Abstract
Aims/hypothesis
Type 2 diabetes mellitus prevalence is increasing globally and the greatest burden is borne by racialised people. However, there are concerns that the enrolment of racialised people into RCTs is limited, resulting in a lack of ethnic and racial diversity. This may differ depending whether an RCT is government funded or industry funded. The aim of this study was to review the proportions of racialised and white participants included in large RCTs of type 2 diabetes pharmacotherapies relative to the disease burden of type 2 diabetes in these groups.
Methods
The Ovid MEDLINE database was searched from 1 January 2000 to 31 December 2020. English language reports of RCTs of type 2 diabetes pharmacotherapies published in select medical journals were included. Studies were included in this review if they had a sample size of at least 100 participants and all participants were adults with type 2 diabetes. Industry-funded trials must have recruited participants from at least two countries. Government-funded trials were not held to the same standard because they are typically conducted in a single country. Data including the numbers and proportions of participants by ethnicity and race were extracted from trial reports. The participation-to-prevalence ratio (PPR) was calculated for each trial by dividing the percentage of white and racialised participants in each trial by the percentage of white and racialised participants with type 2 diabetes, respectively, for the regions of recruitment. A random-effects meta-analysis was used to generate the pooled PPRs and 95% CIs across study types. A PPR <0.80 indicates under-representation and a PPR >1.20 indicates over-representation. Risk of bias assessments were not conducted for this study as the objective was to examine recruitment of racialised and white participants rather than evaluate the trustworthiness of clinical trial outcomes.
Results
A total of 83 trials were included, involving 283,122 participants, of which 15 were government-funded and 68 were industry-funded trials. In government-funded trials, the PPR for white participants was 1.11 (95% CI 0.99, 1.24) and the PPR for racialised participants was 0.72 (95% CI 0.60, 0.86). In industry-funded trials, the PPR for white participants was 1.95 (95% CI 1.74, 2.18) and the PPR for racialised participants was 0.36 (95% CI 0.32, 0.42). The limitations of this study include the reliance on investigator-reported ethnicity and race to classify participants as ‘white’ or ‘racialised’, the use of estimates for type 2 diabetes prevalence and demographic data, and the high levels of heterogeneity of pooled estimates. However, despite these limitations, the results were consistent with respect to direction.
Conclusions/interpretation
Racialised participants are under-represented in government- and industry-funded type 2 diabetes trials. Strategies to improve recruitment and enrolment of racialised participants into RCTs should be developed.
Registration
Open Science Framework registration no. f59mk (https://osf.io/f59mk)
Funding
The authors received no financial support for this research or authorship of the article.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
The burden of type 2 diabetes is disproportionately higher in non-white ethnic and racial groups than in white individuals [1]. However, individuals from non-white ethnic or racial groups, herein referred to as racialised people, are generally under-represented in RCTs, which by design produce the most reliable evidence regarding the efficacy and safety of medical therapies [1, 2]. As such, RCTs inform treatment recommendations in guidelines. Under-representation of racialised people in RCTs can limit the generalisability of the trial findings and uptake of guideline recommendations among racialised groups with the highest disease burden [3].
In the USA, the National Institutes of Health (NIH) provides guidelines for the inclusion of racialised groups in the clinical research they fund, a measure taken to improve the generalisability of research findings [4]. Industry-funded trials do not have the same requirements as the NIH, nor do many other government-established funding agencies [5,6,7,8]. For example, Canada and Australia do not have guidelines for the recruitment of racialised populations into clinical trials. The ‘Guidance for industry: standards for clinical trials in type 2 diabetes in Canada’ (2007) document does not mention the terms ‘race’ or ‘ethnicity,’ nor does it provide guidelines on participant recruitment [7]. Similarly, the Government of Australia’s guidelines for clinical trials do not include any regulations pertaining to recruitment of racialised groups [8]. In the UK, there is also no requirement to record and report ethnicity or race in research studies [5].
The NIH requirements are guided by the Public Health Service Act sec. 492B, 42 U.S.C. sec. 289a-2 and are designed to enhance the inclusion of minority groups in NIH-funded research [4]. Efforts to increase representation of racialised groups in RCTs have been made internationally as well. For example, some research institutions in the UK are taking measures to address the lack of ethnic participant recruitment guidelines for clinical trials. In 2018, the UK’s National Institute for Health and Care Research (NIHR) launched the INCLUDE project, designed to enhance the diversity of research participants in clinical studies [5].
In this study we conducted a meta-epidemiological review of Phase II–IV RCTs published between 1 January 2000 and 31 December 2020 that tested at least one type 2 diabetes pharmacotherapy and investigated (1) the participation of racialised individuals relative to the disease burden of type 2 diabetes in large RCTs in which type 2 diabetes therapies were evaluated and (2) the differences in participation of racialised and white individuals between industry- and government-funded trials.
Methods
This meta-epidemiological review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. This study was registered on Open Science Framework (registration no. f59mk; https://osf.io/f59mk).
Data sources and searches
Guided by a clinical expert, a broad search strategy with keywords related to type 2 diabetes RCTs was developed (electronic supplementary material [ESM] Table 1). Initially, the search was limited to trials published in the New England Journal of Medicine, The Lancet, JAMA, The BMJ and Annals of Internal Medicine. These journals were selected because they have a history of being targeted for publication of large, high-impact, multicountry trials of type 2 diabetes drugs. This criterion was kept for industry-funded trials; however, it was expanded for government-funded trials to include publications from five specialty journals (Diabetes Care, Circulation, Lancet Diabetes and Endocrinology, JAMA Internal Medicine, JAMA Ophthalmology), as we recognise that discipline-specific journals are more likely to publish smaller-scale government-funded trials that recruit fewer participants and/or are conducted in a single country. A complete list of study selection criteria can be found in ESM Table 2. The Ovid MEDLINE database was searched from 1 January 2000 to 31 December 2020 to identify relevant studies. The year 2000 was selected as a starting point because most of the oral hypoglycaemic agents for diabetes (metformin, glimepiride, rosiglitazone) were approved in the mid to late 1990s. As such, their use in clinical trials became more widespread in 2000 and beyond. The endpoint of our time frame was selected to capture trends before the COVID-19 pandemic. The decade-by-decade analysis presents an opportunity to revisit the data and examine trends in 10-year increments. Ovid MEDLINE was used because the identified journals are all indexed in the database, which eliminated the need for multiple database searching. Full-text screening was completed by two reviewers (RA and RJdS) independently and in duplicate based on predetermined study selection criteria (ESM Fig. 1). Covidence (https://www.covidence.org/) was used for data management.
Data extraction
Three reviewers (authors RA and VL, with JW) independently extracted the following information from published studies: title, year of publication, journal, primary funding source, pharmacotherapy intervention, total number of participants, country or region of greatest participant recruitment, and numbers of white and racialised participants. For this review, participants were categorised as ‘racialised’ if they belonged to any race or ethnicity that was not specifically described as ‘white’ or ‘Caucasian’ by the investigators. Discrepancies were cross-checked and, where necessary, were resolved by discussion with the senior author (SSA). The resource ClinicalTrials.gov and other publicly available web resources were consulted to obtain any missing information that was not in the main articles or supplementary materials.
Outcome measures
There were two outcomes of interest for this meta-analysis: the proportion of white participants in government- and industry-funded trials relative to the type 2 diabetes disease burden in the population, and the proportion of racialised participants in government- and industry-funded trials relative to the type 2 diabetes disease burden in the population.
Statistical analysis
The participation-to-prevalence ratio (PPR) metric was used to estimate the representation of white participants and the representation of racialised participants compared with their respective disease burden, separately in industry- and government-funded trials. The PPR for white participants and racialised participants in each trial was calculated using the respective formulas below.
A PPR of 1 suggests that racialised or white people make up the same proportion of participants in the trial as the proportion of racialised or white people, respectively, among the diseased population in the countries from which a trial recruited. For example, if 80% of participants in a trial are racialised and 80% of the cases of diabetes in the country or region occur in racialised groups, the PPR would be 1. The underlying goal for equity should be for the proportion of participants recruited by ethnicity or race to be similar to the disease burden faced by those ethnic or racial groups in that country or region. For this review, a group was considered to be under-represented when the PPR was <0.80 and over-represented when the PPR was >1.20. These decision points are consistent with a 2020 study evaluating the participation of women in cardiovascular RCTs [9].
The numerator for the PPR was known for each trial. The denominator, however, was calculated for each trial using prevalence and demographic data. A detailed explanation of PPR calculations is provided in ESM Methods. A list of estimates used for the PPR calculations is provided in ESM Table 3.
Once the PPRs for white and racialised people were calculated for each trial, a random-effects meta-analysis was used to pool the individual-study PPRs and compute the overall 95% CIs for the pooled PPRs. A random-effects model was used for this analysis because it provides appropriately wider CIs and study weights in the presence of heterogeneity, which we expected to see across varying recruitment approaches, trial conditions and countries of conduct. Under this model, it can be assumed that the true PPR may differ according to the setting, country or type of trial.
We conducted a sensitivity analysis by varying our worldwide population proportion estimates (11.7% white and 88.3% racialised). These estimates were used when a trial that recruited from three or more regions did not provide data on how many participants were recruited from each country/region. We systematically altered the proportion of racialised people to values from 80% to 90% in increments of 2.5%, and of white participants from 10% to 20% in increments of 2.5%.
All study-specific PPR estimates were calculated in Microsoft Excel and the pooled PPRs across studies and 95% CIs were calculated using Review Manager version 5.4 (RevMan; The Cochrane Collaboration, London, UK).
Results
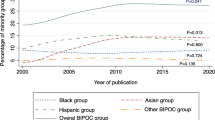
Of the 512 records that were assessed for eligibility, 83 RCTs with either industry funding or government funding with a total of 283,122 participants were included (ESM Fig. 1). Three other studies with dual funding were included in a sensitivity analysis. The RCTs that were excluded after full-text review are listed in ESM Table 4. Of the 83 trials included in the review, 15 (18.1%) were government-funded [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24], 68 (81.9%) were sponsored by industry [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92] and 49 (59.0%) were published between the years 2011 and 2020. The proportion of racialised participants in this set of trials increased significantly from 10.7% in 2000–2005 to 23.6% in 2006–2010 and remained relatively constant between 2006 and 2020. Over one-third of the studies in the review (42.2%) recruited the greatest number of participants from the USA. The most common pharmacotherapy interventions were from the glucagon-like peptide-1 (GLP-1) receptor agonist, sodium–glucose co-transporter 2 (SGLT-2) inhibitor and dipeptidyl peptidase-4 (DPP-4) inhibitor classes. The percentage of racialised participants across all trials was 23.8%. Government-funded trials had a higher overall percentage of racialised participants (26.0%) than industry-funded studies (23.4%) (Table 1). The list of included RCTs is provided in Table 2.
The pooled PPR for white participants was 1.11 (95% CI 0.99, 1.24) for government-funded trials, consistent with proportional representation, and 1.95 (95% CI 1.74, 2.18) for industry-funded trials, consistent with over-representation. The PPR for racialised people was 0.72 (95% CI 0.60, 0.86) for government-funded trials and 0.36 (95% CI 0.32, 0.42) for industry-funded trials, both of which are consistent with under-representation (Fig. 1). Figures 2, 3, 4 and 5 show detailed breakdowns of the pooled PPRs.
The pooled meta-analytic estimates had high levels of heterogeneity (I2>90%). However, despite this, the results were directionally consistent. Only seven of 68 industry-funded trials had a PPR <1 for white people and a PPR >1 for racialised people [29, 53, 57, 60, 78, 79, 89], and these carried approximately 11.1% of the weight in the pooled estimates. Similarly, only four of 15 government-funded trials had a PPR <1 for white people and a PPR >1 for racialised people [18,19,20, 23], and these carried approximately 20.6% of the weight in the pooled estimates.
Sensitivity analysis
Twelve industry-funded trials that recruited from three or more regions did not provide data on how many participants were recruited from each country or region (indicated by ‘Worldwide’ in Table 2). For these trials, worldwide estimates of the proportions of white and racialised people were used (11.7% and 88.3%, respectively) (ESM Methods). A series of sensitivity analyses were conducted in which the estimated proportions of white and racialised people were varied for these trials. The proportion of white participants was varied from 80% to 90% and of racialised participants from 10% to 20%. This did not result in appreciably different estimates from the main analyses. The full data for these sensitivity analyses are provided in ESM Appendix 1 (ESM Figs 2–5).
In addition to the 83 trials that were included in the main analysis, three trials that were funded by both government and industry sources were included in a separate sensitivity analysis [93,94,95]. Because a primary source of funding for these trials could not be determined with confidence, a sensitivity analysis was conducted to determine the effect that the trials would have on the pooled PPRs had they been included and categorised as industry or government funded. In one analysis both trials were included as industry-funded studies and in a second analysis both were included as government-funded trials. The results were not appreciably different from the main analyses (see ESM Appendix 2, ESM Figs 6 and 7).
Discussion
Type 2 diabetes disproportionately affects racialised people worldwide [96]. This meta-epidemiological review shows that white individuals are over-enrolled and racialised individuals are under-enrolled in type 2 diabetes RCTs. Government-funded type 2 diabetes trials tend to have better representation of racialised participants than industry-funded trials.
The finding that government-funded trials recruit more racialised participants may reflect adherence to the more stringent conditions associated with funding from government bodies. For example, the NIH emphasises the inclusion and appropriate representation of minority groups in clinical research [4]. This measure attempts to ensure that racialised populations are proportionately represented in NIH-funded clinical research and that research findings are generalisable across all ethnic groups. Industry-funded trials do not have the same requirements as some government bodies and therefore decisions regarding who to enrol and in what proportion (i.e. by ethnicity, race and sex) are influenced entirely by the trial sponsors, steering committees and research staff. The under-representation of racialised participants limits the ability to generalise efficacy and safety outcomes to racialised people and may limit the uptake of this evidence by racialised communities, which adds to the disadvantages they may face.
For industry-funded trials, white participants were over-represented relative to their disease burden (Fig. 4). However, the pooled PPR estimate was heterogeneous. For example, some studies had PPR values that were notably higher than the others [35, 37, 54, 55, 62, 64, 66, 70, 80, 82, 84, 92]. This is because worldwide estimates were used for both type 2 diabetes prevalence and demographic data for these trials. Prevalence estimates were obtained from Saeedi et al [97] and are listed in ESM Table 3. Although the PPRs for white participants in industry-funded trials may be overestimates, the results of our sensitivity analyses do not suggest any substantial overestimation (ESM Appendix 1). This is because the proportion of racialised participants in these trials was relatively small and therefore varying the worldwide proportion estimates for racialised participants did not greatly affect the pooled PPR. Greater variance was observed among the different proportions of white participants in the trials. Overall, the effects of varying worldwide proportions of both white and racialised people on the PPRs were limited. This is because worldwide estimates were needed for only 12 of the 68 industry-funded trials and the participants in all 12 trials comprised only 3.8% of the total participants across the industry-funded trials in this review.
Racialised participants were under-represented in industry-funded trials relative to their disease burden (Fig. 5). Seven trials [29, 53, 57, 60, 78, 79, 89], however, had a PPR >1, with racialised PPRs of 1.85, 1.47, 1.18, 1.04, 1.15, 1.06, 1.67, respectively. Six of these trials recruited over 1500 participants from at least 24 countries including regions of North America, Europe, South America, Asia and Africa. Future industry-funded trials should consider enrolling participants from diverse communities and regions of the world, especially when the disease burden is higher than in white European-origin individuals.
There are several potential explanations for the patterns of over-representation of white participants and under-representation of racialised participants in government- and industry-funded RCTs. First, inclusion and exclusion criteria for RCTs may favour enrolling white over racialised participants. For example, in the USA, the ability to read and speak English is often an inclusion criterion for clinical trials, which can disproportionately impact the participation of racialised groups. Second, recruitment processes can affect the overall diversity of participants in RCTs. For instance, specialty clinics and hospitals where research is taking place may be located in areas with lower proportions of racialised people. Third, limited screening of racialised people for enrolment may occur because of implicit biases and/or social or medical reasons that make participation difficult for these groups. Fourth, mistrust and fear of medical institutions because of historical mistreatment of racialised groups may result in a lack of willingness of racialised people to participate in clinical trials. Fifth, racialised groups may not enrol because of language barriers, cultural practices or related contextual factors that limit their participation, including socioeconomic disadvantages. Finally, logistical barriers may exist such as inflexible work schedules and additional costs associated with participating in research studies, such as transport costs for attending study visits [98].
Furthermore, a lack of diversity among principal investigators, local investigators and study staff in some RCTs may also be related to lower enrolment rates for racialised participants [98]. For example, in an NIH study on the diversity of the NIH-funded workforce, it was found that 71.9% of principal investigators on NIH-funded research project grants were white [99]. Ethnically or racially diverse representation among study staff might increase the trust of participants from racialised communities and improve communication [98]. Additionally, industry-funded trials might benefit from having racialised participant recruitment guidelines similar to those that exist for the NIH. Regulatory bodies could indicate that proportional representation of participants affected by the disease of interest by ethnicity is strongly recommended or mandatory so that industry trial leaders more carefully consider who and from where to recruit within a given country or region. In future work, reviews of the recruitment of racialised groups should be carried out for trials conducted within single countries to assess country-specific trends. These reviews could guide clinical practice and be used to establish recruitment standards that are reasonable given the prevalence of type 2 diabetes and demographics in a given country. Future studies should also be conducted to analyse racialised participant recruitment trends in RCTs on other health conditions such as hypertension and stroke, which are known to be common in racialised communities.
Our study has several strengths. The meta-analysis included trials that were published in top-tier medical journals and that are likely to be highly cited and used to inform clinical guidelines. Analysing racialised participant recruitment in these studies allows for a finer assessment of the generalisability of the results to certain practice settings. The calculation and presentation of PPRs improves the summary of the findings of over-representation of white participants and under-representation of racialised participants (Figs 2, 3, 4 and 5).
Our study also has certain limitations. First, this analysis relied on investigator-reported ethnicity or race. Participants belonging to any ethnicity or race that was not explicitly defined by trial investigators as ‘white’ were categorised as ‘racialised’. We recognise, however, that the understanding of the terms ‘white’ and ‘racialised’ may vary between countries and trial investigators. Inconsistent interpretations of the term ‘white’ could influence the overall PPR estimates. Second, the PPR denominator calculations were based on prevalence and demographic data for white and racialised participants in different countries and regions of the world. While type 2 diabetes prevalence and demographic data are typically documented for specific countries, limited data exist for type 2 diabetes prevalence and demographics in larger regions of the world. When specific data were not available, these values were estimated (ESM Methods and ESM Table 3). Third, this meta-epidemiological review included studies that were published between 1 January 2000 and 31 December 2020. However, our estimates of type 2 diabetes prevalence and demographic data did not correspond exactly to the prevalence of type 2 diabetes in white and racialised people and the ethnic breakdown of countries or regions at the time that each trial was conducted, although we attempted to match them as closely as possible. Fourth, our pooled meta-analytic estimates had high levels of heterogeneity (I2 >90%). This heterogeneity may stem from many factors, including the study design used, country or region of conduct, study size and period of enrolment. Despite the high levels of heterogeneity, our results were consistent with respect to direction. Thus, statistical heterogeneity influenced the precision of our estimates but not the direction or magnitude to an extent that would cause concern. Finally, we did not conduct risk of bias assessments for the trials used in this review. This is because our goal was to examine recruitment of white and racialised participants as opposed to evaluating the clinical outcomes of included studies. Furthermore, our selection criteria were such that we only included large (n>100) RCTs (lowest risk of bias design) published in top-tier journals, which helped to ensure a comparable and low risk of bias across included studies.
Conclusion
Racialised participants appear to be under-represented in both government- and industry-funded type 2 diabetes RCTs relative to their disease burden, while white participants appear to be over-represented in industry-funded trials. This meta-epidemiological review shows that the greatest disparity in ethnic and racial diversity in RCTs occurs in industry-funded trials. Strategies to improve the recruitment and enrolment of racialised participants into industry- and government-funded RCTs should be developed.
Abbreviations
- DPP-4:
-
Dipeptidyl peptidase-4
- GLP-1:
-
Glucagon-like peptide-1
- NIH:
-
National Institutes of Health
- PPR:
-
Participation-to-prevalence ratio
- SGLT-2:
-
Sodium–glucose co-transporter 2
References
Oldroyd J (2005) Diabetes and ethnic minorities. Postgrad Med J 81(958):486–490. https://doi.org/10.1136/pgmj.2004.029124
Hariton E, Locascio JJ (2018) Randomised controlled trials – the gold standard for effectiveness research: study design: randomised controlled trials. BJOG 125(13):1716–1716. https://doi.org/10.1111/1471-0528.15199
Khan SU, Khan MZ, Raghu Subramanian C et al (2020) Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: a systematic review. JAMA Netw Open 3(5):e205202. https://doi.org/10.1001/jamanetworkopen.2020.5202
National Institutes of Health (2022) Inclusion of women and minorities as participants in research involving human subjects. Available from: https://grants.nih.gov/policy/inclusion/women-and-minorities.htm. Accessed 5 Feb 2023
Treweek S, Forouhi NG, Narayan KMV, Khunti K (2020) COVID-19 and ethnicity: who will research results apply to? Lancet 395(10242):1955–1957. https://doi.org/10.1016/S0140-6736(20)31380-5
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (2016) Integrated addendum to ICH E6(R1): guideline for good clinical practice. Available from: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Accessed 5 Feb 2023
Government of Canada (2007) Guidance for industry: standards for clinical trials in type 2 diabetes in Canada. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/clinical-trials/guidance-industry-standards-clinical-trials-type-2-diabetes-canada.html. Accessed 5 Feb 2023
National Health and Medical Research Council and Department of Industry (2017) Clinical trials toolkit. Available from: https://www.australianclinicaltrials.gov.au/clinical-trials-toolkit. Accessed 5 Feb 2023
Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL (2020) Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation 141(7):540–548. https://doi.org/10.1161/CIRCULATIONAHA.119.043594
Gerstein HC, Miller ME, Byington RP et al (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358(24):2545–2559. https://doi.org/10.1056/NEJMoa0802743
Frye RL, August P, Brooks MM et al (2009) A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 360(24):2503–2515. https://doi.org/10.1056/NEJMoa0805796
Colhoun HM, Betteridge DJ, Durrington PN et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364(9435):685–696. https://doi.org/10.1016/S0140-6736(04)16895-5
Keech A, Simes RJ, Barter P et al (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366(9500):1849–1861. https://doi.org/10.1016/S0140-6736(05)67667-2
Wexler DJ, Krause-Steinrauf H, Crandall JP et al (2019) Baseline characteristics of randomized participants in the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 42(11):2098–2107. https://doi.org/10.2337/dc19-0901
Kadoglou NPE, Iliadis F, Liapis CD, Perrea D, Angelopoulou N, Alevizos M (2007) Beneficial effects of combined treatment with rosiglitazone and exercise on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care 30(9):2242–2244. https://doi.org/10.2337/dc07-0341
Levin SR, Coburn JW, Abraira C et al (2000) Effect of intensive glycemic control on microalbuminuria in type 2 diabetes veterans affairs cooperative study on glycemic control and complications in type 2 diabetes feasibility trial investigators. Diabetes Care 23(10):1478–1485. https://doi.org/10.2337/diacare.23.10.1478
Meyer C, Boron A, Plummer E, Voltchenok M, Vedda R (2010) Glulisine versus human regular insulin in combination with glargine in noncritically ill hospitalized patients with type 2 diabetes. Diabetes Care 33(12):2496–2501. https://doi.org/10.2337/dc10-0957
Feig DS, Donovan LE, Zinman B et al (2020) Metformin in women with type 2 diabetes in pregnancy (MiTy): a multicenter, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 8(10):834–844. https://doi.org/10.1016/S2213-8587(20)30310-7
Hong J, Zhang Y, Lai S et al (2013) Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 36(5):1304–1311. https://doi.org/10.2337/dc12-0719
Abdul-Ghani M, Migahid O, Megahed A et al (2017) Combination therapy with exenatide plus pioglitazone versus basal/bolus insulin in patients with poorly controlled type 2 diabetes on sulfonylurea plus metformin: The Qatar Study. Diabetes Care 40(3):325–331. https://doi.org/10.2337/dc16-1738
Goldfine AB (2010) The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med 152(6):346–357. https://doi.org/10.7326/0003-4819-152-6-201003160-00004
Goldfine AB (2013) Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med 159(1):1–12. https://doi.org/10.7326/0003-4819-159-1-201307020-00003
Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR, for the UK Prospective Diabetes Study Group (2002) Sulfonylurea inadequacy. Diabetes Care 25(2):330–336. https://doi.org/10.2337/diacare.25.2.330
Duckworth W, Abraira C, Moritz T et al (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360(2):129–139. https://doi.org/10.1056/NEJMoa0808431
Pratley RE, Nauck M, Bailey T et al (2010) Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet 375(9724):1447–1456. https://doi.org/10.1016/S0140-6736(10)60307-8
Holman RR, Thorne KI, Farmer AJ et al (2007) Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 357(17):1716–1730. https://doi.org/10.1056/NEJMoa075392
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355(23):2427–2443. https://doi.org/10.1056/NEJMoa066224
Lincoff AM, Tardif JC, Schwartz GG et al (2014) Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA 311(15):1515–1525. https://doi.org/10.1001/jama.2014.3321
Parving HH, Brenner BM, McMurray JJV et al (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367(23):2204–2213. https://doi.org/10.1056/NEJMoa1208799
Bakris GL, Agarwal R, Chan JC et al (2015) Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA 314(9):884–894. https://doi.org/10.1001/jama.2015.10081
Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK (2008) Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358(23):2433–2446. https://doi.org/10.1056/NEJMoa0708379
Blonde L, Jendle J, Gross J et al (2015) Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet 385(9982):2057–2066. https://doi.org/10.1016/S0140-6736(15)60936-9
Dungan KM, Povedano ST, Forst T et al (2014) Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 384(9951):1349–1357. https://doi.org/10.1016/S0140-6736(14)60976-4
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375(9733):2223–2233. https://doi.org/10.1016/S0140-6736(10)60407-2
Barnett AH, Huisman H, Jones R, von Eynatten M, Patel S, Woerle HJ (2013) Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet 382(9902):1413–1423. https://doi.org/10.1016/S0140-6736(13)61500-7
de Zeeuw D, Akizawa T, Audhya P et al (2013) Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369(26):2492–2503. https://doi.org/10.1056/NEJMoa1306033
Garber AJ, King AB, Prato SD et al (2012) Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 379(9825):1498–1507. https://doi.org/10.1016/S0140-6736(12)60205-0
Burant CF, Viswanathan P, Marcinak J et al (2012) TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379(9824):1403–1411. https://doi.org/10.1016/S0140-6736(11)61879-5
Buse JB, Bergenstal RM, Glass LC et al (2011) Use of twice-daily exenatide in basal insulin–treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 154(2):103–112. https://doi.org/10.7326/0003-4819-154-2-201101180-00300
Cefalu WT, Leiter LA, Yoon KH et al (2013) Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382(9896):941–950. https://doi.org/10.1016/S0140-6736(13)60683-2
Neal B, Perkovic V, Mahaffey KW et al (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377(7):644–657. https://doi.org/10.1056/NEJMoa1611925
Rosenstock J, Perkovic V, Johansen OE et al (2019) Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 321(1):69–79. https://doi.org/10.1001/jama.2018.18269
Perkovic V, Jardine MJ, Neal B et al (2019) Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380(24):2295–2306. https://doi.org/10.1056/NEJMoa1811744
(2001) Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 357(9260):905–910. https://doi.org/10.1016/S0140-6736(00)04209-4
Wilding JPH (2012) Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med 156(6):405–415. https://doi.org/10.7326/0003-4819-156-6-201203200-00003
Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J (2017) Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 318(15):1460–1470. https://doi.org/10.1001/jama.2017.14752
Wiviott SD, Raz I, Bonaca MP et al (2019) Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380(4):347–357. https://doi.org/10.1056/NEJMoa1812389
Barnett AH, Bain SC, Bouter P et al (2004) Angiotensin-receptor blockade versus converting–enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351(19):1952–1961. https://doi.org/10.1056/NEJMoa042274
Marso SP, McGuire DK, Zinman B et al (2017) Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 377(8):723–732. https://doi.org/10.1056/NEJMoa1615692
Sjølie AK, Klein R, Porta M et al (2008) Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet 372(9647):1385–1393. https://doi.org/10.1016/S0140-6736(08)61411-7
Lingvay I, Manghi FP, García-Hernández P et al (2016) Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA 315(9):898–907. https://doi.org/10.1001/jama.2016.1252
Drucker DJ, Buse JB, Taylor K et al (2008) Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372(9645):1240–1250. https://doi.org/10.1016/S0140-6736(08)61206-4
Bergenstal RM, Wysham C, MacConell L et al (2010) Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 376(9739):431–439. https://doi.org/10.1016/S0140-6736(10)60590-9
Diamant M, Van Gaal L, Stranks S et al (2010) Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 375(9733):2234–2243. https://doi.org/10.1016/S0140-6736(10)60406-0
Buse JB, Nauck M, Forst T et al (2013) Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 381(9861):117–124. https://doi.org/10.1016/S0140-6736(12)61267-7
Pfeffer MA, Claggett B, Diaz R et al (2015) Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 373(23):2247–2257. https://doi.org/10.1056/NEJMoa1509225
Zinman B, Wanner C, Lachin JM et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373(22):2117–2128. https://doi.org/10.1056/NEJMoa1504720
Gallwitz B, Guzman J, Dotta F et al (2012) Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet 379(9833):2270–2278. https://doi.org/10.1016/S0140-6736(12)60479-6
White WB, Cannon CP, Heller SR et al (2013) Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 369(14):1327–1335. https://doi.org/10.1056/NEJMoa1305889
Holman RR, Bethel MA, Mentz RJ et al (2017) Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 377(13):1228–1239. https://doi.org/10.1056/NEJMoa1612917
Frias JP, Nauck MA, Van J et al (2018) Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392(10160):2180–2193. https://doi.org/10.1016/S0140-6736(18)32260-8
Gallwitz B, Rosenstock J, Rauch T et al (2012) 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 380(9840):475–483. https://doi.org/10.1016/S0140-6736(12)60691-6
Hernandez AF, Green JB, Janmohamed S et al (2018) Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 392(10157):1519–1529. https://doi.org/10.1016/S0140-6736(18)32261-X
Heine RJ, Van Gaal LF, Johns D et al (2005) Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 143(8):559–569. https://doi.org/10.7326/0003-4819-143-8-200510180-00006
Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldánius PM (2013) Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet 382(9890):409–416. https://doi.org/10.1016/S0140-6736(13)60995-2
Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345(12):870–878. https://doi.org/10.1056/NEJMoa011489
Garber A, Henry R, Ratner R et al (2009) Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373(9662):473–481. https://doi.org/10.1016/S0140-6736(08)61246-5
Buse JB, Rosenstock J, Sesti G et al (2009) Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374(9683):39–47. https://doi.org/10.1016/S0140-6736(09)60659-0
Marso SP, Daniels GH, Brown-Frandsen K et al (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
Lewis EJ, Hunsicker LG, Clarke WR et al (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345(12):851–860. https://doi.org/10.1056/NEJMoa011303
Nissen SE, Nicholls SJ, Wolski K et al (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299(13):1561–1573. https://doi.org/10.1001/jama.299.13.1561
Rosenstock J, Allison D, Birkenfeld AL et al (2019) Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA 321(15):1466–1480. https://doi.org/10.1001/jama.2019.2942
Pratley R, Amod A, Hoff ST et al (2019) Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 394(10192):39–50. https://doi.org/10.1016/S0140-6736(19)31271-1
Husain M, Birkenfeld AL, Donsmark M et al (2019) Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 381(9):841–851. https://doi.org/10.1056/NEJMoa1901118
Dormandy JA, Charbonnel B, Eckland DJ et al (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366(9493):1279–1289. https://doi.org/10.1016/S0140-6736(05)67528-9
Ray KK, Nicholls SJ, Buhr KA et al (2020) Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: a randomized clinical trial. JAMA 323(16):1565–1573. https://doi.org/10.1001/jama.2020.3308
Home PD, Pocock SJ, Beck-Nielsen H et al (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373(9681):2125–2135. https://doi.org/10.1016/S0140-6736(09)60953-3
Brenner BM, Cooper ME, de Zeeuw D et al (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345(12):861–869. https://doi.org/10.1056/NEJMoa011161
Gerstein HC, Colhoun HM, Dagenais GR et al (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193):121–130. https://doi.org/10.1016/S0140-6736(19)31149-3
Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF (2006) Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368(9548):1660–1672. https://doi.org/10.1016/S0140-6736(06)69571-8
Haller H, Ito S, Izzo JL et al (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364(10):907–917. https://doi.org/10.1056/NEJMoa1007994
Rosenstock J, Lorber DL, Gnudi L et al (2010) Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet 375(9733):2244–2253. https://doi.org/10.1016/S0140-6736(10)60632-0
Scirica BM, Bhatt DL, Braunwald E et al (2013) Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 369(14):1317–1326. https://doi.org/10.1056/NEJMoa1307684
Davies MJ, Bergenstal R, Bode B et al (2015) Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA 314(7):687–699. https://doi.org/10.1001/jama.2015.9676
Heerspink HJL, Parving HH, Andress DL et al (2019) Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 393(10184):1937–1947. https://doi.org/10.1016/S0140-6736(19)30772-X
Green JB, Bethel MA, Armstrong PW et al (2015) Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 373(3):232–242. https://doi.org/10.1056/NEJMoa1501352
Steg PG, Bhatt DL, Simon T et al (2019) Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 381(14):1309–1320. https://doi.org/10.1056/NEJMoa1908077
Pfeffer MA, Burdmann EA, Chen CY et al (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361(21):2019–2032. https://doi.org/10.1056/NEJMoa0907845
Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S (2019) Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet 394(10208):1519–1529. https://doi.org/10.1016/S0140-6736(19)32131-2
Cannon CP, Pratley R, Dagogo-Jack S et al (2020) Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 383(15):1425–1435. https://doi.org/10.1056/NEJMoa2004967
Zinman B, Hoogwerf BJ, Durán García S et al (2007) The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 146(7):477–485. https://doi.org/10.7326/0003-4819-146-7-200704030-00003
Zinman B, Fulcher G, Rao PV et al (2011) Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: a 16-week, randomised, open-label, phase 2 trial. Lancet 377(9769):924–931. https://doi.org/10.1016/S0140-6736(10)62305-7
Fayfman M, Galindo RJ, Rubin DJ et al (2019) A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diabetes Care 42(3):450–456. https://doi.org/10.2337/dc18-1760
Dailey G, Rosenstock J, Moses RG, Ways K (2004) Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 27(10):2363–2368. https://doi.org/10.2337/diacare.27.10.2363
Zhu D, Gan S, Liu Y et al (2018) Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol 6(8):627–636. https://doi.org/10.1016/S2213-8587(18)30105-0
Vos T, Lim SS, Abbafati C et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Chastain DB, Osae SP, Henao-Martínez AF, Franco-Paredes C, Chastain JS, Young HN (2020) Racial disproportionality in Covid clinical trials. N Engl J Med 383(9):2486–2490. https://doi.org/10.1056/NEJMp2021971
Rockey S (2011) New NIH study on diversity. Available from: https://nexus.od.nih.gov/all/2011/08/18/new-nih-study-on-diversity/. Accessed 6 Feb 2023
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
We thank J. Wu (McMaster University, ON, Canada) for his assistance with extracting data and creating summary tables.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Demographic and prevalence data sourced from resources in the public domain are referenced in the ESM.
Funding
SSA holds a Canada Research Chair in Ethnic Diversity and Cardiovascular Disease (Tier 1) and Michael G. DeGroote Heart and Stroke Chair in Population Health.
Authors’ relationships and activities
SSA holds a Canada Research Chair in Ethnic Diversity and Cardiovascular Disease and the Heart and Stroke Michael G DeGroote Chair in Population Health. SSA also has received honoraria from Bayer, Janssen Pharmaceuticals, Novartis, and Daiichi Sankyo (consulting and speaker fees). RJdS has served as an external resource person to the WHO’s Nutrition Guidelines Advisory Group on trans fats, saturated fats and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012 to 2017 to present and discuss this work. He presented updates of this work to the WHO in 2022. He has also carried out contract research for the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes, Health Canada and the WHO for which he received remuneration. He has received speaker’s fees from the University of Toronto and McMaster Children’s Hospital. He has held grants from the Canadian Institutes of Health Research, Canadian Foundation for Dietetic Research, Population Health Research Institute and Hamilton Health Sciences Corporation as a principal investigator and is a co-investigator on several funded team grants from the Canadian Institutes of Health Research. He has served as an independent director of the Helderleigh Foundation (Canada) and as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada). He is a co-opted member of the Scientific Advisory Committee on Nutrition (SACN) Subgroup on the Framework for the Evaluation of Evidence (Public Health England).
Contribution statement
SSA, RJdS and RA were responsible for the conception and design of the study. LB developed the search strategy. RA and RJdS screened the articles and RA and VL carried out the data extraction. RA, RJdS, VL and SSA were responsible for the analysis and interpretation of the results. RA, RJdS and SSA drafted the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors reviewed and approved the final version of the manuscript to be published. RJdS and SSA are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, R., de Souza, R.J., Li, V. et al. Twenty years of participation of racialised groups in type 2 diabetes randomised clinical trials: a meta-epidemiological review. Diabetologia 67, 443–458 (2024). https://doi.org/10.1007/s00125-023-06052-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-06052-w