312–324. https://doi.org/10.1002/JRSM.1058
Riley RD, Higgins JPT, Deeks JJ (2011) Interpretation of random effects meta-analyses. BMJ 342(7804):964–967. https://doi.org/10.1136/BMJ.D549
Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G (2020) CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev 16(1):e1080. https://doi.org/10.1002/CL2.1080
Dankers M, Nelissen-Vrancken MHJMG, Hart BH, Lambooij AC, van Dijk L, Mantel-Teeuwisse AK (2021) Alignment between outcomes and minimal clinically important differences in the Dutch type 2 diabetes mellitus guideline and healthcare professionals’ preferences. Pharmacol Res Perspect 9(3):e00750. https://doi.org/10.1002/PRP2.750
Dias S, Welton NJ, Caldwell DM, Ades AE (2010) Checking consistency in mixed treatment comparison meta-analysis. Stat Med 29(7–8):932–944. https://doi.org/10.1002/SIM.3767
Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR (2012) Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 3(2):98–110. https://doi.org/10.1002/JRSM.1044
Rücker G, Schwarzer G (2015) Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 15(1):58. https://doi.org/10.1186/S12874-015-0060-8
Balduzzi S, Rücker G, Nikolakopoulou A et al (2023) netmeta: an R Package for network meta-analysis using frequentist methods. J Stat Softw 106(2):1–40. https://doi.org/10.18637/JSS.V106.I02
Chaimani A, Yu T (2023) A hands-on introduction to NMAstudio: a web-application to produce and visualize network meta-analyses. Abstracts accepted for the 27th Cochrane Colloquium, London, UK. Cochrane Database of Systematic Reviews 2023; (1 Supp 1):36690 (Abstract)
NMAstudio (2020) Welcome to NMAstudio 2.0. Available from www.nmastudioapp.com/. Accessed: 19 November 2023
Nikolakopoulou A, Higgins JPT, Papakonstantinou T et al (2020) CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med 17(4):e1003082. https://doi.org/10.1371/JOURNAL.PMED.1003082
Sorli C, Harashima S-I, Tsoukas GM et al (2017) Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 5(4):251–260. https://doi.org/10.1016/S2213-8587(17)30013-X
Ahrén B, Masmiquel L, Kumar H et al (2017) Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 5(5):341–354. https://doi.org/10.1016/S2213-8587(17)30092-X
Ahmann AJ, Capehorn M, Charpentier G et al (2018) Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 41(2):258–266. https://doi.org/10.2337/dc17-0417
Aroda VR, Bain SC, Cariou B et al (2017) Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 5(5):355–366. https://doi.org/10.1016/S2213-8587(17)30085-2
Rodbard H, Lingvay I, Reed J et al (2018) Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 103(6):2291–2301. https://doi.org/10.1210/jc.2018-00070
Marso SP, Bain SC, Consoli A et al (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375:1834–1844. https://doi.org/10.1056/NEJMoa1607141
Pratley RE, Aroda VR, Lingvay I et al (2018) Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 6(4):275–286. https://doi.org/10.1016/S2213-8587(18)30024-X
Zinman B, Bhosekar V, Busch R et al (2019) Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 7(5):356–367. https://doi.org/10.1016/S2213-8587(19)30066-X
Capehorn MS, Catarig A-M, Furberg JK et al (2020) Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 46(2):100–109. https://doi.org/10.1016/j.diabet.2019.101117
Kellerer M, Kaltoft MS, Lawson J et al (2022) Effect of once-weekly semaglutide versus thrice-daily insulin aspart, both as add-on to metformin and optimized insulin glargine treatment in participants with type 2 diabetes (SUSTAIN 11): a randomized, open-label, multinational, phase 3b trial. Diabetes Obes Metab 24(9):1788–1799. https://doi.org/10.1111/dom.14765
Ji L, Dong X, Li Y et al (2021) Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: a 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab 23(2):404–414. https://doi.org/10.1111/dom.14232
Frías JP, Auerbach P, Bajaj HS et al (2021) Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol 9(9):563–574. https://doi.org/10.1016/S2213-8587(21)00174-1
Davies M, Pieber TR, Hartoft-Nielsen M-L, Hansen OKH, Jabbour S, Rosenstock J (2017) Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 318(15):1460–1470. https://doi.org/10.1001/jama.2017.14752
Iijima T, Shibuya M, Ito Y, Terauchi Y (2023) Effects of switching from liraglutide to semaglutide or dulaglutide in patients with type 2 diabetes: a randomized controlled trial. J Diabetes Investig 14(6):774–781. https://doi.org/10.1111/JDI.14000
Seino Y, Terauchi Y, Osonoi T et al (2018) Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab 20(2):378–388. https://doi.org/10.1111/dom.13082
Takahashi Y, Nomoto H, Yokoyama H et al (2023) Improvement of glycaemic control and treatment satisfaction by switching from liraglutide or dulaglutide to subcutaneous semaglutide in patients with type 2 diabetes: a multicentre, prospective, randomized, open-label, parallel-group comparison study (SWITCH-SEMA 1 study). Diabetes Obes Metab 25(6):1503–1511. https://doi.org/10.1111/DOM.14998
Rosenstock J, Wysham C, Frías JP et al (2021) Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398(10295):143–155. https://doi.org/10.1016/S0140-6736(21)01324-6
Ludvik B, Giorgino F, Jódar E et al (2021) Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 398(10300):583–598. https://doi.org/10.1016/S0140-6736(21)01443-4
Del Prato S, Kahn SE, Pavo I et al (2021) Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398(10313):1811–1824. https://doi.org/10.1016/S0140-6736(21)02188-7
Dahl D, Onishi Y, Norwood P et al (2022) Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 327(6):534–545. https://doi.org/10.1001/jama.2022.0078
Rosenstock J, Frías JP, Rodbard HW et al (2023) Tirzepatide vs insulin lispro added to basal insulin in type 2 diabetes: the SURPASS-6 randomized clinical trial. JAMA 330(17):1631–1640. https://doi.org/10.1001/JAMA.2023.20294
Gao L, Lee BW, Chawla M et al (2023) Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: the SURPASS-AP-Combo trial. Nat Med 29(6):1500–1510. https://doi.org/10.1038/S41591-023-02344-1
Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y (2022) Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol 10(9):634–644. https://doi.org/10.1016/S2213-8587(22)00187-5
Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y (2022) Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol 10(9):623–633. https://doi.org/10.1016/S2213-8587(22)00188-7
Garvey WT, Frias JP, Jastreboff AM et al (2023) Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 402(10402):613–626. https://doi.org/10.1016/S0140-6736(23)01200-X
Frias JP, Nauck MA, Van J et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193. https://doi.org/10.1016/S0140-6736(18)32260-8
Guan R, Yang Q, Yang X, Du W, Li X, Ma G (2022) Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a Bayesian network meta-analysis. Front Pharmacol 13:998816. https://doi.org/10.3389/FPHAR.2022.998816
Zaazouee MS, Hamdallah A, Helmy SK et al (2022) Semaglutide for the treatment of type 2 diabetes mellitus: a systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes Metab Syndr 16(6):102511. https://doi.org/10.1016/J.DSX.2022.102511
Caruso I, Di Gioia L, Di Molfetta S et al (2023) Glucometabolic outcomes of GLP-1 receptor agonist-based therapies in patients with type 2 diabetes: a systematic review and network meta-analysis. EClinicalMedicine 64:102181. https://doi.org/10.1016/J.ECLINM.2023.102181
Shi Q, Nong K, Vandvik PO et al (2023) Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 381:e074068. https://doi.org/10.1136/BMJ-2022-074068
Ding Y, Shi Y, Guan R et al (2024) Evaluation and comparison of efficacy and safety of tirzepatide and semaglutide in patients with type 2 diabetes mellitus: a Bayesian network meta-analysis. Pharmacol Res 199:107031. https://doi.org/10.1016/J.PHRS.2023.107031
Vadher K, Patel H, Mody R et al (2022) Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: an adjusted indirect treatment comparison. Diabetes Obes Metab 24(9):1861–1868. https://doi.org/10.1111/DOM.14775
Yabe D, Seino Y, Fukushima M, Seino S (2015) β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 15(6):36. https://doi.org/10.1007/S11892-015-0602-9
Kautzky-Willer A, Leutner M, Harreiter J (2023) Sex differences in type 2 diabetes. Diabetologia 66(6):986–1002. https://doi.org/10.1007/s00125-023-05891-x
Nicholls SJ, Bhatt DL, Buse JB et al (2023) Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am Heart J 267:1–11. https://doi.org/10.1016/J.AHJ.2023.09.007
Sattar N, McGuire DK, Pavo I et al (2022) Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med 28(3):591–598. https://doi.org/10.1038/S41591-022-01707-4
Rodriguez PJ, Goodwin Cartwright BM, Gratzl S et al (2023) Comparative effectiveness of semaglutide and tirzepatide for weight loss in adults with overweight and obesity in the US: a real-world evidence study. medRxiv 2023.11.21.23298775 (Preprint). 22 Nov 2023. Available from: https://doi.org/10.1101/2023.11.21.23298775
Karagiannis T, Bekiari E, Tsapas A (2023) Socioeconomic aspects of incretin-based therapy. Diabetologia 66(10):1859–1868. https://doi.org/10.1007/S00125-023-05962-Z
Valentine WJ, Hoog M, Mody R, Belger M, Pollock R (2023) Long-term cost-effectiveness analysis of tirzepatide versus semaglutide 1.0 mg for the management of type 2 diabetes in the United States. Diabetes Obes Metab 25(5):1292–1300. https://doi.org/10.1111/DOM.14979
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Authors’ relationships and activities
AL has received speaker honoraria and educational support from Novo Nordisk. EB has received research support and speaker honoraria, and has served on an advisory board for Novo Nordisk. All other authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors significantly contributed to the manuscript. TK, AT and EB designed the study. KM, AS, PK, AL, DV, NK and TK contributed to data acquisition. TK, IA and KM did the statistical analysis. TK, AL, DV, NK, AT and EB contributed to interpretation of data. TK wrote the first manuscript draft. All authors edited the final manuscript draft and approved the final version for publication. TK is the guarantor of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karagiannis, T., Malandris, K., Avgerinos, I. et al. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetologia 67, 1206–1222 (2024). https://doi.org/10.1007/s00125-024-06144-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-024-06144-1